Application of Fractal Dimension Analysis of Sublingual Blood Vessel Patterns in Correlation with Cardiovascular Diseases—A Pilot Study Title
Abstract
1. Introduction
- There are no differences in the value of the fractal dimension of large sublingual vessels between the control and examined group;
- There are no differences in the value of the fractal dimension of capillary vessels between the control and examined group;
- There are no differences in the value of the fractal dimension of large sublingual vessels between the examined diseases and control group;
- There are no differences in the value of the fractal dimension of capillary vessels between the examined diseases and control group.
2. Materials and Methods
2.1. Patients
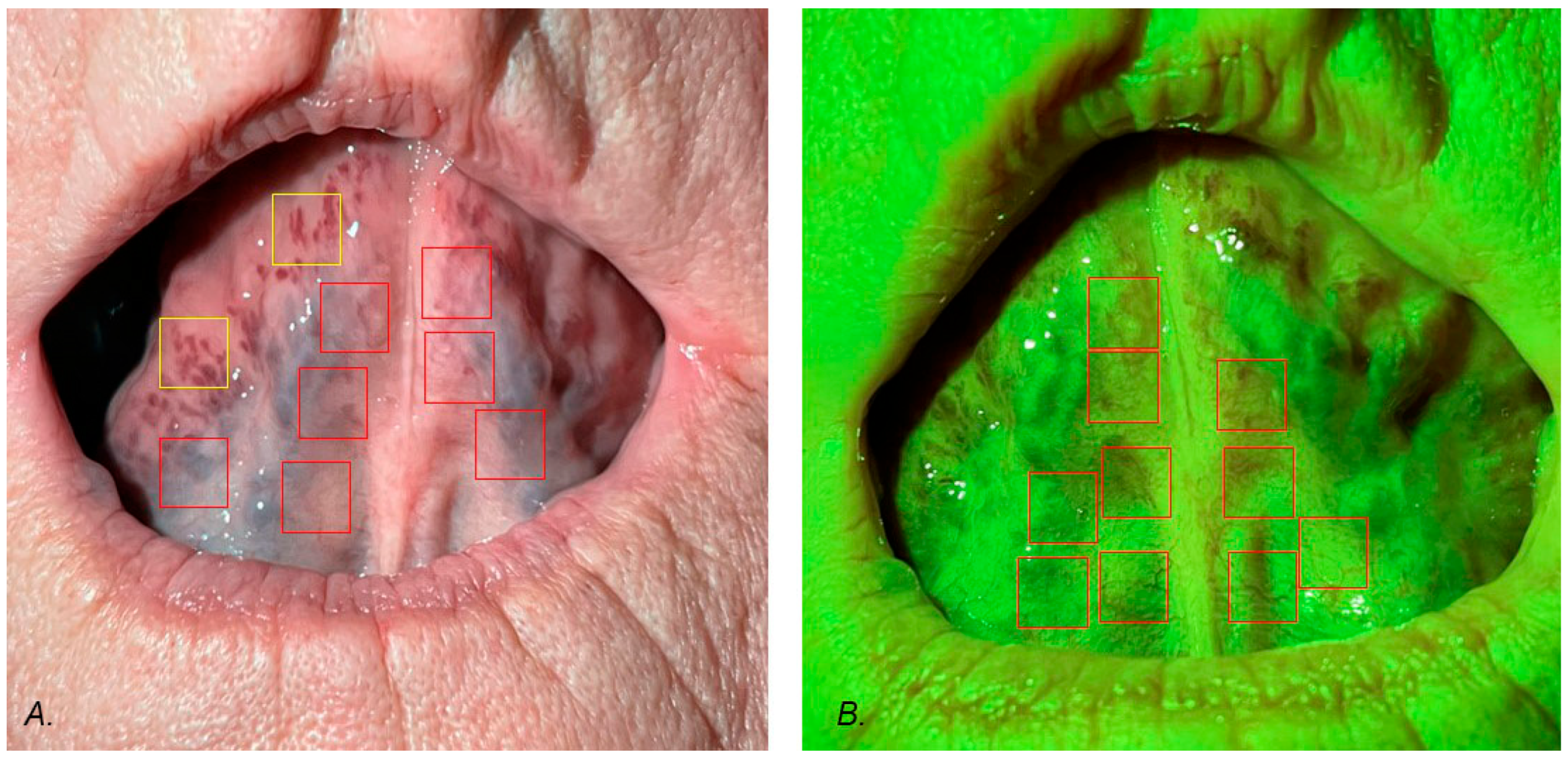
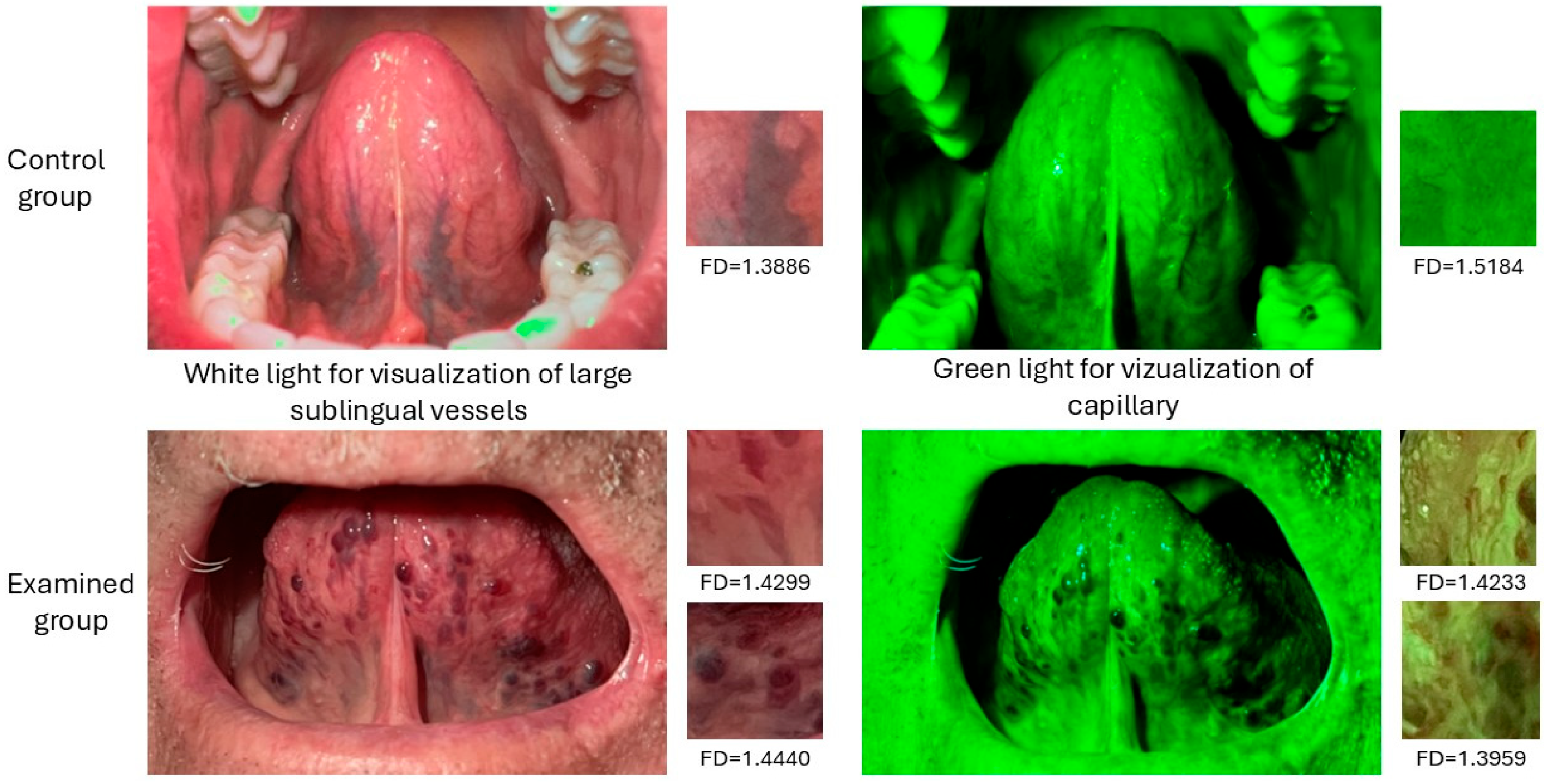
2.2. Taking Photos and Region of Interest Selection
2.3. Fractal Dimension Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- There are statistical differences between the study group and the control group in relation to the fractal dimension of large sublingual vessels. Null hypothesis rejected.
- There are statistical differences between the examined group and the control group in relation to the fractal dimension of capillary vessels, but the p value is on the edge of statistical significance. Null hypothesis rejected.
- There are differences in the value of the fractal dimension of large sublingual vessels between the examined diseases and the control group in all of the diseases: hypertension, heart coronary disease, atherosclerosis, atrial fibrillation, and heart valve defects. Null hypothesis rejected.
- There are differences in the value of the fractal dimension of capillary vessels between hypertension and the control group. Null hypothesis rejected.
- Our results show that the fractal dimension analysis of sublingual vessels can be used in screening programs.
6. Study Limitations
- In the examined group, some patients suffered from more than one disease, which potentially could have influenced the value of the fractal dimension.
- One limitation is that the study only looks back in time; it cannot prove cause and effect, only associations.
- Small group sizes but data increased by examination of more regions of interests (ROIs) than patients.
- Discrepancy in age between the control and examined groups may affect our results, but creating a control group without any cardiovascular diseases in similar age to the examined group is almost impossible.
- It is a retrospective study design, so causality could not be established, only associations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, J.; Chin, C.W.L.; Hong, J.; Chee, M.L.; Le, T.T.; Ting, D.S.W.; Wong, T.Y.; Schmetterer, L. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hyypertens 2019, 37, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, L.F.; Bergamo, V.C.; Conti, M.L.; Fares, N.T.; Costa, L.A.; Ambrogini, O., Jr.; De Moraes, N.S.B. The retinal foveal avascular zone as a systemic biomarker to evaluate inflammatory bowel disease control. Int. J. Retin. Vitr. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Azuma, M.; Nakayama, N.; Fukui, K.; Ito, M.; Saito, N.; Horita, N.; Utsunomiya, D. Diagnostic accuracy of whole heart coronary magnetic resonance angiography: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. 2023, 25, 36. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Akram, M.; Laila, U.; Khalil, M.T.; Zainab, R.; Iftikhar, M.; Ozdemir, F.A.; Sołowski, G.; Alinia-Ahandani, E.; Chukwuebuka, E.; et al. The analysis of retinal blood vessels and systemic diseases includes the relationship between retinal blood vessels and myocardial infarction (heart disease), and retinal blood vessels and cerebrovascular diseases. IAIM 2023, 10, 61–68. [Google Scholar]
- Newsome, I.G.; Dayton, P.A. Visualization of Microvascular Angiogenesis Using Dual-Frequency Contrast-Enhanced Acoustic Angiography: A Review. Ultrasoudn Med. Biol. 2020, 46, 2625–2635. [Google Scholar] [CrossRef]
- Popecki, P.; Kozakiewicz, M.; Ziętek, M.; Jurczyszyn, K. Fractal Dimension Analysis of Melanocytic Nevi and Melanomas in Normal and Polarized Light-A Preliminary Report. Life 2022, 12, 1008. [Google Scholar] [CrossRef]
- Casamassimo, P.S.; Flaitz, C.M.; Hammersmith, K.; Sangvai, S.; Kumar, A. Recognizing the Relationship Between Disorders in the Oral Cavity and Systemic Disease. Pediatr. Clin. N. Am. 2018, 65, 1007–1032. [Google Scholar] [CrossRef]
- Randall, D.A.; Westmark, N.L.; Neville, B.W. Common Oral Lesions. Am. Fam. Physician 2022, 105, 369–376. [Google Scholar]
- Zahid, E.; Bhatti, O.; Zahid, M.A.; Stubbs, M. Overview of common oral lesions. Malays. Fam. Physician 2022, 17, 9–21. [Google Scholar] [CrossRef]
- Akkaya, N.; Ölmez, D.; Özkan, G. Evaluation of the factors associated with sublingual varices: A descriptive clinical study. Folia Morphol. 2019, 78, 325–330. [Google Scholar] [CrossRef]
- Hedström, L.; Albrektsson, M.; Bergh, H. Is there a connection between sublingual varices and hypertension? BMC Oral Health 2015, 15, 78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleinman, H.Z. Lingual varicosities. Oral Surg. Oral Med. Oral Pathol. 1967, 23, 546–548. [Google Scholar] [CrossRef]
- Hedström, L.; Bergh, H. Sublingual varices in relation to smoking and cardiovascular diseases. Br. J. Oral Maxillofac. Surg. 2010, 48, 136–138. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Nauntofte, B.; Smidt, D.; Torpet, L.A. Oral mucosal lesions in older people: Relation to salivary secretion, systemic diseases and medications. Oral Dis. 2015, 21, 721–729. [Google Scholar] [CrossRef]
- Shivakumar, K.M.; Raje, V.; Kadashetti, V. Assessing the correlation between sublingual varices and hypertension among the adults of Satara district, Maharashtra: An observational study. J. Int. Oral Health 2020, 12, 349–354. [Google Scholar] [CrossRef]
- Bergh, H.; Kastberg, C.; Albrektson, M.; Hedström, L. Persistence and stability of sublingual varices over time and their connection to underlying factors: An 8 year follow up study. BMC Oral Health 2022, 22, 346. [Google Scholar] [CrossRef]
- Eslami, H.; Halimi Milani, F.; Salehnia, F.; Kourehpaz, N.; Katebi, K. Relationship between sublingual varices and hypertension: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 240. [Google Scholar] [CrossRef]
- Accardo, A.; Pascazio, L.; Costantinides, F.; Gorza, F.; Silveri, G. Influence of hypertension and other risk factors on the onset of sublingual varices. BMC Oral Health 2021, 21, 235. [Google Scholar] [CrossRef]
- Khalilzada, M.; Dogan, K.; Ince, C.; Stam, J. Sublingual microvascular changes in patients with cerebral small vessel disease. Stroke 2011, 42, 2071–2073. [Google Scholar] [CrossRef]
- Al-Shayyab, M.H.; Baqain, Z.H. Sublingual varices in relation to smoking, cardiovascular diseases, denture wearing, and consuming vitamin rich foods. Saudi Med. J. 2015, 36, 310–315. [Google Scholar] [CrossRef]
- Bergh, H.; Albrektson, M.; Kastberg, C.; Hedström, L. Association of Sublingual Varices with Heart- and Cerebrovascular Disease. Int. Dent. J. 2024, 74, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Thomas, G.N.; Tay, W.; Ikram, M.K.; Hsu, W.; Lee, M.L.; Lau, Q.P.; Wong, T.Y. Retinal vascular fractal dimension and its relationship with cardiovascular and ocular risk factors. Am. J. Ophthalmol. 2012, 154, 663–674.e1. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Mitchell, P.; Rochtchina, E.; Wong, T.Y.; Hsu, W.; Lee, M.L.; Wainwright, A.; Wang, J.J. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur. Heart J. 2011, 32, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Gopinath, B.; White, A.J.; Burlutsky, G.; Yin Wong, T.; Mitchell, P. Retinal Vasculature Fractal and Stroke Mortality. Stroke 2021, 52, 1276–1282. [Google Scholar] [CrossRef]

| Disease | Hypertension | Heart Coronary Disease | Atherosclerosis | Atrial Fibrillation | Heart Valve Defects |
|---|---|---|---|---|---|
| Amount of | 19 | 10 | 9 | 5 | 4 |
| Power of Test | Mean (1) Examined Group | Mean (2) Control Group | t | df | p | SD (1) | SD (2) | |
|---|---|---|---|---|---|---|---|---|
| FD of large sublingual vessels (N = 42) | 0.987 | 1.4019 | 1.3282 | 6.06 | 198 | 0.000000 | 0.07340 | 0.08619 |
| FD of capillary vessels (N = 193) | 0.764 | 1.4184 | 1.4369 | −2.09 | 288 | 0.037463 | 0.06788 | 0.07640 |
| Power of Test | Mean (1) Examined Group | Mean (2) Control Group N = 119 | t | df | p | SD 1 | SD 2 | ||
|---|---|---|---|---|---|---|---|---|---|
| FD of large sublingual vessels | 0.999 | Hypertension (N = 91) | 1.3830 | 1.3282 | 4.1522 | 145 | 0.0001 | 0.0721 | 0.0862 |
| 1.000 | Heart coronary disease (N = 73) | 1.4078 | 5.3464 | 127 | 0.0000 | 0.0819 | |||
| 0.999 | Atherosclerosis (N = 60) | 1.4156 | 5.7255 | 114 | 0.0000 | 0.0782 | |||
| 0.999 | Atrial fibrillation (N = 23) | 1.4414 | 5.8904 | 77 | 0.0000 | 0.0501 | |||
| 0.999 | Heart valve defects (N = 20) | 1.4357 | 5.1618 | 74 | 0.0000 | 0.0581 |
| Power of Test | Mean (1) Examined Group | Mean (2) Control Group N = 119 | t | df | p | SD 1 | SD 2 | ||
|---|---|---|---|---|---|---|---|---|---|
| FD of capillary vessels | 0.951 | Hypertension (N = 134) | 1.4031 | 1.4369 | −3.6885 | 229 | 0.0003 | 0.0623 | 0.0764 |
| 0.320 | Heart coronary disease (N = 76) | 1.4182 | −1.5334 | 171 | 0.1270 | 0.0834 | |||
| 0.050 | Atherosclerosis (N = 61) | 1.4374 | 0.0486 | 156 | 0.9613 | 0.0687 | |||
| 0.247 | Atrial fibrillation (N = 25) | 1.4572 | 1.2407 | 120 | 0.2171 | 0.0569 | |||
| 0.054 | Heart valve defects (N = 25) | 1.4333 | −0.2038 | 120 | 0.8389 | 0.0790 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, A.; Jurczyszyn, K.; Wojtowicz, A.; Zieliński, F.; Trafalski, M. Application of Fractal Dimension Analysis of Sublingual Blood Vessel Patterns in Correlation with Cardiovascular Diseases—A Pilot Study Title. J. Clin. Med. 2025, 14, 1429. https://doi.org/10.3390/jcm14051429
Janik A, Jurczyszyn K, Wojtowicz A, Zieliński F, Trafalski M. Application of Fractal Dimension Analysis of Sublingual Blood Vessel Patterns in Correlation with Cardiovascular Diseases—A Pilot Study Title. Journal of Clinical Medicine. 2025; 14(5):1429. https://doi.org/10.3390/jcm14051429
Chicago/Turabian StyleJanik, Anastazja, Kamil Jurczyszyn, Andrzej Wojtowicz, Fryderyk Zieliński, and Mateusz Trafalski. 2025. "Application of Fractal Dimension Analysis of Sublingual Blood Vessel Patterns in Correlation with Cardiovascular Diseases—A Pilot Study Title" Journal of Clinical Medicine 14, no. 5: 1429. https://doi.org/10.3390/jcm14051429
APA StyleJanik, A., Jurczyszyn, K., Wojtowicz, A., Zieliński, F., & Trafalski, M. (2025). Application of Fractal Dimension Analysis of Sublingual Blood Vessel Patterns in Correlation with Cardiovascular Diseases—A Pilot Study Title. Journal of Clinical Medicine, 14(5), 1429. https://doi.org/10.3390/jcm14051429







