Sex-Specific Risk Factors and Predictors of Major Adverse Cardiac and Cerebrovascular Events in Heart Failure with Preserved Ejection Fraction with SARS-CoV-2 Infection: A Nationwide Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Statistical Analysis
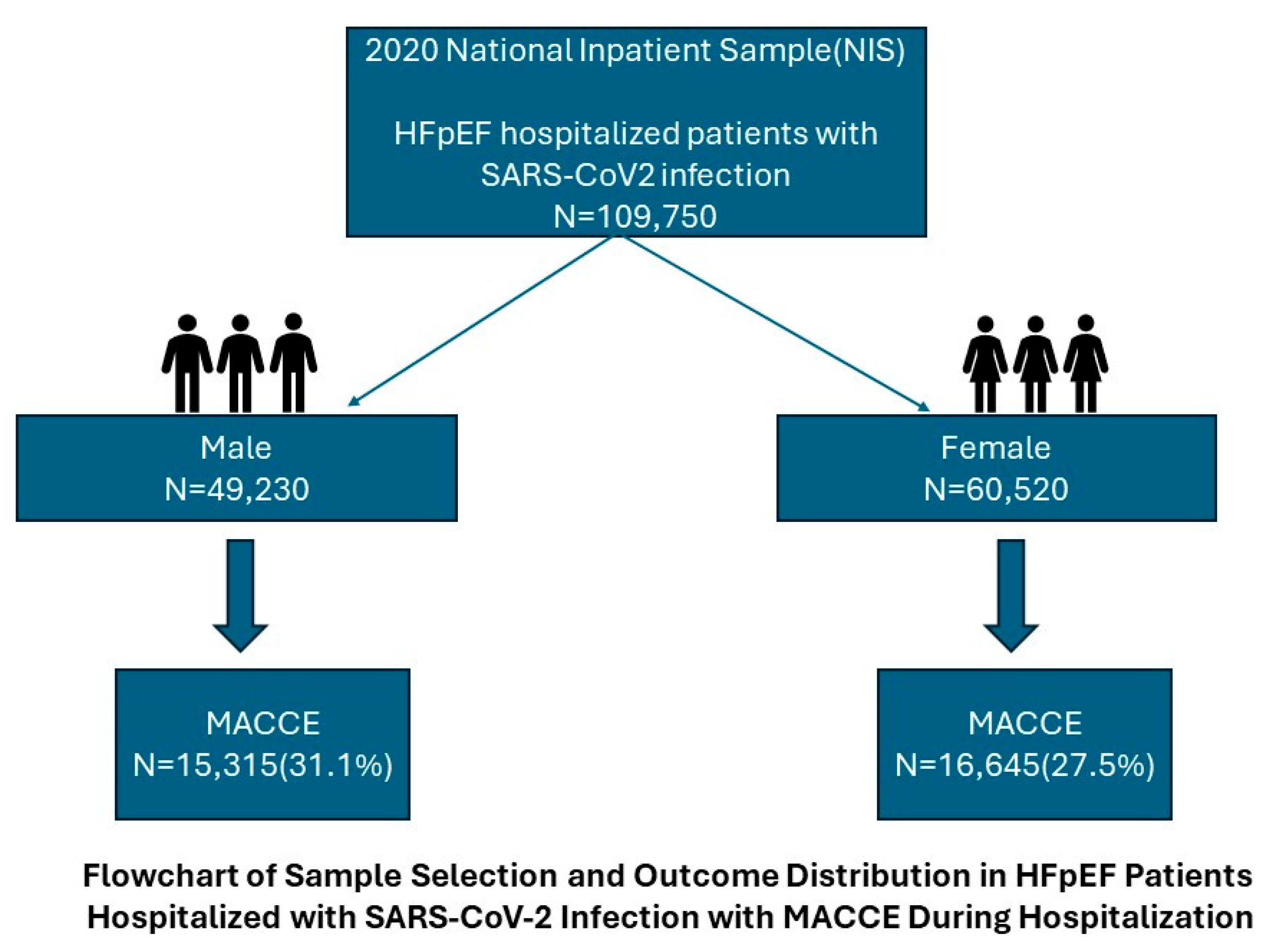
3. Results
3.1. Demographic Characteristics
3.2. Predictors of MACCEs in the Overall Cohort
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Universal Definition and Classification of Heart Failure: A Step in the Right Direction from Failure to Function. American College of Cardiology. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2021/07/12/12/31/http%3a%2f%2fwww.acc.org%2fLatest-in-Cardiology%2fArticles%2f2021%2f07%2f12%2f12%2f31%2fUniversal-Definition-and-Classification-of-Heart-Failure (accessed on 31 August 2023).
- CDC. Heart Failure | cdc.gov. Centers for Disease Control and Prevention. Published 5 January 2023. Available online: https://www.cdc.gov/heartdisease/heart_failure.htm (accessed on 31 August 2023).
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Gangu, K.; Cannon, H.R.; Khan, U.A.; Shumway, N.B.; Bobba, A.; Sagheer, S.; Chourasia, P.; Shuja, H.; Avula, S.R.; et al. COVID-19 and Heart Failure with Preserved and Reduced Ejection Fraction Clinical Outcomes among Hospitalized Patients in the United States. Viruses 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Zaccone, G.; Tomasoni, D.; Tomasoni, D.; Italia, L.; Italia, L.; Lombardi, C.M.; Lombardi, C.M.; Metra, M.; Metra, M. Myocardial Involvement in COVID-19: An Interaction Between Comorbidities and Heart Failure with Preserved Ejection Fraction. A Further Indication of the Role of Inflammation. Curr. Heart Fail. Rep. 2021, 18, 99–106. [Google Scholar] [CrossRef]
- HCUP-US NIS Overview. Available online: https://hcup-us.ahrq.gov/nisoverview.jsp (accessed on 31 August 2023).
- Panagides, V.; Vincent, F.; Weizman, O.; Jonveaux, M.; Trimaille, A.; Pommier, T.; Cellier, J.; Geneste, L.; Marsou, W.; Deney, A.; et al. History of heart failure in patients with coronavirus disease 2019: Insights from a French registry. Arch. Cardiovasc. Dis. 2021, 114, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.d.P.; Del Carlo, C.H.; Gonçalinho, G.H.F.; Avakian, S.D.; Ribeiro, L.C.; Ianni, B.M.; Fernandes, F.; César, L.A.M.; Bocchi, E.A.; Pereira-Barretto, A.C. Sex Differences in Heart Failure Mortality with Preserved, Mildly Reduced and Reduced Ejection Fraction: A Retrospective, Single-Center, Large-Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 16171. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Jering, K.S.; Vaduganathan, M.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Signorovitch, J.; Thune, J.J.; Vardeny, O.; Solomon, S.D. Clinical Outcomes in Patients With Heart Failure Hospitalized With COVID-19. JACC Heart Fail. 2021, 9, 65–73. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Gialluisi, A.; Antinori, A.; Berselli, N.; Blandi, L.; Bruno, R.; Cauda, R.; Guaraldi, G.; et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: Survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 1899–1913. [Google Scholar] [CrossRef] [PubMed]
- Akwo, E.A.; Kabagambe, E.K.; Harrell, F.E.; Blot, W.J.; Bachmann, J.M.; Wang, T.J.; Gupta, D.K.; Lipworth, L. Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004052. [Google Scholar] [CrossRef] [PubMed]
- Raifman, M.A.; Raifman, J.R. Disparities in the Population at Risk of Severe Illness From COVID-19 by Race/Ethnicity and Income. Am. J. Prev. Med. 2020, 59, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, I.; Patella, G.; Michael, A.; Serra, R.; Provenzano, M.; Andreucci, M. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J. Clin. Med. 2020, 9, 2506. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Stretti, L.; Zippo, D.; Coats, A.J.; Anker, M.S.; von Haehling, S.; Metra, M.; Tomasoni, D. A year in heart failure: An update of recent findings. ESC Heart Fail. 2021, 8, 4370–4393. [Google Scholar] [CrossRef] [PubMed]
- Aboueshia, M.; Hussein, M.H.; Attia, A.S.; Swinford, A.; Miller, P.; Omar, M.; Toraih, E.A.; Saba, N.; Safah, H.; Duchesne, J.; et al. Cancer and COVID-19: Analysis of patient outcomes. Future Oncol. Lond. Engl. 2021, 17, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Leslie, A.; Hewitt, J.N.; Kovoor, J.G.; Ovenden, C.D.; Edwards, S.; Chan, J.C.Y.; Worthington, M.G. Cardiac surgery on patients with COVID-19: A systematic review and meta-analysis. ANZ J. Surg. 2022, 92, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Kosiborod, M.N.; Claggett, B.L.; Miao, Z.M.; Vaduganathan, M.; Lam, C.S.P.; Hernandez, A.F.; Martinez, F.A.; Inzucchi, S.E.; Shah, S.J.; et al. Impact of COVID-19 in patients with heart failure with mildly reduced or preserved ejection fraction enrolled in the DELIVER trial. Eur. J. Heart Fail. 2023, 25, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, M.S.; Kalra, A.; Gupta, T.; Kolte, D.; Khera, S.; Bhatt, D.L.; Ginwalla, M. Effect of Influenza on Outcomes in Patients With Heart Failure. JACC Heart Fail. 2019, 7, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Patel, S.; Thapi, S.; Jaladanki, S.K.; Rao, A.; Nirenberg, S.; Lala, A. Association of Reduced Hospitalizations and Mortality Rates Among COVID-19-Vaccinated Patients With Heart Failure. J. Card. Fail. 2022, 28, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Maddox, T.M.; Ferdinand, K.C.; Kirkpatrick, J.N.; Ky, B.; Morris, A.A.; Mullen, J.B.; Parikh, S.A.; Philbin, D.M., Jr.; Vaduganathan, M. ACC Health Policy Statement on Cardiovascular Disease Considerations for COVID-19 Vaccine Prioritization: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.; Jankowska, E.A.; Ray, R.; Metra, M.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Bayes-Genis, A.; Belenkov, Y.; Ben Gal, T.; et al. COVID-19 vaccination in patients with heart failure: A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 1806–1818. [Google Scholar] [CrossRef]
| Variable | Male (49,230) | Female (60,520) | Total Inpatient Encounters with HFpEF with SARS-CoV-2 | p-Value | |
|---|---|---|---|---|---|
| Age (years) at admission, median [IQR] | 75 (65–83) | 77 (67–85) | 76 (67–84) | <0.05 | |
| 18–44 | 2.4% | 1.8% | 2.1% | ||
| 45–64 | 20.8% | 17.5% | 19.0% | ||
| ≥65 | 76.7% | 80.7% | 78.9% | ||
| Race | White | 66.8% | 63.1% | 64.8% | <0.05 |
| Black | 18.4% | 23.4% | 21.1% | ||
| Hispanic | 11.8% | 10.7% | 11.2% | ||
| Asian/PI | 2.2% | 2.1% | 2.2% | ||
| Native American | 0.7% | 0.7% | 0.7% | ||
| Median household income national quartile for patient ZIP code | 0–25th | 32.2% | 33.8% | 33.1% | <0.05 |
| 26–50th | 28.1% | 27.7% | 27.9% | ||
| 51–75th | 23.8% | 22.3% | 23.0% | ||
| 76–100th | 15.8% | 16.2% | 16.1% | ||
| Payer type | Medicare | 78.7% | 81.5% | 80.2% | <0.05 |
| Medicaid | 7.2% | 7.6% | 7.4% | ||
| Private | 12.9% | 9.8% | 11.2% | ||
| Self-pay | 1.1% | 1.0% | 1.0% | ||
| No charge | 0.1% | 0.1% | 0.1% | ||
| Hospital region | Northeast | 19.7% | 19.4% | 19.5% | <0.05 |
| Midwest | 29.7% | 28.8% | 29.2% | ||
| South | 36.1% | 38.8% | 37.6% | ||
| West | 14.5% | 12.9% | 13.6% | ||
| Comorbidities | |||||
| Hypertension | 88.2% | 88.8% | 88.6% | 0.02 | |
| Diabetes | 55.5% | 53.5% | 54.4% | <0.05 | |
| Hyperlipidemia | 57.0% | 54.0% | 55.3% | <0.05 | |
| Obesity | 29.9% | 36.2% | 33.3% | <0.05 | |
| Peripheral vascular disease | 9.2% | 7.0% | 8.0% | <0.05 | |
| Smoking | 32.7% | 22.6% | 27.1% | <0.05 | |
| Prior MI | 9.6% | 7.6% | 8.5% | <0.05 | |
| Prior TIA/stroke | 9.8% | 10.9% | 10.4% | <0.05 | |
| Prior VTE | 6.6% | 8.2% | 7.5% | <0.05 | |
| Cancer | 6.1% | 4.5% | 5.2% | <0.05 | |
| Chronic kidney disease | 51.1% | 47.0% | 48.8% | <0.05 | |
| Alcohol abuse | 2.8% | 0.6% | 1.6% | <0.05 | |
| Drug abuse | 2.3% | 1.5% | 1.8% | <0.05 | |
| Depression | 11.6% | 17.7% | 14.9% | <0.05 | |
| Chronic pulmonary disease | 36.8% | 42.2% | 39.7% | <0.05 | |
| Hypothyroidism | 12.7% | 25.7% | 19.9% | <0.05 | |
| Other thyroid disorders | 0.9% | 1.5% | 1.3% | <0.05 | |
| Valvular disease | 4.5% | 3.4% | 3.9% | <0.05 | |
| Metastatic cancer | 1.4% | 0.9% | 1.1% | <0.05 | |
| Predictor | Odds Ratio | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age at admission | 18–44 vs. 45–64 | 0.62 | 0.47 | 0.82 | <0.001 |
| ≥65 vs. 45–64 | 1.47 | 1.33 | 1.62 | ||
| Sex | Male vs. female | 1.20 | 1.12 | 1.28 | <0.001 |
| Race | Black vs. White | 1.01 | 0.92 | 1.10 | |
| Hispanic vs. White | 1.27 | 1.14 | 1.42 | <0.001 | |
| Asian/Pacific Islander vs. White | 1.37 | 1.12 | 1.67 | ||
| Native American vs. White | 1.65 | 1.15 | 2.38 | ||
| Median household income national quartile for patient ZIP code | 0–25th vs. 76–100th | 1.16 | 1.05 | 1.30 | 0.012 |
| 26–50th vs. 76–100th | 1.11 | 1.00 | 1.23 | ||
| 51–75th vs. 76–100th | 1.2 | 0.92 | 1.14 | ||
| Payer type | Medicaid vs. Medicare | 0.93 | 0.81 | 1.07 | 0.034 |
| Private vs. Medicare | 1.02 | 0.91 | 1.15 | ||
| Self-pay vs. Medicare | 1.63 | 1.18 | 2.25 | ||
| No charge vs. Medicare | 1.23 | 0.31 | 4.92 | ||
| Type of admission | Non-elective vs. elective | 1.07 | 0.85 | 1.34 | 0.557 |
| Hospital bed size | Medium vs. small | 1.13 | 1.02 | 1.25 | 0.001 |
| Large vs. small | 1.18 | 1.08 | 1.29 | ||
| Hospital location and teaching status | Urban non-teaching vs. rural | 1.28 | 1.10 | 1.49 | 0.001 |
| Urban teaching vs. rural | 1.33 | 1.16 | 1.53 | ||
| Hospital region | Midwest vs. Northeast | 0.88 | 0.79 | 0.98 | 0.009 |
| South vs. Northeast | 0.84 | 0.76 | 0.93 | ||
| West vs. Northeast | 0.93 | 0.82 | 1.05 | ||
| Hypertension | 1.02 | 0.92 | 1.13 | 0.687 | |
| Diabetes | 0.99 | 0.92 | 1.06 | 0.747 | |
| Hyperlipidemia | 0.79 | 0.74 | 0.84 | <0.001 | |
| Obesity | 0.91 | 0.85 | 0.98 | 0.011 | |
| Peripheral vascular disease | 0.96 | 0.85 | 1.08 | 0.492 | |
| Tobacco use disorder | 0.76 | 0.71 | 0.82 | <0.001 | |
| Prior MI | 1.06 | 0.95 | 1.18 | 0.301 | |
| Prior PCI | 0.82 | 0.55 | 1.21 | 0.316 | |
| Prior CABG | 1.15 | 1.02 | 1.30 | 0.026 | |
| Prior TIA | 0.81 | 0.72 | 0.90 | <0.001 | |
| Prior SCA | 0.86 | 0.48 | 1.56 | 0.627 | |
| Prior VTE | 0.72 | 0.63 | 0.82 | <0.001 | |
| Cancer | 1.24 | 1.08 | 1.42 | 0.002 | |
| CKD | 1.15 | 1.08 | 1.23 | <0.001 | |
| AIDS | 0.61 | 0.32 | 1.15 | 0.124 | |
| Alcohol abuse | 0.76 | 0.58 | 1.01 | 0.060 | |
| Drug abuse | 0.96 | 0.75 | 1.23 | 0.746 | |
| Depression | 0.78 | 0.71 | 0.86 | <0.001 | |
| COPD | 0.98 | 0.92 | 1.05 | 0.543 | |
| Hypothyroidism | 0.96 | 0.88 | 1.04 | 0.272 | |
| Valvular heart disease | 0.77 | 0.65 | 0.91 | 0.002 | |
| Autoimmune conditions | 1.00 | 0.87 | 1.16 | 0.963 | |
| MACCE | Odds Ratio | 95 Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Male subpopulation | ||||
| Hyperlipidemia | 1.31 | 1.18 | 1.44 | <0.01 |
| Obesity | 1.13 | 1.01 | 1.27 | 0.028 |
| TUD | 1.37 | 1.23 | 1.53 | <0.01 |
| Prior stroke/TIA | 1.30 | 1.10 | 1.53 | 0.002 |
| Prior VTE | 1.47 | 1.20 | 1.80 | <0.001 |
| Alcohol abuse | 1.53 | 1.10 | 2.12 | 0.011 |
| Depression | 1.27 | 1.09 | 1.48 | 0.002 |
| Valvular disease | 1.50 | 1.17 | 1.92 | 0.001 |
| Female subpopulation | ||||
| Hyperlipidemia | 1.24 | 1.14 | 1.35 | <0.001 |
| TUD | 1.25 | 1.13 | 1.39 | <0.001 |
| Prior stroke/TIA | 1.19 | 1.04 | 1.37 | 0.014 |
| Prior VTE | 1.33 | 1.13 | 1.57 | 0.001 |
| Depression | 1.29 | 1.14 | 1.45 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekkala, S.P.; Mohammed, A.S.; Ahmed, H.; Al-Sulami, M.; Khan, J.; Desai, R.; Ghantasala, P.; Singh, H.; Ali, S.S.; Bianco, C. Sex-Specific Risk Factors and Predictors of Major Adverse Cardiac and Cerebrovascular Events in Heart Failure with Preserved Ejection Fraction with SARS-CoV-2 Infection: A Nationwide Analysis. J. Clin. Med. 2025, 14, 1469. https://doi.org/10.3390/jcm14051469
Lekkala SP, Mohammed AS, Ahmed H, Al-Sulami M, Khan J, Desai R, Ghantasala P, Singh H, Ali SS, Bianco C. Sex-Specific Risk Factors and Predictors of Major Adverse Cardiac and Cerebrovascular Events in Heart Failure with Preserved Ejection Fraction with SARS-CoV-2 Infection: A Nationwide Analysis. Journal of Clinical Medicine. 2025; 14(5):1469. https://doi.org/10.3390/jcm14051469
Chicago/Turabian StyleLekkala, Sai Prasanna, Adil Sarvar Mohammed, Hafeezuddin Ahmed, Meshal Al-Sulami, Jahangir Khan, Rupak Desai, Paritharsh Ghantasala, Hemindermeet Singh, Syed Sohail Ali, and Christopher Bianco. 2025. "Sex-Specific Risk Factors and Predictors of Major Adverse Cardiac and Cerebrovascular Events in Heart Failure with Preserved Ejection Fraction with SARS-CoV-2 Infection: A Nationwide Analysis" Journal of Clinical Medicine 14, no. 5: 1469. https://doi.org/10.3390/jcm14051469
APA StyleLekkala, S. P., Mohammed, A. S., Ahmed, H., Al-Sulami, M., Khan, J., Desai, R., Ghantasala, P., Singh, H., Ali, S. S., & Bianco, C. (2025). Sex-Specific Risk Factors and Predictors of Major Adverse Cardiac and Cerebrovascular Events in Heart Failure with Preserved Ejection Fraction with SARS-CoV-2 Infection: A Nationwide Analysis. Journal of Clinical Medicine, 14(5), 1469. https://doi.org/10.3390/jcm14051469






