Abstract
Background/Objectives: Hyponatremia is associated with increased mortality in the general hospital population. We sought to investigate whether hyponatremia affects the long-term survival of patients following a myocardial infarction (MI) in both ST-segment elevation (STEMI) and non-ST elevation (NSTEMI) presentations. Methods: In this study, 862 MI patients who were hospitalized between 2012 and 2017 were retrospectively followed-up within the median time period of 41.9 [28.2–73.5] months. All participants were assigned to a hyponatremic or normonatremic group with hyponatremia defined as a sodium level of less than 135 mEq/L on admission. Results: In the acute phase of an MI, hyponatremia was diagnosed in 31 (3.6%) patients. The patients with hyponatremia were less often male (38.7 vs. 70.4%, p < 0.001), and less frequently had Killip class I (63.3 vs. 80%) but more often had Killip class IV on admission (16.7 vs. 4.2%, p = 0.024) and more often had a history of impaired renal function (32.3 vs. 15.5%, p = 0.013) than those with normonatremia. Hyponatremic patients had higher troponin T levels on admission by 75.1% (p = 0.003), a higher isoenzyme MB of creatine kinase level by 34.4% (p = 0.006), and lower hemoglobin (by 8.5%, p = 0.001) levels as compared to the normonatremia group. Long-term mortality was significantly higher in the patients with hyponatremia versus normonatremia (18 [58.1%] vs. 243 [29.2%], log-rank p < 0.001). This was driven by differences in the NSTEMI population (65 vs. 30.5%, p < 0.001). By a Cox proportional hazard regression analysis, hyponatremia was associated with a higher long-term mortality (hazard ratio [HR] of 2.222, a 95% confidence interval [CI] of 1.309–3.773, and p = 0.003). Conclusions: Hyponatremia rarely identified in acute phase of MI was associated with higher long-term mortality, particularly in the NSTEMI population.
1. Introduction
Hyponatremia, defined as a sodium serum concentration below 135 mmol/L, is a quite common electrolyte abnormality, affecting up to 30% of hospitalized patients [1,2]. It may appear clinically significant in many conditions including dehydration and fluid loss, volume overload, hyperglycemia, nephrotic syndrome, cirrhosis, the syndrome of inappropriate antidiuretic hormone, psychogenic polydipsia, hypothyroidism, adrenal insufficiency, renal failure, a subarachnoid hemorrhage, or the use of loop diuretics in the treatment of patients with heart failure [2]. Hyponatremia has been considered an important marker of poor prognosis in various clinical settings including heart failure [3,4,5], stroke [6], chronic kidney disease [7], and many other conditions [8].
Hyponatremia was found to be a significant predictor of mortality in patients with an acute myocardial infarction (MI) but the disparities in its prognostic value in specific MI presentations remain not well established. According to the results of a meta-analysis conducted by Ma et al., numerous studies revealed significantly higher 30-day mortality rates in acute coronary syndrome patients with hyponatremia (with a relative risk [RR] of 2.18, a 95% confidence interval [CI] of 1.96 to 2.42, and p < 0.001) [9]. In addition, similar outcomes were obtained for long-term mortality during the up to 18-year follow-ups (hazard ratio [HR] of 1.74, a 95% CI of 1.56–1.942, and p < 0.001) [9]. According to the current knowledge, an MI is divided into two main subtypes: an ST segment elevation myocardial infarction (STEMI) and a non-ST segment elevation myocardial infarction (NSTEMI) [10]. These two groups of patients differ in clinical manifestations, treatment, and short- and long-term prognoses [11,12,13,14,15]. In several studies, it has been proven that hyponatremia is an important prognostic factor in STEMI and MI patients without specifications about its presentation. Sanchez et al. found that the differences in the prevalence of NSTEMI and STEMI presentation in hyponatremic patients were not statistically significant; however, hyponatremia on admission and during hospitalization was associated with increased mortality, regardless of MI type [9,16,17]. Interestingly, the non-osmotic release of arginine vasopressin with subsequent dilutional hyponatremia in the patients with STEMI complicated by symptoms of congestive heart failure was observed [17]. Moreover, a significantly increased 30-day mortality in the NSTEMI patients with hyponatremia (13.8 vs. 7.5%) was found by Singla et al., suggesting that hyponatremia may also be an unfavorable prognostic factor in this group of patients [18].
The aim of this study was to identify the association between as measured on admission serum hyponatremia and the long-term mortality rate of the cohort of Polish MI patients with STEMI and NSTEMI.
2. Materials and Methods
According to the European Society of Cardiology (ESC) guidelines, an MI is defined as myocardial cell death due to prolonged ischemia. In our current study, we focused on patients with type 1 MI, which are caused by atherothrombotic coronary artery disease (CAD). We enrolled 862 type 1 MI patients who were hospitalized between 2012 and 2017. The diagnosis of an MI was based on the clinical presentation and the detection of a rise and/or fall in cTn values, with at least one value above the 99th percentile URL. The mandatory inclusion criterion was a performed coronary angiography. Among these patients, based on the ECG records, we distinguish two types—STEMI and NSTEMI. STEMI presentation was diagnosed when the new ST-segment elevation at the J-point in at least two contiguous leads amounted to ≥2.5 mm in men <40 years of age, ≥2 mm in men ≥40 years of age, or ≥1.5 mm in women regardless of age for leads V2–V3 and/or ≥1 mm in the other leads. STEMI presentation also included patients presenting with a new left or right bundle branch block. No changes in ECG or other than those mentioned above enhanced for NSTEMI diagnosis. All of the patients underwent an assessment of their on-admission electrolyte levels as a standard procedure. We incorporated patients whose sodium level did not exceed 145 mEq/L. The baseline characteristics included anthropometric data, risk factors, comorbidities, laboratory results obtained on admission, data regarding the course of hospitalization, and medications on discharge [19]. Hyponatremia was defined as a serum sodium level of less than 135 mEq/L. All patients were assigned to the hypo- or normonatremia group. Renal function was assessed with the Cockcroft–Gault formula and a creatinine clearance level below 60 mL/min indicated impaired renal function.
In all patients, immediately after admission, a coronary angiography was performed with a subsequent revascularization if needed [20]. All coronary angiographics afterwards were thoroughly evaluated by two blinded physicians to determine infarct-related artery (IRA) patency and to assess critical lesions along with the results of the primary percutaneous coronary intervention (PCI). In case of a lack of obstructive lesions, narrowing the epicardial coronary segments by more than 50% during an angiography myocardial infarction with non-obstructive coronary arteries (MINOCA) was recognized [20]. Every patient underwent a two-dimensional transthoracic echocardiography at rest between the 2nd and 4th day of hospitalization after the stabilization of the patients to assess left ventricular ejection fraction (LVEF) with the Simpson’s method [19].
Data on the long-term outcomes, all-cause mortality rate, and its date were obtained from the Polish National Death Registry [21]. In our study, the long-term observation period was a median time of 41.9 [28.2–73.5] months. None of the patients were lost to follow-up. The study protocol complied with the Declaration of Helsinki and was approved by the relevant local Ethics Committee. Informed consent was provided by all the patients participating in this study.
Statistical Analysis
A statistical analysis was performed using IBM SPSS Statistics Version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables were first checked for normal distribution using the Shapiro–Wilk test. Due to the vast majority of continuous variables being characterized by a non-normal distribution, they were expressed as medians (interquartile range), and categorical variables as numbers (percentages). The differences in continuous variables were compared by a Student’s t-test or Mann–Whitney U test if the distribution was normal or different than normal, respectively [19]. Categorical variables were analyzed with the chi-square test or Fisher’s exact test. Kaplan–Meier curves for overall mortality were constructed to estimate the survival rates in the overall population as well as in the NSTEMI and STEMI subgroups. A log-rank test was performed to assess the differences in survival rates between the studied groups [19]. A multivariable linear regression was performed to find the independent determinants of baseline sodium levels. Finally, all independent variables potentially associated with an outcome were included in the Cox proportional hazard regression model to determine the independent predictors of long-term mortality. A two-sided p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Clinical Characteristics
Of the 862 analyzed patients, hyponatremia was diagnosed in 31 participants (3.6%) (Figure 1). The sodium levels of those patients were significantly lower (132 [130–133] vs. 140 [138–142] mEq/L, p < 0.001) than in the normonatremic patients.
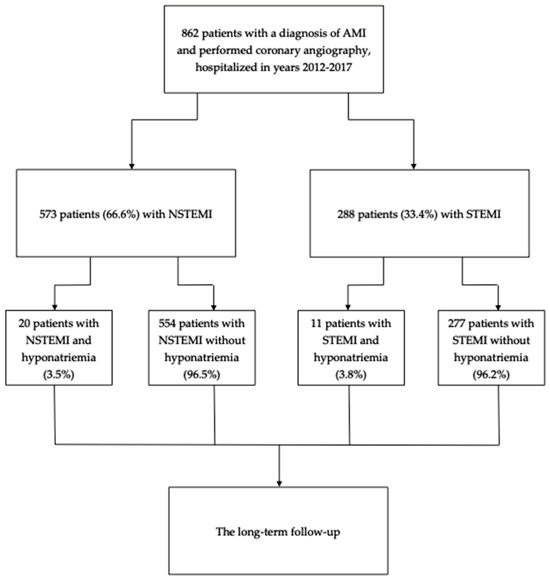
Figure 1.
The study flow-chart.
The hyponatremic group had a higher proportion of women in comparison to the group of normonatremic patients (61.3 vs. 29.6%, p < 0.001) (Table 1). Significant differences in age, history of stroke, a prior MI incidence, prior revascularization, a left ventricular ejection fraction, as well as clinical presentation and medications on discharge between the compared groups were not identified. Both groups were also similar in terms of the majority of cardiovascular risk factors, except for impaired renal function which occurred more frequently in the hyponatremic patients (32.3 vs. 15.5%, p = 0.013). In addition, the patients without hyponatremia were characterized by less severe clinical symptoms on admission. Patients with Killip class I were more frequently found in the normo- versus hyponatremic patients (80 vs. 63.3%) while those with Killip class IV appeared less frequently (4.2 vs. 16.7%, p = 0.024).

Table 1.
Characteristics of the studied patients.
Some differences in the laboratory parameters on admission were identified (Table 1). Myocardial necrotic markers including troponin T (by 75.1%, p = 0.003) and the isoenzyme MB of creatine kinase (by 34.4%, p = 0.006) levels were higher in the hyponatremic group. In this group, higher levels of white blood cells, glucose, and creatinine were also found. In contrast, lower levels of hemoglobin, hematocrit, and glomerular filtration rate were observed in that group (Table 1).
3.2. Angiography and Revascularization Strategy
The angiographic analysis did not reveal significant differences in the distribution of IRA and the frequency of an MINOCA diagnosis (Table 2). There was also a lack of differences in the revascularization strategy. The most performed revascularization therapy in hypo- and normonatremic groups was a primary PCI (87.1 and 81.1%, respectively) (Table 2).

Table 2.
Angiography and revascularization in-the studied-groups.
3.3. Long-Term Mortality
During the median time of 41.9 [28.2–73.5] months, the long-term all-cause mortality rate was significantly higher in the patients with hyponatremia versus normonatremia (18 [58.1%] vs. 243 [29.2%], log-rank p < 0.001) (Figure 2A). In the analysis of MI subtypes, hyponatremia in the NSTEMI patients was associated with a significantly higher long-term mortality (65 vs. 30.5%, log-rank p < 0.001) (Figure 2B). In contrast, a similar difference was not observed in the STEMI patients (45.5 vs. 26.7%, log-rank p = 0.13) (Figure 2C).
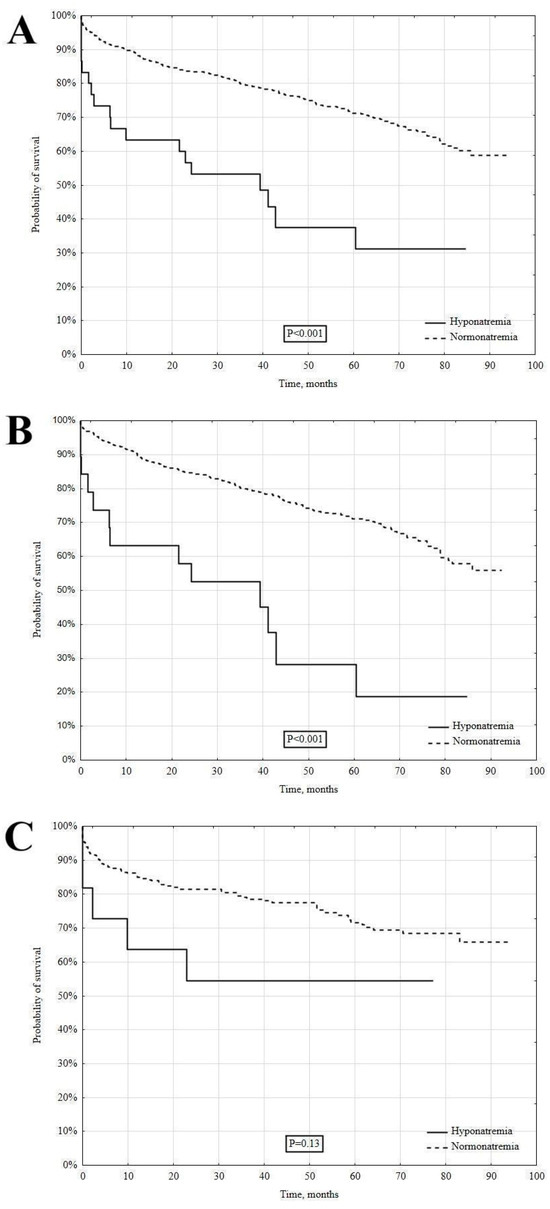
Figure 2.
The long-term survival in hypo- and normonatremic patients. Total group (A), NSTEMI (B), and STEMI (C).
As determined by multivariable linear regression, there are several factors associated with the baseline sodium levels of the whole studied group (R2 = 0.133, p < 0.001, Table 3). As has been shown, lower hematocrit levels were associated with lower sodium levels. In turn, an inverse relationship was observed for creatinine, the isoenzyme MB of creatinine kinase, and glucose levels (Table 3).

Table 3.
The independent factors associated with baseline serum sodium level.
As determined by the Cox proportional hazard regression analysis, the independent determinants of mortality were a higher age, a lower LVEF, a higher Killip class, and lower hemoglobin levels (Table 4). Apart from these, a hyponatremia diagnosis was also an independent predictor of long-term all-cause mortality (hazard ratio [HR] of 2.222, a 95% confidence interval [CI] of 1.309–3.773, and p = 0.003) (Table 4).

Table 4.
The independent predictors of long-term mortality.
4. Discussion
This study demonstrated that hyponatremia in MI patients is associated with a significantly higher long-term mortality, particularly in NSTEMI patients. The hyponatremic patients presented more severe clinical symptoms on admission, including a worse Killip class and higher levels of troponin T and the isoenzyme MB of creatinine kinase. Despite similar coronary revascularization treatments between groups, the patients with hyponatremia had unfavorable outcomes during the median follow-up period of almost 5 years. Finally, the multivariable model confirmed hyponatremia as an independent predictor, doubling the mortality risk when compared with the normonatremic patients.
The proper identifying of high-risk patients may mean opting for more aggressive management, such as early invasive strategies or enhanced pharmacological interventions. One of the major causes of hyponatremia in AMI patients is fluid overload due to progressive heart failure. Knowing this, practicians might be more cautious with fluid administration or consider the intensification of heart failure treatment. Patients with hyponatremia may need to be admitted to an intensive care unit (ICU) for closer monitoring of their fluid and electrolyte statuses, as well as their cardiac and renal functions. This enables a timely intervention if complications arise. It is also an indication of the need to optimize the patient’s pharmacotherapy (especially those diagnosed with heart failure) upon discharge. Hyponatremia can indicate more severe underlying organ damage. Therefore, post-AMI management may require a more comprehensive approach, including rehabilitation, a closer follow-up, and monitoring for heart failure progression or recurrent events.
It is interesting that the prevalence of hyponatremia in our MI population was almost 10-times lower than the estimated rate of low sodium levels in the general hospitalized patients [1,2]. We find it difficult to explain, considering that acute MI occurs more often in the group of older patients as in our study, and MI represents an acute condition, in which we expected lower sodium levels. In our analysis, as has been proven in previous studies, hyponatremia occurs more frequently in female patients [22]. The underlying mechanism of it has not been fully understood. The exposure to estrogen and progesterone which changes during the menopause period affects arginine vasopressin (AVP) regulation in women [23]. It was also hypothesized that the distribution of renal transporters along the nephron is different between men and women. The greater amount of renal Na+ transporters in the proximal tubules in females may be connected with their need for adaptation to increase fluid retention during pregnancy and lactation [24]. More research is required to understand the sex differences and their clinical implications.
In the existing literature, there are some data regarding the influence of hyponatremia on prognosis in acute MI patients. In the study by Choi et al., hyponatremia was found to have a significant influence on long-term mortality only when present upon discharge [25]. The results of the Goldberg et al. study revealed findings in line with ours; however, only patients with STEMI were assessed [26]. In this study, hyponatremia as measured on admission increased mortality in the long-term, particularly within the almost 5-year follow-up period. In addition, hyponatremia remained an independent predictor of long-term all-cause mortality (HR of 2.0, 95% CI of 1.3–3.2, and p = 0.002) [26]. Consistent results were received by Havránek et al. during the mean follow-up period of 39 months [27]. The STEMI patients with hyponatremia on admission had a lower LVEF, an elevated Killip class, and a higher death rate when compared with the normonatremic group (34.7% vs. 20.5%, p = 0.02) [27]. Furthermore, Burkhardt et al. analyzed the influence of hyponatremia on STEMI and NSTEMI patients separately, discovering that there was no significant difference in mortality between the different MI types. Both groups with hyponatremia had a lower long-term survival rate than the normonatremic participants [28].
The mechanisms dependent on hyponatremia and responsible for a worse prognosis in acute MI patients can be associated with the release of vasopressin, and the activation of the sympathetic and renin– angiotensin–aldosterone systems [29]. According to the current knowledge, these mechanisms lead to peripheral vasoconstriction and myocardial hypertrophy [30,31]. Hyponatremia also affects the myocardium directly by altering the action of the sodium–calcium pump in cardiomyocytes, which leads to a calcium overload and may induce myocardial oedema, contractile dysfunction, and coronary vasoconstriction [32]. Moreover, it enhances reactive oxygen species production which increases myocardial injury. In this context, hyponatremia can worsen cardiac vulnerability to ischemia and reperfusion injury in the acute phase of an MI [33].
The relatively high mortality rate observed in the MI patients—both with hypo- and normonatremia—who were admitted to our clinic in 2012–2017 can be partly attributed to factors such as delayed diagnoses due to the lack of sufficient public education on cardiovascular diseases or limited access to specialized care, and suboptimal management practices. Additionally, this period predates the introduction of key treatments for heart failure management, such as flozins (SGLT2 inhibitors) and sacubitril/valsartan, which have since become integral to improving outcomes. The lack of these advanced therapeutic options likely contributed to the poor prognosis of many MI patients, particularly those with heart failure and a reduced ejection fraction or other comorbidities. However, in more recent years, significant improvements have been made in patient care following an MI, with the development of numerous monitoring programs, such as the “KOS-MI” initiative, which tracks and supports patients after their hospitalization [15,34]. These programs have proven to be highly effective in improving long-term outcomes and survival rates, demonstrating the positive impact of more structured follow-up care and early intervention [15,34]. As a result, the overall prognosis for MI patients in Poland has markedly improved in recent years.
Our study has several limitations. Firstly, the analyzed patients were recruited in a single center; therefore, the results possibly do not represent the whole Polish population adequately. Secondly, patients’ sodium levels were assessed only on admission and were not evaluated throughout their hospital stay, thus the comparison of mortality depending on natremia fluctuations could not be performed. We also did not consider all the possible causes of hyponatremia and their impact on the results of our study. Moreover, we did not classify them as primary and secondary. Finally, we were not able to provide the causes of patients’ death due to the limitations of the analyzed death registry.
5. Conclusions
Although relatively rare, hyponatremia in the acute phase of MI remains an independent predictor of increased long-term all-cause mortality, particularly with NSTEMI presentation. For clinicians, it is important to consider electrolyte imbalance when assessing a patient’s prognosis and possibly adjusting a treatment regimen. Further studies concerning this relationship need to be performed with a representative number of participants to understand the underlying mechanisms and plan the optimal management of the condition to ensure favorable long-term clinical outcomes as frequently as possible.
Author Contributions
Conceptualization, K.E. and K.S.; methodology, K.E. and K.S.; software, K.N. and J.Z.; validation, K.E., K.S. and J.Z.; formal analysis, K.S.; investigation, K.E., K.S. and J.Z.; resources, K.E., K.S., K.N., K.K. and M.P.; data curation, M.W., O.A., K.M., G.M., G.H. and J.P.; writing—original draft preparation, M.W., O.A., K.M., G.H. and J.P.; writing—review and editing, K.E., K.S., K.K., M.P. and K.N.; visualization, K.E. and K.S.; supervision, J.N. and J.Z.; project administration, K.E., K.S., J.N. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Jagiellonian University Medical College (protocol code 1072.6120.59.2018 and the date of approval was 1 June 2018).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in this study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tazmini, K.; Nymo, S.H.; Louch, W.E.; Ranhoff, A.H.; Øie, E. Electrolyte imbalances in an unselected population in an emergency department: A retrospective cohort study. PLoS ONE 2019, 14, e0215673. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Epidemiology of hyponatremia. Semin. Nephrol. 2009, 29, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Choi, D.J.; Yoon, C.H.; Oh, I.Y.; Jeon, E.S.; Kim, J.J.; Cho, M.C.; Chae, S.C.; Ryu, K.H.; Oh, B.H. Improvement of hyponatraemia during hospitalisation for acute heart failure is not associated with improvement of prognosis: An analysis from the Korean Heart Failure (KorHF) registry. Heart 2012, 98, 1798–1804. [Google Scholar] [CrossRef]
- Kapłon-Cieślicka, A.; Ozierański, K.; Balsam, P.; Tymińska, A.; Peller, M.; Galas, M.; Wyzgał, M.; Marchel, M.; Drożdż, J.; Opolski, G. Clinical characteristics and 1-year outcome of hyponatremic patients hospitalized for heart failure. Pol. Arch. Intern. Med. 2015, 125, 120–131. [Google Scholar] [CrossRef]
- Rywik, T.M.; Wiśniewska, A.; Cegłowska, U.; Drohomirecka, A.; Topór-Mądry, R.; Łazarczyk, H.; Połaska, P.; Zieliński, T.; Doryńska, A. Heart failure with reduced, mildly reduced, and preserved ejection fraction: Outcomes and predictors of prognosis. Pol. Arch. Intern. Med. 2023, 133, 16522. [Google Scholar] [CrossRef]
- Huang, W.Y.; Weng, W.C.; Peng, T.I.; Chien, Y.Y.; Wu, C.L.; Lee, M.; Hung, C.C.; Chen, K.H. Association of hyponatremia in acute stroke stage with three-year mortality in patients with first-ever ischemic stroke. Cerebrovasc. Dis. 2012, 34, 55–62. [Google Scholar] [CrossRef]
- Waikar, S.S.; Curhan, G.C.; Brunelli, S.M. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am. J. Med. 2011, 124, 77–84. [Google Scholar] [CrossRef]
- Funk, G.C.; Lindner, G.; Druml, W.; Metni, B.; Schwarz, C.; Bauer, P.; Metni, P.G. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010, 36, 304–311. [Google Scholar] [CrossRef]
- Ma, Q.Q.; Fan, X.D.; Li, T.; Hao, Y.Y.; Ma, F. Short- and long-term prognostic value of hyponatremia in patients with acute coronary syndrome: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0193857. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Commi ee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, P.; Kowalik, I.; Piotrowicz, E. Feasibility of hybrid telerehabilitation as a component of the Managed Care after Acute Myocardial Infarction (MC-AMI) program in a 12-month follow-up: Experience from a single center. Pol. Arch. Intern. Med. 2023, 133, 16456. [Google Scholar] [CrossRef] [PubMed]
- Kaziród-Wolski, K.; Sielski, J.; Gąsior, M.; Bujak, K.; Hawranek, M.; Pyka, Ł.; Gierlotka, M.; Pawłowski, T.; Siudak, Z. Factors affecting short- and long-term survival of patients with acute coronary syndrome treated invasively using intravascular ultrasound and fractional flow reserve: Analysis of data from the Polish Registry of Acute Coronary Syndromes 2017–2020. Kardiol. Pol. 2023, 81, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Pruc, M.; Kubica, J.; Banach, M.; Swieczkowski, D.; Rafique, Z.; Peacock, W.F.; Siudak, Z.; Kurek, K.; Nanayakkara, P.; Szarpak, Ł. Diagnostic and prognostic performance of the neutrophil-to-lymphocyte ratio in acute coronary syndromes: A meta-analysis of 90 studies including 45,990 patients. Kardiol. Pol. 2024, 82, 276–284. [Google Scholar] [CrossRef]
- Kolarczyk-Haczyk, A.; Konopko, M.; Mazur, M.; Żurakowski, A.; Gąsior, M.; Rogala, M.; Jankowski, P.; Kaźmierczak, P.; Milewski, K.P.; Buszman, P.E.; et al. Long-term outcomes of the Coordinated Care Program in Patients after Myocardial Infarction (KOS-MI). Kardiol. Pol. 2023, 81, 587–596. [Google Scholar] [CrossRef]
- Cordova Sanchez, A.; Bhuta, K.; Shmorgon, G.; Angeloni, N.; Murphy, R.; Chaudhuri, D. The association of hyponatremia and clinical outcomes in patients with acute myocardial infarction: A cross-sectional study. BMC Cardiovasc. Disord. 2022, 22, 276. [Google Scholar] [CrossRef]
- Tada, Y.; Nakamura, T.; Funayama, H.; Sugawara, Y.; Ako, J.; Ishikawa, S.E.; Momomura, S. Early development of hyponatremia implicates short- and long-term outcomes in ST-elevation acute myocardial infarction. Circ. J. 2011, 75, 1927–1933. [Google Scholar] [CrossRef]
- Singla, I.; Zahid, M.; Good, C.B.; Macioce, A.; Sonel, A.F. Effect of hyponatremia (<135 mEq/L) on outcome in patients with non-ST-elevation acute coronary syndrome. Am. J. Cardiol. 2007, 100, 406–408. [Google Scholar] [CrossRef]
- Stępień, K.; Nowak, K.; Kachnic, N.; Karcińska, A.; Del Carmen Yika, A.; Furczyński, J.; Platschek, M.; Skorupa, M.; Wyleciał, Z.; Zalewski, J.; et al. Clinical characteristics and long-term outcomes of patients with heart failure with improved ejection fraction. First Polish experience from LECRA-HF registry. Adv. Med. Sci. 2024, 69, 132–138. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Szlosarczyk, B.; Nessler, J.; Zalewski, J. Clinical Characteristics and Long-Term Outcomes of MINOCA Accompanied by Active Cancer: A Retrospective Insight Into a Cardio-Oncology Center Registry. Front. Cardiovasc. Med. 2022, 9, 785246. [Google Scholar] [CrossRef]
- Krawczyk, K.; Stepien, K.; Nowak, K.; Nessler, J.; Zalewski, J. ST-segment re-elevation following primary angioplasty in acute myocardial infarction with patent infarct-related artery: Impact on left ventricular function recovery and remodeling. Adv. Interv. Cardiol. 2019, 15, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gu, S.; Parikh, A.; Radhakrishnan, J. Prevalence of hyponatremia and association with mortality: Results from NHANES. Am. J. Med. 2013, 126, 1127–1137.e1. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, N.S. Hormonal changes during menopause and the impact on fluid regulation. Reprod. Sci. 2014, 21, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; McDonough, A.A.; Layton, A.T. Sex differences in solute and water handling in the human kidney: Modeling and functional implications. iScience 2021, 24, 102667. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Ahn, Y.K.; Jeong, M.H.; Kim, S.W. Prognostic impact of hyponatremia occurring at various time points during hospitalization on mortality in patients with acute myocardial infarction. Medicine 2017, 96, e7023. [Google Scholar] [CrossRef]
- Goldberg, A.; Hammerman, H.; Petcherski, S.; Nassar, M.; Zdorovyak, A.; Yalonetsky, S.; Kapeliovich, M.; Agmon, Y.; Beyar, R.; Markiewicz, W.; et al. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch. Intern. Med. 2006, 166, 781–786. [Google Scholar] [CrossRef]
- Havránek, Š.; Bělohlávek, J.; Škulec, R.; Kovárník, T.; Dytrych, V.; Linhart, A. Long-term prognostic impact of hyponatremia in the ST-elevation myocardial infarction. Scand. J. Clin. Lab. Investig. 2011, 71, 38–44. [Google Scholar] [CrossRef]
- Burkhardt, K.; Kirchberger, I.; Heier, M.; Zirngibl, A.; Kling, E.; von Scheidt, W.; Kuch, B.; Meisinger, C. Hyponatraemia on admission to hospital is associated with increased long-term risk of mortality in survivors of myocardial infarction. Eur. J. Prev. Cardiol. 2015, 22, 1419–1426. [Google Scholar] [CrossRef]
- Palmer, B.R.; Pilbrow, A.P.; Frampton, C.M.; Yandle, T.G.; Skelton, L.; Nicholls, M.G.; Richards, A.M. Plasma aldosterone levels during hospitalization are predictive of survival post-myocardial infarction. Eur. Heart J. 2008, 29, 2489–2496. [Google Scholar] [CrossRef]
- Correale, M.; Tricarico, L.; Fortunato, M.; Mazzeo, P.; Nodari, S.; Di Biase, M.; Brune, N.D. New Targets in Heart Failure Drug Therapy. Front. Cardiovasc. Med. 2021, 8, 665797. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Mota, K.O.; Elimban, V.; Shah, A.K.; de Vasconcelos, C.M.L.; Bhullar, S.K. Role of Vasoactive Hormone-Induced Signal Transduction in Cardiac Hypertrophy and Heart Failure. Cells 2024, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Movafagh, S.; Cleemann, L.; Morad, M. Regulation of cardiac Ca2+ channel by extracellular Na+. Cell Calcium 2011, 49, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Oniki, T.; Teshima, Y.; Nishio, S.; Ishii, Y.; Kira, S.; Abe, I.; Yufu, K.; Takahashi, N. Hyponatraemia aggravates cardiac susceptibility to ischaemia/reperfusion injury. Int. J. Exp. Pathol. 2019, 100, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kułach, A.; Wilkosz, K.; Wybraniec, M.; Wieczorek, P.; Gąsior, Z.; Mizia-Stec, K.; Wojakowski, W.; Zdrojewski, T.; Wojtyniak, B.; Gąsior, M.; et al. Managed Care after Acute Myocardial Infarction (MC-AMI)—Poland’s nationwide program of comprehensive post-MI care improves prognosis in 2-year follow-up. A single high-volume center intention-to-treat analysis. Kardiol. Pol. 2023, 81, 123–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).