Insights from Real-World Evidence on the Use of Inhalers in Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
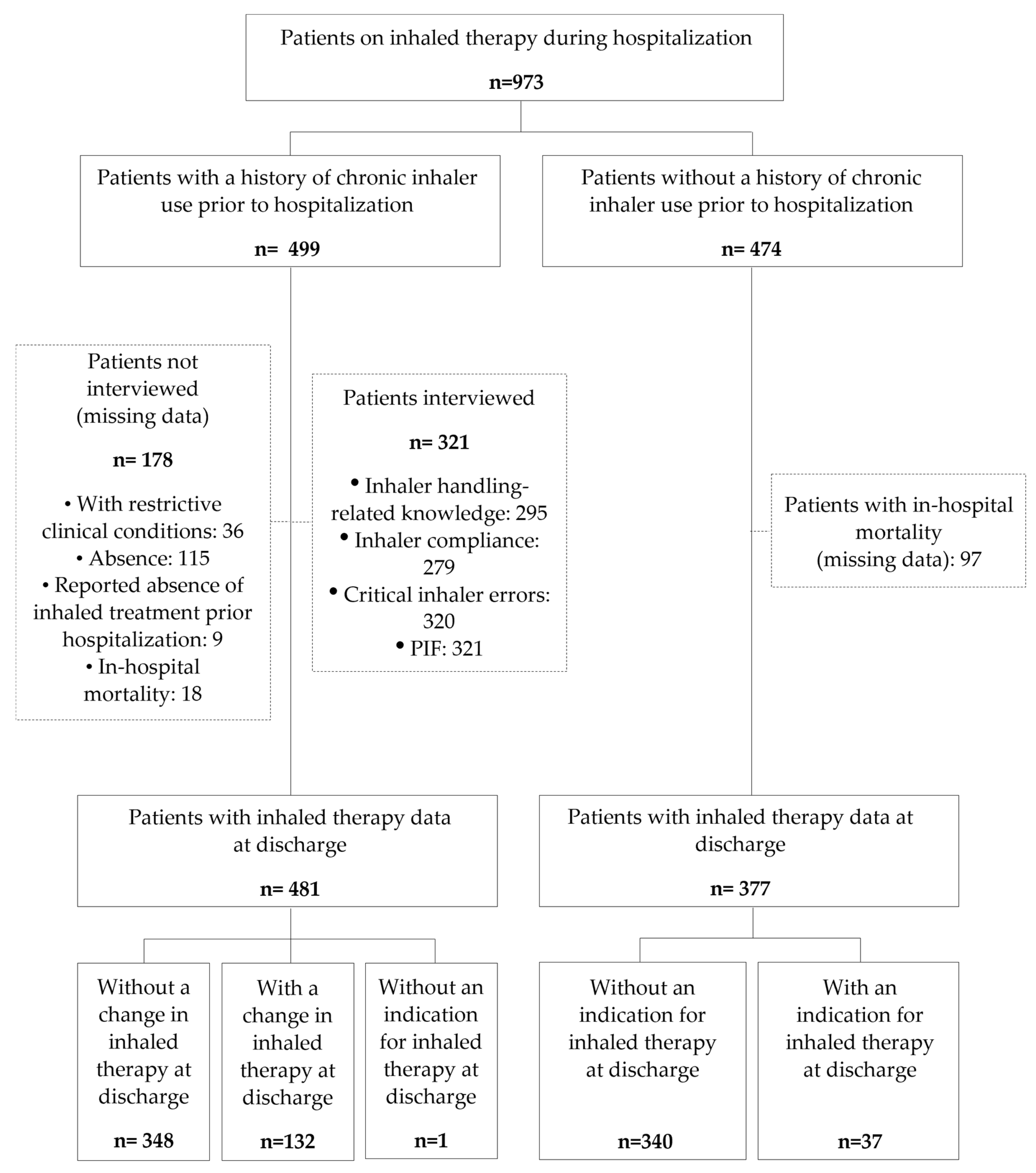
2.2. Data Source and Patient Selection
2.3. Data and Clinical Variables Collected
2.4. Data Analysis
3. Results
3.1. Population Studied According to Inhaler Use History
3.1.1. Patient Characteristic
3.1.2. Inhaled Therapy Used and Reason
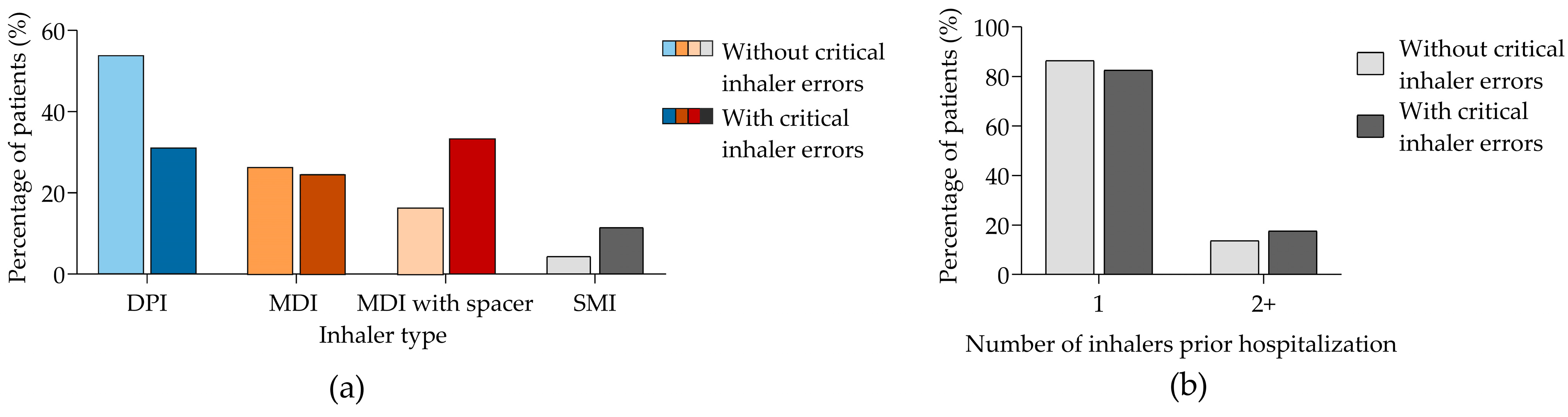
3.2. Critical Inhaler Errors, Treatment Compliance, and Inhaler Handling-Related Knowledge in Patients with a Previous History of At-Home Inhaler Use
3.3. Inhaled Therapy Based on Maximum PIF Levels
3.4. Patient Adherence to Inhaler Treatments
3.5. Change of Pre-Hospitalization Inhaled Therapy Device at Discharge
4. Discussion
4.1. Inhaler Selection
4.2. Critical Errors in Inhaled Therapies
4.3. Compliance to Inhaled Treatment
4.4. Clinical Inertia
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Noncommunicable Diseases 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Collaborators, G.C.R.D. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Cataldo, D.; Hanon, S.; Peché, R.V.; Schuermans, D.J.; Degryse, J.M.; De Wulf, I.A.; Elinck, K.; Leys, M.H.; Rummens, P.L.; Derom, E. How to Choose the Right Inhaler Using a Patient-Centric Approach? Adv. Ther. 2022, 39, 1149–1163. [Google Scholar] [CrossRef]
- Rigby, D. Inhaler Device Selection for People with Asthma or Chronic Obstructive Pulmonary Disease. Aust. Prescr. 2024, 47, 140–147. [Google Scholar] [PubMed]
- Melani, A.S.; Bonavia, M.; Cilenti, V.; Cinti, C.; Lodi, M.; Martucci, P.; Serra, M.; Scichilone, N.; Sestini, P.; Aliani, M.; et al. Inhaler Mishandling Remains Common in Real Life and Is Associated with Reduced Disease Control. Respir. Med. 2011, 105, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Gich, I.; Pedersen, S. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 2016, 150, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Usmani, O.S.; Lavorini, F.; Marshall, J.; Dunlop, W.C.N.; Heron, L.; Farrington, E.; Dekhuijzen, R. Critical Inhaler Errors in Asthma and COPD: A Systematic Review of Impact on Health Outcomes. Respir. Res. 2018, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Leving, M.T.; Kocks, J.; Bosnic-Anticevich, S.; Dekhuijzen, R.; Usmani, O.S. Relationship between Peak Inspiratory Flow and Patient and Disease Characteristics in Individuals with COPD—A Systematic Scoping Review. Biomedicines 2022, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Molimard, M.; Raherison, C.; Lignot, S.; Balestra, A.; Lamarque, S.; Chartier, A.; Droz-Perroteau, C.; Lassalle, R.; Moore, N.; Girodet, P.O. Chronic Obstructive Pulmonary Disease Exacerbation and Inhaler Device Handling: Real-Life Assessment of 2935 Patients. Eur. Respir. J. 2017, 49, 1601794. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Inhaler Treatment Options in COPD. Eur. Respir. Rev. 2005, 14, 102–108. [Google Scholar] [CrossRef]
- Amin, A.N.; Ganapathy, V.; Roughley, A.; Small, M. Confidence in Correct Inhaler Technique and Its Association with Treatment Adherence and Health Status among US Patients with Chronic Obstructive Pulmonary Disease. Patient Prefer. Adherence 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahler, D.A.; Halpin, D.M.G. Consideration and Assessment of Patient Factors When Selecting an Inhaled Delivery System in COPD. Chest 2024, 165, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Kumar, L.; Vohra, V.; Sarin, R.; Jaiswal, A.; Puri, M.M.; Rathee, D.; Chakraborty, P. Evaluating the Technique of Using Inhalation Device in COPD and Bronchial Asthma Patients. Respir. Med. 2014, 108, 992–998. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Plaza, V.; López-Viña, A.; Cosio, B.G. Test of Adherence to Inhalers. Arch. Bronconeumol. 2017, 53, 360–361. [Google Scholar] [CrossRef]
- Ghosh, S.; Ohar, J.A.; Drummond, M.B. Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: Implications for Dry Powder Inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 381–387. [Google Scholar] [CrossRef]
- Sistema de Información y Análisis de La Prestación Farmacéutica de La Comunidad de Madrid. Available online: http://www.eco.uc3m.es/servicios/sesam/actividades/jornada_datos/MonicaAusejoTexto.pdf (accessed on 11 December 2024).
- Mahler, D.A.; Halpin, D.M.G. Personalizing Selection of Inhaled Delivery Systems in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2023, 20, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Couillard, S.; Digby, G.; Tse, S.M.; Green, S.; Aceron, R.; Carlsten, C.; Hubick, J.; Penz, E. Canadian Thoracic Society Position Statement on Climate Change and Choice of Inhalers for Patients with Respiratory Disease. Can. J. Respir. Crit. Care Sleep Med. 2023, 7, 232–239. [Google Scholar] [CrossRef]
- Calle Rubio, M.; López-Campos, J.L.; Miravitlles, M.; Soler Cataluña, J.J.; Alcázar Navarrete, B.; Fuentes Ferrer, M.E.; Rodríguez Hermosa, J.L. Variations in Chronic Obstructive Pulmonary Disease Outpatient Care in Respiratory Clinics: Results From the 2021 EPOCONSUL Audit. Arch. Bronconeumol. 2023, 59, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ohar, J.A.; Ferguson, G.T.; Mahler, D.A.; Drummond, M.B.; Dhand, R.; Pleasants, R.A.; Anzueto, A.; Halpin, D.M.; Price, D.B.; Drescher, G.S.; et al. Measuring Peak Inspiratory Flow in Patients with Chronic Obstructive Pulmonary Disease. Int. J. COPD 2022, 17, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Huang, C.K.; Peng, H.C.; Tsai, H.C.; Huang, S.Y.; Yu, C.J.; Chien, J.Y. Peak-Inspiratory-Flow-Rate Guided Inhalation Therapy Reduce Severe Exacerbation of COPD. Front. Pharmacol. 2021, 12, 704316. [Google Scholar] [CrossRef]
- Chrystyn, H.; Lavorini, F. The Dry Powder Inhaler Features of the Easyhaler That Benefit the Management of Patients. Expert Rev. Respir. Med. 2020, 14, 345–351. [Google Scholar] [CrossRef]
- de Koning, J.P.; van der Mark, T.W.; Coenegracht, P.M.J.; Tromp, T.F.J.; Frijlink, H.W. Effect of an External Resistance to Airflow on the Inspiratory Flow Curve. Int. J. Pharm. 2002, 234, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Wells, B.J.; Saha, A.K.; Franchino-Elder, J.; Shaikh, A.; Donato, B.M.K.; Ohar, J.A. Low Peak Inspiratory Flow Rates Are Common Among COPD Inpatients and Are Associated with Increased Healthcare Resource Utilization: A Retrospective Cohort Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 1483–1494. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Kim, S.H.; Kim, Y.; Lee, H.; Rhee, C.K.; Ra, S.W.; Lee, C.Y.; Park, J.H.; Park, Y.B.; Yoo, K.H. Suboptimal Peak Inspiratory Flow Rate in Dry-Powder Inhaler Users for Chronic Obstructive Pulmonary Disease in Korea. Pulm. Pharmacol. Ther. 2024, 85, 102298. [Google Scholar] [CrossRef] [PubMed]
- Usmani, O.S.; Hickey, A.J.; Guranlioglu, D.; Rawson, K.; Stjepanovic, N.; Siddiqui, S.; Dhand, R. The Impact of Inhaler Device Regimen in Patients with Asthma or COPD. J. Allergy Clin. Immunol. Pract. 2021, 9, 3033–3040.e1. [Google Scholar] [CrossRef]
- Volerman, A.; Carpenter, D.; Press, V. What Can Be Done to Impact Respiratory Inhaler Misuse: Exploring the Problem, Reasons, and Solutions. Expert Rev. Respir. Med. 2020, 14, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Zaeh, S.E.; Ramsey, R.; Bender, B.; Hommel, K.; Mosnaim, G.; Rand, C. The Impact of Adherence and Health Literacy on Difficult-to-Control Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kocks, J.; Bosnic-Anticevich, S.; van Cooten, J.; Correia de Sousa, J.; Cvetkovski, B.; Dekhuijzen, R.; Dijk, L.; Garcia Pardo, M.; Gardev, A.; Gawlik, R.; et al. Identifying Critical Inhalation Technique Errors in Dry Powder Inhaler Use in Patients with COPD Based on the Association with Health Status and Exacerbations: Findings from the Multi-Country Cross-Sectional Observational PIFotal Study. BMC Pulm. Med. 2023, 23, 302. [Google Scholar] [CrossRef] [PubMed]
- Leving, M.T.; van Boven, J.F.M.; Bosnic-Anticevich, S.Z.; van Cooten, J.; Correia de Sousa, J.; Cvetkovski, B.; Dekhuijzen, R.; Dijk, L.; García Pardo, M.; Gardev, A.; et al. Suboptimal Peak Inspiratory Flow and Critical Inhalation Errors Are Associated with Higher COPD-Related Healthcare Costs. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.J.; Wielders, P.; van der Palen, J.; Heyes, L.; Midwinter, D.; Collison, K.; Preece, A.; Barnes, N.; Sharma, R. Critical Error Frequency and the Impact of Training with Inhalers Commonly Used for Maintenance Treatment in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Siler, T.M.; Jain, R.; Collison, K.; Sharma, R.; Sutton, L.; Rees, J.; Bernstein, D.I. Correct Use and Ease-of-Use of Placebo ELLIPTA Dry-Powder Inhaler in Adult Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE 2022, 17, e0273170. [Google Scholar] [CrossRef]
- Chrischilles, E.; Gilden, D.; Kubisiak, J.; Rubenstein, L.; Shah, H. Delivery of Ipratropium and Albuterol Combination Therapy for Chronic Obstructive Pulmonary Disease: Effectiveness of a Two-in-One Inhaler versus Separate Inhalers. Am. J. Manag. Care 2002, 8, 902–911. [Google Scholar] [PubMed]
- George, J.; Kong, D.C.M.; Thoman, R.; Stewart, K. Factors Associated with Medication Nonadherence in Patients with COPD. Chest 2005, 128, 3198–3204. [Google Scholar] [CrossRef]
- Chiu, K.C.; Boonsawat, W.; Cho, S.H.; Cho, Y.J.; Hsu, J.Y.; Liam, C.K.; Muttalif, A.R.; Nguyen, H.D.; Nguyen, V.N.; Wang, C.; et al. Patients’ Beliefs and Behaviors Related to Treatment Adherence in Patients with Asthma Requiring Maintenance Treatment in Asia. J. Asthma 2014, 51, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.; Giner, J.; Calle, M.; Rytilä, P.; Campo, C.; Ribó, P.; Valero, A. Impact of Patient Satisfaction with His or Her Inhaler on Adherence and Asthma Control. Allergy Asthma Proc. 2018, 39, 437–444. [Google Scholar] [CrossRef]
- Grandmaison, G.; Grobéty, T.; Dumont, P.; Vaucher, J.; Hayoz, D.; Suter, P. An In-Hospital Intervention to Reduce the Proportion of Misused Inhalers at Hospital Discharge among Patients with COPD: A Non-Randomised Intervention Study. Swiss Med. Wkly. 2024, 154, 3394. [Google Scholar] [CrossRef]
- Villamañán, E.; Sobrino, C.; Bilbao, C.; Fernández, J.; Herrero, A.; Calle, M.; Alvaro, D.; Segura, M.; Picazo, G.; Rodríguez, J.M.; et al. Off-Label Use of Inhaled Bronchodilators in Hospitalised Patients in Spain: A Multicentre Observational Study. Eur. J. Hosp. Pharm. 2021, 28, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Macie, C.; Wooldrage, K.; Manfreda, J.; Anthonisen, N.R. Inhaled Corticosteriods and Mortality in COPD. Chest 2006, 130, 640–646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shams, I.; Ajorlou, S.; Yang, K. A Predictive Analytics Approach to Reducing 30-Day Avoidable Readmissions among Patients with Heart Failure, Acute Myocardial Infarction, Pneumonia, or COPD. Health Care Manag. Sci. 2015, 18, 19–34. [Google Scholar] [CrossRef]
- Chassin, M.R.; Loeb, J.M.; Schmaltz, S.P.; Wachter, R.M. Accountability Measures—Using Measurement to Promote Quality Improvement. N. Engl. J. Med. 2010, 363, 683–688. [Google Scholar] [CrossRef] [PubMed]


| With Previous History of Inhaler Use Before Hospitalization | Without Previous History of Inhaler Use Before Hospitalization | p-Value | |
|---|---|---|---|
| Patients included, n (%) | 499 (51.3) | 474 (48.7) | |
| Age, median (SD) | 75.4 (12.4) | 79.2 (12.7) | <0.001 |
| Gender (men), n (%) | 243 (59.6) | 167 (49) | 0.005 |
| Current smoker, n (%) | 54 (10.8) | 25 (5.8) | 0.381 |
| Charlson index, median (SD) | 3 (1–4) | 2 (1–4) | 0.002 |
| Patients with Charlson index ≥ 2, n (%) | 340 (67.9) | 301 (63.5) | 0.151 |
| Respiratory comorbidities, n (%) | <0.001 | ||
| Absence | 66 (13.2) | 361 (76.2) | |
| COPD | 268 (53.7) | 32 (6.8) | |
| Bronchiectasis | 40 (8) | 4 (0.8) | |
| Asthma | 89 (17.8) | 16 (3.4) | |
| Other | 36 (7.2) | 61 (12.9) | |
| Number of hospitalizations in previous year, median (IQR) | 2 (1–3) | 1 (0–2) | <0.001 |
| Hospitalizations previous year ≥ 1, n (%) | 388 (77.4) | 271 (57.2) | <0.001 |
| Antibiotic/corticosteroid courses in previous year, median (IQR) | 1 (0–3) | 0 (0–1) | <0.001 |
| Number of courses ≥ 2, n (%) | 232 (46.5) | 103 (21.7) | <0.001 |
| Cause for therapy during admission, n (%) | <0.001 | ||
| COPD exacerbation | 205 (40.9) | 23 (4.9) | |
| Asthma exacerbation | 17 (3.4) | 5 (1.1) | |
| Bronchiectasis | 10 (2) | 0 | |
| Respiratory infection | 218 (43.5) | 304 (64.1) | |
| Cardiac insufficiency | 43 (8.6) | 125 (26.4) | |
| Inpatient service, n (%) | <0.001 | ||
| Internal medicine | 260 (52.1) | 279 (58.9) | |
| Pulmonology | 125 (25) | 35 (7.4) | |
| Geriatrics | 114 (22.8) | 160 (33.8) | |
| Inhaled therapy during hospitalization, n (%) | 0.434 | ||
| SABD | 246 (49.3) | 325 (68.6) | |
| ICS + SABD | 96 (19.2) | 67 (14.1) | |
| LAMA | 30 (6) | 14 (3) | |
| LABA + LAMA | 17 (3.4) | 5 (1.1) | |
| LABA + ICS | 35 (7) | 27 (5.7) | |
| LABA + LAMA + ICS (single inhaler) | 66 (13.2) | 27 (5.7) | |
| LABA + LAMA + ICS (multiple inhalers) | 9 (3.4) | 9 (1.9) | |
| Inhaler devices during hospitalization, n (%) | 0.212 | ||
| MDI (with spacer) | 260 (51.9) | 219 (46.2) | |
| Nebulizer | 209 (41.7) | 248 (52.3) | |
| MDI | 2 (0.4) | 1 (0.2) | |
| DPI | 14 (2.8) | 1 (0.2) | |
| SMI | 13 (2.6) | 5 (1.0) | |
| Mortality, n (%) | <0.001 | ||
| In-hospital | 18 (3.6) | 97 (20.5) | |
| 90 days | 108 (21.6) | 51 (10.8) |
| All Patients | Patients Using DPI | Patients Using MDI | Patients Using MDI with Spacer | Patients Using SMI | |
|---|---|---|---|---|---|
| Patients included, n (%) | 279 (100) | 135 (48.4) | 68 (24.4) | 61 (21.9) | 15 (5.3) |
| Inhaled therapy compliance, n (%) | |||||
| Poor | 106 (38) | 51 (37.8) | 24 (35.3) | 23 (37.7) | 8 (53.3) |
| Intermediate | 89 (31.9) | 47 (34.8) | 23 (33.8) | 16 (26.2) | 3 (20) |
| Good | 84 (30.1) | 37 (27.4) | 21 (30.9) | 22 (36.1) | 4 (26.7) |
| Type of inhaler noncompliance, n (%) | |||||
| Erratic | 165 (59.1) | 76 (56.3) | 43 (63.2) | 35 (57.4) | 11 (73.3) |
| Deliberate | 35 (12.5) | 9 (6.6) | 7 (10.3) | 13 (21.3) | 6 (40) |
| Unconscious | 266 (95.4) | 126 (93.3) | 66 (97.0) | 60 (98.3) | 14 (93.3) |
| Without Critical Inhaler Errors | With Critical Inhaler Errors | p-Value | |
|---|---|---|---|
| PIF, median (SD) | 60.8 (17.3) | 52.1 (17.8) | <0.001 |
| Inhaler compliance, n/N (%) | 0.007 | ||
| Poor | 57/184 (31) | 46/91 (50.5) | |
| Intermediate | 66/184 (35.9) | 22/91 (24.2) | |
| Good | 61/184 (33.2) | 23/91 (25.3) | |
| Inhaler handling-related knowledge, n/N (%) | <0.001 | ||
| Good | 172/195 (88.2) | 49/99 (49.5) | |
| Regular or poor | 23/195 (11.8) | 50/99 (50.5) |
| PIF ≥ 30 L/min | PIF < 30 L/min | |
|---|---|---|
| Inhaler device, n/N (%) | ||
| DPI | 133/294 (45.2) | 11/27 (40.7) |
| MDI | 74/294 (25.2) | 7/27 (25.9) |
| MDI with spacer | 67/294 (22.8) | 3/27 (11.1) |
| SMI | 17/294 (6.8) 5.8 | 5/27 (18.5) |
| Unknown | 3/294 (1.0) | 1/27 (3.7) |
| Number of inhalers, n/N (%) | ||
| 1 | 252/294 (85.7) | 22/27 (81.5) |
| 2 | 42/294 (14.3) | 5/27 (18.5) |
| Treatment period, median (IQR) | 15 (7–40) | 25 (7–63) |
| Patients with critical inhaler errors, n/N (%) | 96/294 (32.6) | 18/27 (66.7) |
| Type of inhaler compliance, n/N (%) | ||
| Poor | 98/253 (38.7) | 8/24 (33.3) |
| Intermediate | 84/253 (33.2) | 5/24 (20.8) |
| Good | 71/253 (28.1) | 11/24 (45.8) |
| Inhaler handling-related knowledge, n/N (%) | ||
| Good | 212/272 (77.9) | 9/24 (37.5) |
| Regular or poor | 60/272 (22.1) | 15/24 (62.5) |
| Good Inhaler Treatment Adherence | Regular/Poor Inhaler Treatment Adherence | p-Value | |
|---|---|---|---|
| Inhaler device, n/N (%) | 0.512 | ||
| DPI | 37/84 (44) | 98/195 (50.3) | |
| MDI | 21/84 (25) | 47/195 (24.1) | |
| MDI with spacer | 22/84 (26.2) | 39/195 (20) | |
| SMI | 4/84 (4.8) | 11/195 (5.6) | |
| Number of inhalers, n/N (%) | 0.052 | ||
| 1 | 74/84 (88.1) | 166/195 (8.1) | |
| 2 | 10/84 (11.9) | 29/195 (14.9) | |
| Patients with maximum PIF ≥ 30 L/min, n/N (%) | 70/84 (86.4) | 180 (93.8) | 0.868 |
| Patients with critical inhaler errors, n/N (%) | 23/84 (27.4) | 68/195 (34.9) | 0.182 |
| Inhaler handling-related knowledge, n/N (%) | 0.099 | ||
| Good | 71/84 (84.5) | 146/193 (75.6) | |
| Regular or poor | 13/84 (15.5) | 47/193 (24.4) |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| LAMA (ref) | 1 | - | - |
| LAMA + LABA | 0.876 | 0.422–1.817 | 0.722 |
| LABA + ICS | 0.391 | 0.182–0.838 | 0.016 |
| LABA + LAMA + ICS | 0.369 | 0.178–0.764 | 0.007 |
| 1 single inhaler | 1.817 | 0.549–6.011 | 0.328 |
| Do Not Change Inhaler Type | Change Inhaler Type | |||
|---|---|---|---|---|
| DPI Prior Hospitalization | DPI Prior Hospitalization | |||
| Change to MDI | Change to MDI with Spacer | Change to SMI | ||
| Patients included, n/N | 162/348 (46.6) | 35/45 (77.8) | 10/45 (22.2) | 0/45 (0) |
| Patients with critical inhaler errors, n/N (%) | 28/110 (25.5) | 6/35 (22.2) | 1/10 (14.3) | - |
| Patients with maximum PIF < 30 L/min, n/N (%) | 10/160 (6.2) | 1/35 (3.7) | 0/10 | - |
| Regular or poor inhaler handling-related knowledge, n (%) | 16/106 (15.1) | 4/35 (13.8) | 0/10 | - |
| MDI prior hospitalization | MDI prior hospitalization | |||
| Change to DPI | Change to MDI with spacer | Change to SMI | ||
| Patients included, n/N | 113/348 (32.5) | 14/26 (53.9) | 11/26 (42.3) | 1/26 (3.8) |
| Patients with critical inhaler errors, n/N (%) | 22/64 (34.4) | 0 (0) | 4/11 (50) | 0 |
| Patients with PIF <30 L/min, n/N (%) | 5/63 (7.9) | 0 (0) | 1/11 (12.5) | 0 |
| Regular or poor inhaler handling-related knowledge, n (%) | 21/61 (34.4) | 1/14 (16.7) | 4/11 (50) | 1 (100) |
| MDI with spacer prior hospitalization | MDI with spacer prior hospitalization | |||
| Change to DPI | Change to MDI | Change to SMI | ||
| Patients included, n/N | 73/348 (21.0) | 8/21 (38.1) | 13/21 (61.9) | 0/21 |
| Patients with critical inhaler errors, n/N (%) | 29/54 (53.7) | 2/7 (28.6) | 6/9 (66.7) | - |
| Patients with PIF <30 L/min, n/N (%) | 3/54 (5.6) | 0 (0) | 0 (0) | - |
| Regular or poor inhaler handling-related knowledge, n (%) | 17/49 (34.7) | 1/6 (16.7) | 2/6 (33.3) | - |
| SMI prior hospitalization | SMI prior hospitalization | |||
| Change to DPI | Change to MDI | Change to MDI with spacer | ||
| Patients included, n/N | - | 13/40 (32.5) | 6/40 (15.0) | 21/40 (52.5) |
| Patients with critical inhaler errors, n/N (%) | - | 4/8 (50) | 1/3 (33.3) | 8/11 (72.7) |
| Patients with PIF < 30 L/min, n/N (%) | - | 1/8 (12.5) | 1/3 (33.3) | 3/11 (27.3) |
| Regular or poor inhaler handling-related knowledge, n (%) | - | 3/8 (37.5) | 0 (0) | 4/7 (57.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle Rubio, M.; Adami Teppa, P.J.; Rodríguez Hermosa, J.L.; García Carro, M.; Tallón Martínez, J.C.; Riesco Rubio, C.; Fernández Cortés, L.; Morales Dueñas, M.; Chamorro del Barrio, V.; Sánchez-del Hoyo, R.; et al. Insights from Real-World Evidence on the Use of Inhalers in Clinical Practice. J. Clin. Med. 2025, 14, 1217. https://doi.org/10.3390/jcm14041217
Calle Rubio M, Adami Teppa PJ, Rodríguez Hermosa JL, García Carro M, Tallón Martínez JC, Riesco Rubio C, Fernández Cortés L, Morales Dueñas M, Chamorro del Barrio V, Sánchez-del Hoyo R, et al. Insights from Real-World Evidence on the Use of Inhalers in Clinical Practice. Journal of Clinical Medicine. 2025; 14(4):1217. https://doi.org/10.3390/jcm14041217
Chicago/Turabian StyleCalle Rubio, Myriam, Pedro José Adami Teppa, Juan Luis Rodríguez Hermosa, Miriam García Carro, José Carlos Tallón Martínez, Consolación Riesco Rubio, Laura Fernández Cortés, María Morales Dueñas, Valeria Chamorro del Barrio, Rafael Sánchez-del Hoyo, and et al. 2025. "Insights from Real-World Evidence on the Use of Inhalers in Clinical Practice" Journal of Clinical Medicine 14, no. 4: 1217. https://doi.org/10.3390/jcm14041217
APA StyleCalle Rubio, M., Adami Teppa, P. J., Rodríguez Hermosa, J. L., García Carro, M., Tallón Martínez, J. C., Riesco Rubio, C., Fernández Cortés, L., Morales Dueñas, M., Chamorro del Barrio, V., Sánchez-del Hoyo, R., & Aragón, J. G. (2025). Insights from Real-World Evidence on the Use of Inhalers in Clinical Practice. Journal of Clinical Medicine, 14(4), 1217. https://doi.org/10.3390/jcm14041217







