Prediction of Postoperative Pain and Side Effects of Patient-Controlled Analgesia in Pediatric Orthopedic Patients Using Machine Learning: A Retrospective Study
Abstract
1. Introduction
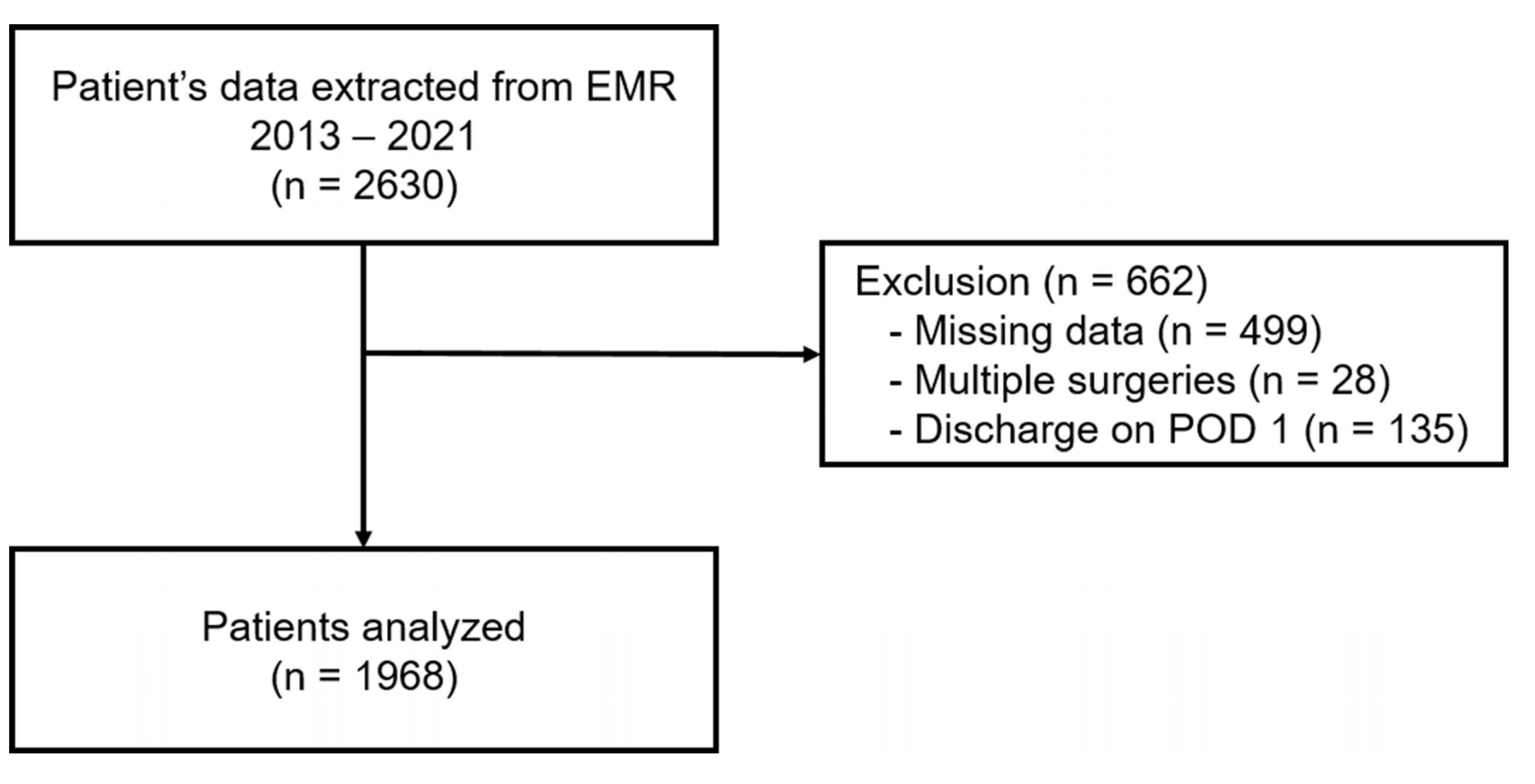
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Anesthetic Management and PCA Composition
2.3. Variables and Outcome Measures
2.4. Statistical Analysis
2.4.1. Machine Learning Methods and Model Selection
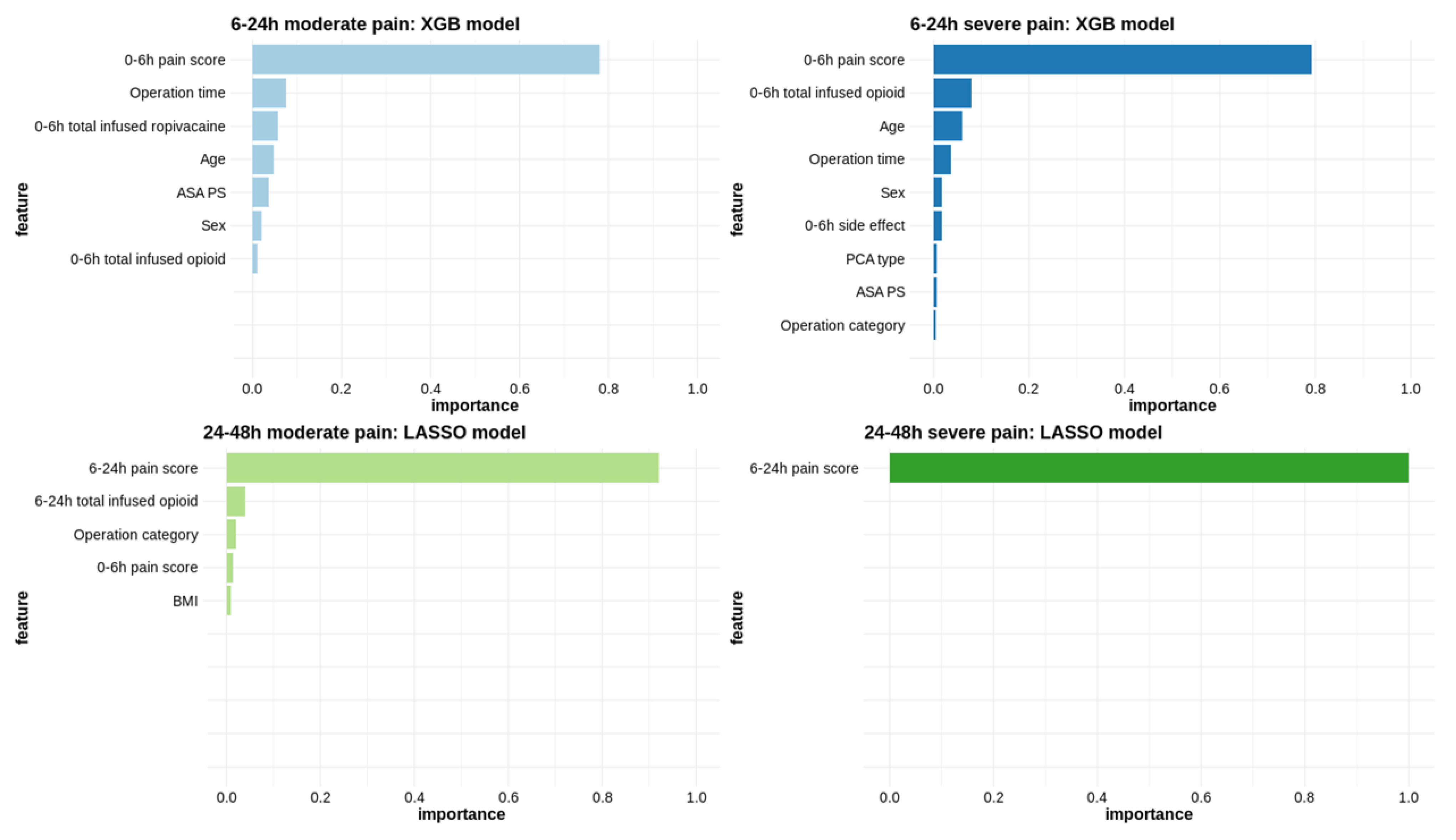
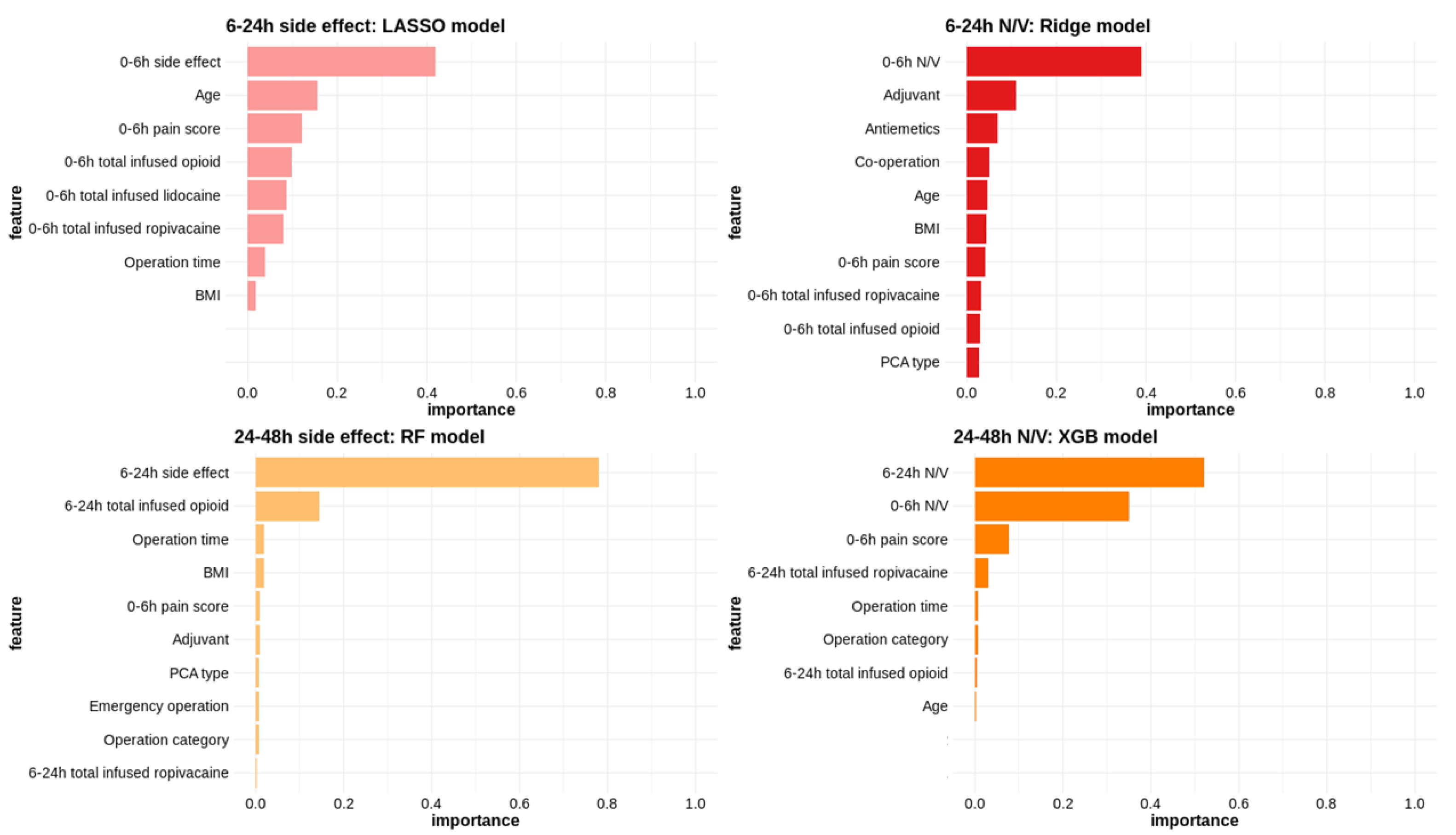
2.4.2. Variable Importance
2.4.3. Descriptive Analysis
3. Results
3.1. Patient Characteristics
3.2. Outcome Measures
3.2.1. Pain Score
3.2.2. Side Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brasher, C.; Gafsous, B.; Dugue, S.; Thiollier, A.; Kinderf, J.; Nivoche, Y.; Grace, R.; Dahmani, S. Postoperative pain management in children and infants: An update. Paediatr. Drugs 2014, 16, 129–140. [Google Scholar] [CrossRef]
- Taddio, A.; Katz, J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr. Drugs 2005, 7, 245–257. [Google Scholar] [CrossRef]
- Fortier, M.A.; Chou, J.; Maurer, E.L.; Kain, Z.N. Acute to chronic postoperative pain in children: Preliminary findings. J. Pediatr. Surg. 2011, 46, 1700–1705. [Google Scholar] [CrossRef]
- Frizzell, K.H.; Cavanaugh, P.K.; Herman, M.J. Pediatric Perioperative Pain Management. Orthop. Clin. North Am. 2017, 48, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Andras, L.M.; Andras, L.E.; Ellington, M.D.; Upasani, V.V.; Shah, A.S. What’s New in Pain Management for Pediatric Orthopaedic Surgery. J. Pediatr. Orthop. 2021, 41, e923–e928. [Google Scholar] [CrossRef] [PubMed]
- Tyler, D.C.; Pomietto, M.; Womack, W. Variation in opioid use during PCA in adolescents. Pediatr. Anesth. 1996, 6, 33–38. [Google Scholar] [CrossRef]
- Woodhouse, A.; Ward, E.M.; E Mather, L. Intra-subject variability in post-operative patient-controlled analgesia (PCA): Is the patient equally satisfied with morphine, pethidine and fentanyl? Pain 1999, 80, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Franson, H.E. Postoperative patient-controlled analgesia in the pediatric population: A literature review. Aana J. 2010, 78, 374–378. [Google Scholar]
- Berde, C.B.; Lehn, B.M.; Yee, J.D.; Sethna, N.F.; Russo, D. Patient-controlled analgesia in children and adolescents: A randomized, prospective comparison with intramuscular administration of morphine for postoperative analgesia. J. Pediatr. 1991, 118, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Jang, Y.E.; Kim, E.H.; Lee, J.H.; Kim, J.T.; Kim, H.S. Comparison of the Effects of Sufentanil and Fentanyl in Intravenous Patient-Controlled Analgesia after Pediatric Moyamoya Surgery: A Retrospective Study. Pediatr. Neurosurg. 2020, 55, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, W.K.; Lee, S.J.; Bai, S.J.; Lee, S.H.; Park, B.Y.; Min, K.T. Parent-controlled analgesia in children undergoing cleft palate repair. J. Korean Med. Sci. 2008, 23, 122–125. [Google Scholar] [CrossRef]
- Chiaretti, A.; Genovese, O.; Antonelli, A.; Tortorolo, L.; Ruggiero, A.; Focarelli, B.; Di Rocco, C. Patient-controlled analgesia with fentanil and midazolam in children with postoperative neurosurgical pain. Childs Nerv. Syst. 2008, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ocay, D.D.; Otis, A.; Teles, A.R.; Ferland, C.E. Safety of Patient-Controlled Analgesia After Surgery in Children And Adolescents: Concerns And Potential Solutions. Front. Pediatr. 2018, 6, 336. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.A.; Witkowski, E.; Gao, L.; Meireles, O.; Rosman, G. Artificial Intelligence in Anesthesiology: Current Techniques, Clinical Applications, and Limitations. Anesthesiology 2020, 132, 379–394. [Google Scholar] [CrossRef]
- Ramgopal, S.; Horvat, C.M.; Yanamala, N.; Alpern, E.R. Machine Learning to Predict Serious Bacterial Infections in Young Febrile Infants. Pediatrics 2020, 146, e20194096. [Google Scholar] [CrossRef]
- Dong, J.; Feng, T.; Thapa-Chhetry, B.; Cho, B.G.; Shum, T.; Inwald, D.P.; Newth, C.J.L.; Vaidya, V.U. Machine learning model for early prediction of acute kidney injury (AKI) in pediatric critical care. Crit. Care 2021, 25, 288. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Arshad, A.; Mazer, M.B.; Carroll, C.L.; Shein, S.L.; Remy, K.E. The use of machine learning and artificial intelligence within pediatric critical care. Pediatr. Res. 2023, 93, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Lamperti, M.; Romero, C.S.; Guarracino, F.; Cammarota, G.; Vetrugno, L.; Tufegdzic, B.; Lozsan, F.; Macias Frias, J.J.; Duma, A.; Bock, M.; et al. Preoperative assessment of adults undergoing elective noncardiac surgery: Updated guidelines from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2025, 42, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R.; Narasimhan, B.; Tay, K.; Simon, N.; Qian, J.; Package ‘glmnet’. CRAN R Repositary 2021. Available online: https://cran.r-project.org/web/packages/glmnet/index.html (accessed on 15 November 2022).
- Karatzoglou, A.; Smola, A.; Hornik, K.; Karatzoglou, M.A.; Package ‘kernlab’. CRAN R Project 2019. Available online: https://www.vps.fmvz.usp.br/CRAN/web/packages/kernlab/kernlab.pdf (accessed on 15 November 2022).
- Liaw, M.A. Package ‘Randomforest’; University of California: Berkeley, CA, USA, 2018. [Google Scholar]
- Liu, B.; Ou, G.; Chen, Y.; Zhang, J. Inhibition of protein tyrosine phosphatase 1B protects against sevoflurane-induced neurotoxicity mediated by ER stress in developing brain. Brain Res. Bull. 2019, 146, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Prusty, S.; Patnaik, S.; Dash, S.K. SKCV: Stratified K-fold cross-validation on ML classifiers for predicting cervical cancer. Front. Nanotechnol. 2022, 4, 972421. [Google Scholar] [CrossRef]
- Parker, B.J.; Günter, S.; Bedo, J. Stratification bias in low signal microarray studies. BMC Bioinform. 2007, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Cortez, P.; Cortez, M.P.; Package ‘rminer’. Teaching Report 2016, 59. Available online: http://r.meteo.uni.wroc.pl/web/packages/rminer/rminer.pdf (accessed on 15 November 2022).
- Alba, A.C.; Agoritsas, T.; Walsh, M.; Hanna, S.; Iorio, A.; Devereaux, P.J.; McGinn, T.; Guyatt, G. Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA 2017, 318, 1377–1384. [Google Scholar] [CrossRef]
- Koh, J.C.; Lee, J.; Kim, S.Y.; Choi, S.; Han, D.W. Postoperative Pain and Intravenous Patient-Controlled Analgesia-Related Adverse Effects in Young and Elderly Patients: A Retrospective Analysis of 10,575 Patients. Medicine 2015, 94, e2008. [Google Scholar] [CrossRef] [PubMed]
- Charalampidis, A.; Rundberg, L.; Möller, H.; Gerdhem, P. Predictors of persistent postoperative pain after surgery for idiopathic scoliosis. J. Child. Orthop. 2021, 15, 458–463. [Google Scholar] [CrossRef]
- Seki, H.; Ideno, S.; Ishihara, T.; Watanabe, K.; Matsumoto, M.; Morisaki, H. Postoperative pain management in patients undergoing posterior spinal fusion for adolescent idiopathic scoliosis: A narrative review. Scoliosis 2018, 13, 17. [Google Scholar] [CrossRef]
- Wong, G.T.C.; Yuen, V.M.Y.; Chow, B.F.M.; Irwin, M.G. Persistent pain in patients following scoliosis surgery. Eur. Spine J. 2007, 16, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
| Patient-related | Anesthesia-related |
| Age | Type of PCA (IV, epidural) |
| Sex | PCA-related |
| Height | Total amount of opioid infused (mcg) |
| Weight | Adjuvant pain killer mixed |
| BMI (categorical: <18.5, 18.5–25, ≥25) | Anti-emetics mixed |
| ASA PS | |
| Surgery-related | Post-operative |
| Operation time | Pain score in PACU |
| Complexity of surgery | Pain score 0–6 h postoperatively |
| Co-operation | Pain score 6–24 h postoperatively |
| Emergency operation | Analgesia used in ward |
| Variable | n = 1968 |
|---|---|
| Age (years) | 8.06 ± 3.16 |
| Female | 893 (45.4) |
| Height (cm) | 126.68 ± 21.87 |
| Weight (kg) | 30.84 ± 14.87 |
| BMI (kg/m2) | |
| <18 | 1234 (62.7) |
| 18–25 | 611 (31.0) |
| ≥25 | 123 (6.2) |
| ASA PS | |
| 1 | 869 (44.2) |
| 2 | 928 (47.2) |
| 3 | 171 (8.7) |
| Operation time (min) | 154.75 ± 113.73 |
| Anesthesia time (min) | 174.63 ± 129.26 |
| Overall | Sex Group | Age Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | p | Age ≤ 1 | 1 < Age ≤ 6 | Age > 6 | p | ||
| n | 1968 | 1075 | 893 | 81 | 503 | 1384 | ||
| Postoperative period 0–6 h | ||||||||
| Pain score | 2.33 ± 2.44 | 2.33 ± 2.39 | 2.34 ± 2.49 | 0.939 | 0.70 ± 1.57 | 1.44 ± 2.03 | 2.75 ± 2.49 | <0.001 |
| Severity of pain | 0.283 | <0.001 | ||||||
| Mild (<4) | 1384 (70.3) | 765 (71.2) | 619 (69.3) | 77 (95.1) | 415 (82.5) | 892 (64.5) | ||
| Moderate (≥4, <7) | 466 (23.7) | 254 (23.6) | 212 (23.7) | 3 (3.7) | 78 (15.5) | 385 (27.8) | ||
| Severe (≥7) | 117 (5.9) | 55 (5.1) | 62 (6.9) | 1 (1.2) | 10 (2.0) | 106 (7.7) | ||
| Any side effect | 172 (8.7) | 102 (9.5) | 70 (7.8) | 0.226 | 3 (3.7) | 29 (5.8) | 140 (10.1) | 0.003 |
| Nausea/vomiting | 121 (6.1) | 69 (6.4) | 52 (5.8) | 0.65 | 3 (3.7) | 18 (3.6) | 100 (7.2) | 0.009 |
| Postoperative period 6–24 h | ||||||||
| Pain score | 1.58 ± 2.39 | 1.48 ± 1.97 | 1.69 ± 2.81 | 0.05 | 0.41 ± 1.51 | 0.96 ± 2.96 | 1.87 ± 2.13 | <0.001 |
| Severity of pain | 0.244 | <0.001 | ||||||
| Mild (<4) | 1607 (81.7) | 894 (83.2) | 713 (79.8) | 78 (96.3) | 453 (90.1) | 1076 (77.7) | ||
| Moderate (≥4, <7) | 306 (15.5) | 155 (14.4) | 151 (16.9) | 0 (0.0) | 39 (7.8) | 267 (19.3) | ||
| Severe (≥7) | 50 (2.5) | 23 (2.1) | 27 (3.0) | 3 (3.7) | 9 (1.8) | 38 (2.7) | ||
| Any side effect | 121 (6.1) | 71 (6.6) | 50 (5.6) | 0.406 | 1 (1.2) | 10 (2.0) | 110 (7.9) | <0.001 |
| Nausea/vomiting | 90 (4.6) | 50 (4.7) | 40 (4.5) | 0.942 | 1 (1.2) | 9 (1.8) | 80 (5.8) | <0.001 |
| Postoperative period 24–48 h | ||||||||
| Pain score | 0.99 ± 1.69 | 1.02 ± 1.74 | 0.96 ± 1.64 | 0.558 | 0.35 ± 1.35 | 0.48 ± 1.26 | 1.21 ± 1.80 | <0.001 |
| Severity of pain | 0.125 | 0.023 | ||||||
| Mild (<4) | 846 (43.0) | 448 (41.7) | 398 (44.6) | 38 (46.9) | 226 (44.9) | 582 (42.1) | ||
| Moderate (≥4, <7) | 90 (4.6) | 42 (3.9) | 48 (5.4) | 1 (1.2) | 11 (2.2) | 78 (5.6) | ||
| Severe (≥7) | 10 (0.5) | 7 (0.7) | 3 (0.3) | 1 (1.2) | 1 (0.2) | 8 (0.6) | ||
| Any side effect | 33 (1.7) | 21 (2.0) | 12 (1.3) | 0.383 | 0 (0.0) | 5 (1.0) | 28 (2.0) | 0.149 |
| Nausea/vomiting | 23 (1.2) | 13 (1.2) | 10 (1.1) | 1 | 0 (0.0) | 3 (0.6) | 20 (1.4) | 0.192 |
| Specificity | Sensitivity | ACC | AUC | ||
|---|---|---|---|---|---|
| Moderate pain 6–24 h | XGB | 0.79 | 0.79 | 0.79 | 0.85 |
| LASSO | 0.87 | 0.67 | 0.83 | 0.84 | |
| RF | 0.85 | 0.7 | 0.83 | 0.84 | |
| Severe pain 6–24 h | XGB | 0.75 | 0.88 | 0.76 | 0.88 |
| RF | 0.79 | 0.76 | 0.79 | 0.85 | |
| LASSO | 0.92 | 0.64 | 0.91 | 0.83 | |
| Moderate pain 24–48 h | LASSO | 0.87 | 0.88 | 0.87 | 0.89 |
| Ridge | 0.8 | 0.89 | 0.81 | 0.89 | |
| XGB | 0.85 | 0.89 | 0.86 | 0.88 | |
| Severe pain 24–48 h | LASSO | 0.96 | 1 | 0.96 | 0.98 |
| XGB | 0.93 | 1 | 0.93 | 0.98 | |
| RF | 0.94 | 0.9 | 0.94 | 0.96 | |
| Side effect 6–24 h | LASSO | 0.63 | 0.74 | 0.64 | 0.75 |
| XGB | 0.66 | 0.71 | 0.66 | 0.74 | |
| Ridge | 0.63 | 0.74 | 0.64 | 0.74 | |
| Side effect 24–48 h | RF | 0.87 | 0.82 | 0.87 | 0.91 |
| Ridge | 0.86 | 0.88 | 0.86 | 0.9 | |
| XGB | 0.8 | 0.91 | 0.8 | 0.89 | |
| Nausea/vomiting 6–24 h | Ridge | 0.78 | 0.52 | 0.77 | 0.72 |
| XGB | 0.5 | 0.86 | 0.52 | 0.72 | |
| LASSO | 0.38 | 0.92 | 0.4 | 0.72 | |
| Nausea/vomiting 24–48 h | XGB | 0.81 | 0.87 | 0.81 | 0.91 |
| Ridge | 0.9 | 0.83 | 0.9 | 0.89 | |
| RF | 0.78 | 0.78 | 0.78 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joe, Y.-E.; Ha, N.; Lee, W.; Byon, H.-J. Prediction of Postoperative Pain and Side Effects of Patient-Controlled Analgesia in Pediatric Orthopedic Patients Using Machine Learning: A Retrospective Study. J. Clin. Med. 2025, 14, 1459. https://doi.org/10.3390/jcm14051459
Joe Y-E, Ha N, Lee W, Byon H-J. Prediction of Postoperative Pain and Side Effects of Patient-Controlled Analgesia in Pediatric Orthopedic Patients Using Machine Learning: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(5):1459. https://doi.org/10.3390/jcm14051459
Chicago/Turabian StyleJoe, Young-Eun, Nayoung Ha, Woojoo Lee, and Hyo-Jin Byon. 2025. "Prediction of Postoperative Pain and Side Effects of Patient-Controlled Analgesia in Pediatric Orthopedic Patients Using Machine Learning: A Retrospective Study" Journal of Clinical Medicine 14, no. 5: 1459. https://doi.org/10.3390/jcm14051459
APA StyleJoe, Y.-E., Ha, N., Lee, W., & Byon, H.-J. (2025). Prediction of Postoperative Pain and Side Effects of Patient-Controlled Analgesia in Pediatric Orthopedic Patients Using Machine Learning: A Retrospective Study. Journal of Clinical Medicine, 14(5), 1459. https://doi.org/10.3390/jcm14051459






