Post-Hoc Analysis of a Multicenter Clinical Trial: Correlation of Coagulation Factor Changes and MRI-Defined Treatment Outcomes After Sclerotherapy for Venous Malformations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. MRI Imaging Methods and Volumetric Measurements
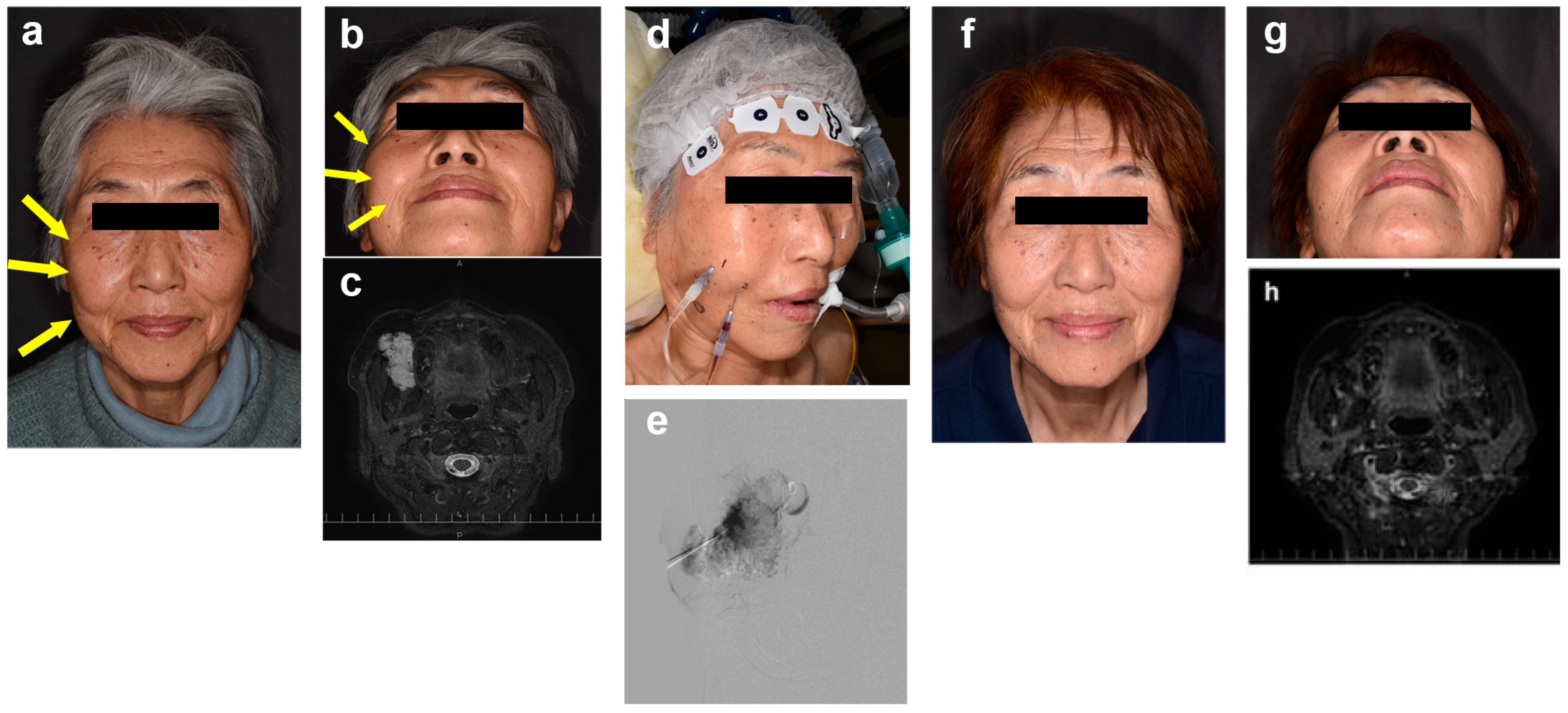
2.3. Sclerotherapy Technique
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics and Lesion Characteristics
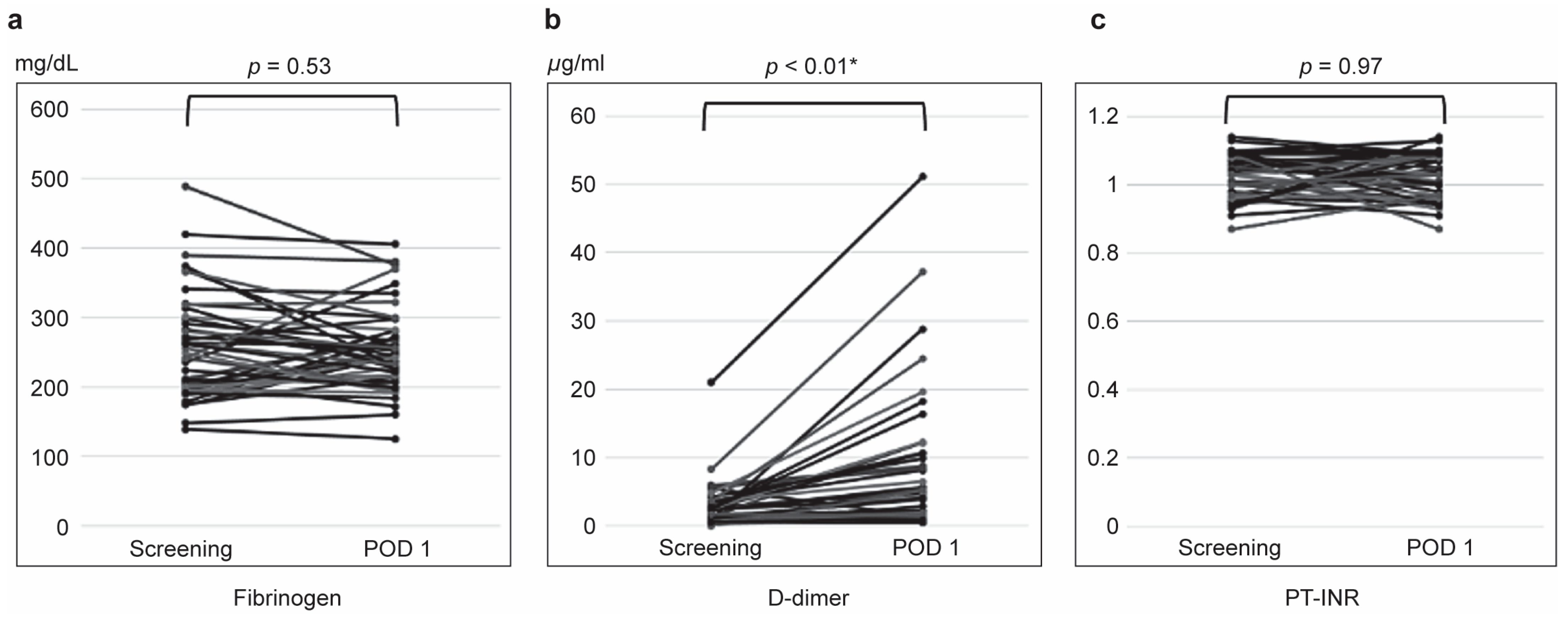
3.2. Coagulation Parameter Changes
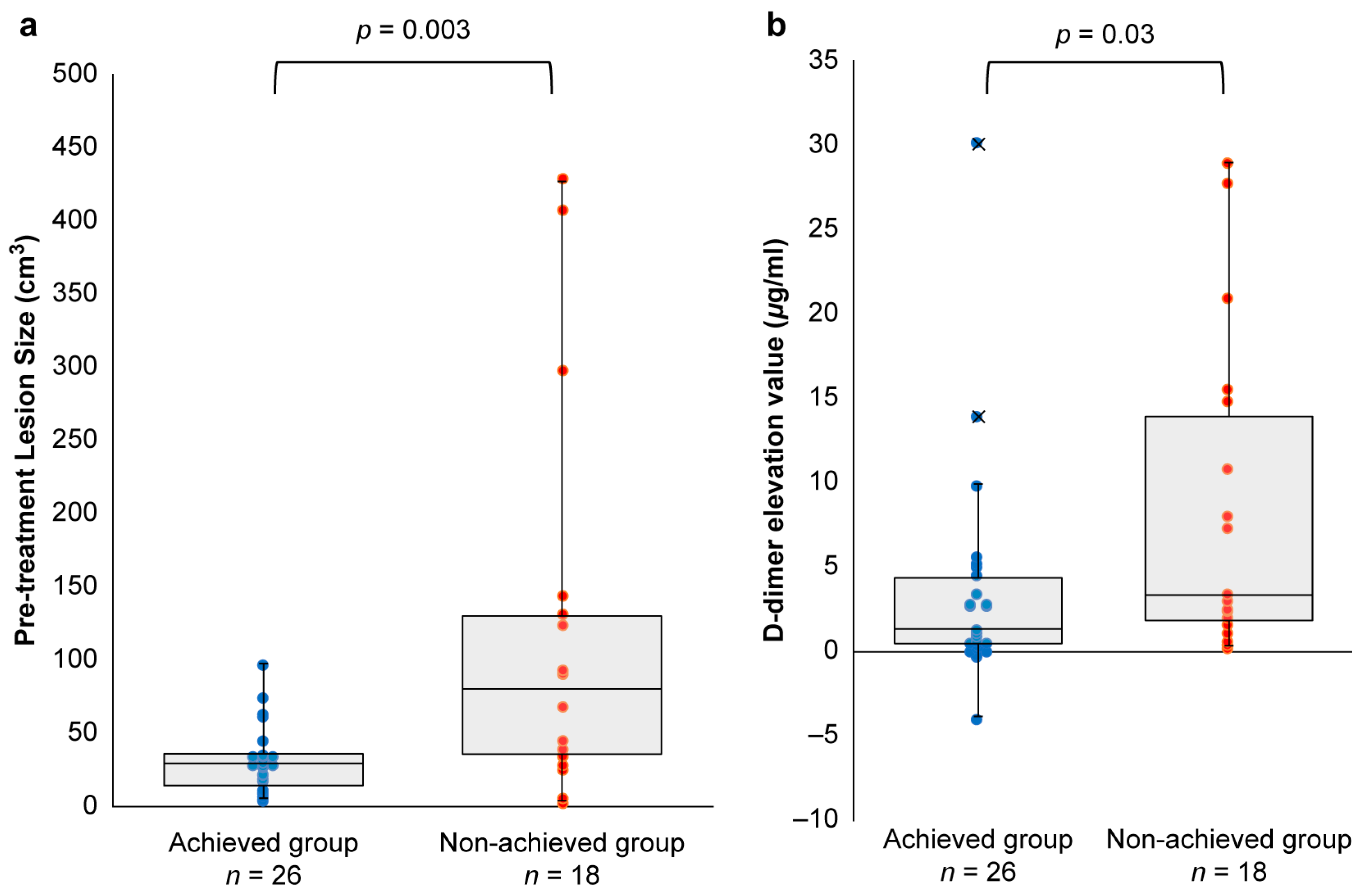
3.3. Achieved vs. Non-Achieved Groups
3.4. Safety Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulliken, J.B.; Glowacki, J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Hein, K.D.; Mulliken, J.B.; Kozakewich, H.P.; Upton, J.; Burrows, P.E. Venous malformations of skeletal muscle. Plast. Reconstr. Surg. 2002, 110, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Carabin, J.; Bouhamama, A.; Vaz, G.; Cuinet, M.; Ricoeur, A.; Thibaut, A.; Beji, H.; Mastier, C.; Pilleul, F. Percutaneous Cryoablation of Symptomatic Intramuscular Venous Malformation. J. Vasc. Interv. Radiol. 2020, 31, 558–563 e553. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.B.; Bae, Y.C.; Nam, S.B.; Bae, S.H.; Sung, J.Y. The Usefulness of Surgical Treatment in Slow-Flow Vascular Malformation Patients. Arch. Plast. Surg. 2017, 44, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.N.; Do, Y.S.; Park, K.B.; Park, H.S.; Kim, Y.W.; Lee, B.B.; Pyon, J.K.; Lim, S.Y.; Mun, G.H.; Kim, D.I. The results of surgical treatment for patients with venous malformations. Ann. Vasc. Surg. 2012, 26, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Do, Y.S.; Byun, H.S.; Choo, I.W.; Kim, D.I.; Huh, S.H. Advanced management of venous malformation with ethanol sclerotherapy: Mid-term results. J. Vasc. Surg. 2003, 37, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Kaji, N.; Kurita, M.; Ozaki, M.; Takushima, A.; Harii, K.; Narushima, M.; Wakita, S. Experience of sclerotherapy and embolosclerotherapy using ethanolamine oleate for vascular malformations of the head and neck. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2009, 43, 126–136. [Google Scholar] [CrossRef]
- Gorriz, E.; Carreira, J.M.; Reyes, R.; Gervas, C.; Pulido, J.M.; Pardo, M.D.; Maynar, M. Intramuscular low flow vascular malformations: Treatment by means of direct percutaneous embolization. Eur. J. Radiol. 1998, 27, 161–165. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; de Mattos Camargo Grossmann, S.; do Amaral, M.B.F.; de Castro, W.H.; Navarro, T.P.; Procopio, R.J.; da Silva, T.A.; de Nazare Alves de Oliveira Kato, C.; Mesquita, R.A. Effectiveness and safety of foam sclerotherapy with 5% ethanolamine oleate in the treatment of low-flow venous malformations in the head and neck region: A case series. Int. J. Oral Maxillofac. Surg. 2018, 47, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Mimura, H.; Akita, S.; Fujino, A.; Jinnin, M.; Ozaki, M.; Osuga, K.; Nakaoka, H.; Morii, E.; Kuramochi, A.; Aoki, Y.; et al. Japanese Clinical Practice Guidelines for Vascular Anomalies 2017. J. Dermatol. 2020, 47, e138–e183. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, M.; Kokubu, S.; Hirota, S.; Koizumi, J.; Nishida, N.; Yasumoto, T.; Mochida, S.; Hidaka, H.; Tanaka, N.; Tajima, T. Short-Term Safety and Efficacy of Balloon-Occluded Retrograde Transvenous Obliteration Using Ethanolamine Oleate: Results of a Prospective, Multicenter, Single-Arm Trial. J. Vasc. Interv. Radiol. 2017, 28, 1108–1115 e1102. [Google Scholar] [CrossRef][Green Version]
- Ozaki, M.; Kurita, M.; Kaji, N.; Fujino, T.; Narushima, M.; Takushima, A.; Harii, K. Efficacy and evaluation of safety of sclerosants for intramuscular venous malformations: Clinical and experimental studies. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2010, 44, 75–87. [Google Scholar] [CrossRef]
- Yamaki, T.; Nozaki, M.; Sakurai, H.; Takeuchi, M.; Soejima, K.; Kono, T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J. Vasc. Surg. 2008, 47, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.; Das, B.K. Treatment of venous malformations with ethanolamine oleate: A descriptive study of 83 cases. Pediatr. Surg. Int. 2011, 27, 527–531. [Google Scholar] [CrossRef]
- Vollherbst, D.F.; Gebhart, P.; Kargus, S.; Burger, A.; Kuhle, R.; Gunther, P.; Hoffmann, J.; Bendszus, M.; Mohlenbruch, M.A. Image-guided percutaneous sclerotherapy of venous malformations of the head and neck: Clinical and MR-based volumetric mid-term outcome. PLoS ONE 2020, 15, e0241347. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Pannier, F. Sclerotherapy in venous malformation. Phlebology 2013, 28 (Suppl. 1), 188–191. [Google Scholar] [CrossRef]
- Horbach, S.E.; Lokhorst, M.M.; Saeed, P.; de Goüyon Matignon de Pontouraude, C.M.; Rothová, A.; van der Horst, C.M. Sclerotherapy for low-flow vascular malformations of the head and neck: A systematic review of sclerosing agents. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 295–304. [Google Scholar] [CrossRef]
- Leung, Y.C.; Leung, M.W.; Yam, S.D.; Hung, J.W.; Liu, C.S.; Chung, L.Y.; Tang, M.Y.; Fung, H.S.; Poon, W.L.; Chao, N.S.; et al. D-dimer level correlation with treatment response in children with venous malformations. J. Pediatr. Surg. 2018, 53, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.P.; Neufeld, E.J.; Karian, V.E.; Zurakowski, D.; Koka, B.V.; Burrows, P.E. Coagulation abnormalities in pediatric and adult patients after sclerotherapy or embolization of vascular anomalies. AJR Am. J. Roentgenol. 2001, 177, 1359–1363. [Google Scholar] [CrossRef]
- Yu, M.W.; Han, Y.Y.; Wang, Q.; Wang, M.; Chen, Y.; Yuan, S.M. Treatment Outcomes and Effects of Ethanol Sclerotherapy on Systemic Coagulation Profile of Patients with Venous Malformation. Ann. Vasc. Surg. 2022, 85, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.; Obara, K.; Suzuki, S.; Orikasa, K.; Mitsuhashi, H.; Iwasaki, K.; Sakamoto, H.; Morito, T.; Kasukawa, R. The destructive effects of sclerosant ethanolamine oleate on mammalian vessel endothelium. Gastroenterol. Jpn. 1990, 25, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Ozaki, M.; Kurachi, I.; Iwashina, Y.; Takushima, A. Risk Factors for Macroscopic Haemoglobinuria After Sclerotherapy Using Ethanolamine Oleate for Venous Malformations. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Kitano, S.; Yamaga, H.; Sugimachi, K. Haptoglobin to protect against renal damage from ethanolamine oleate sclerosant. Lancet 1988, 2, 340–341. [Google Scholar] [CrossRef] [PubMed]
| All (n = 44) | Venous Malformations | p-Value | ||||
|---|---|---|---|---|---|---|
| Cystic-Type | Diffuse-Type | |||||
| (n = 22) | (n = 22) | |||||
| Sex | Male, n (%) | 13 (59.1) | 9 (40.9) | 0.37 | ||
| Female, n (%) | 9 (40.9) | 13 (59.1) | ||||
| Age, median, year (range) | 16.5 (3−78) | 16.5 (5−59) | 0.91 | |||
| Weight, median, kg (range) | 49.1 (14.7−92.0) | 50.5 (20.3−75.2) | 0.24 | |||
| Lesion, n (%) | Trunk | 9 | 5 (22.7) | 4 (18.2) | ||
| Extremity | Upper | 11 | 3 (13.6) | 8 (36.4) | ||
| Lower | 15 | 5 (22.7) | 10 (45.5) | |||
| Head and neck | Head | 0 | 0 (0.0) | 0 (0.0) | ||
| Face (except for intraoral) | 5 | 5 (22.7) | 0 (0.0) | |||
| Intraoral | 1 | 1 (4.5) | 0 (0.0) | |||
| Neck | 3 | 3 (13.6) | 0 (0.0) | |||
| Size, median (min, max) cm3 | 28.1 (2.25, 429) | 27.9 (2.25, 131) | 62.1 (3.85, 429) | 0.017 * | ||
| Coagulation markers | Fibrinogen, median (min, max) mg/dL | 246 (139, 489) | 211 (139, 489) | 262 (175, 374) | 0.39 | |
| D-dimer, median (min, max) μg/mL | 1.40 (0, 21.0) | 2.05 (0.5, 21.0) | 1.10 (0, 8.30) | 0.35 | ||
| PT-INR, median (min, max) | 1.03 (0, 1.14) | 1.03 (0.91, 1.13) | 1.03 (0.87, 1.14) | 0.98 | ||
| Previous treatment for VM, n (%) | Sclerotherapy | 22 (50.0) | 10 (45.5) | 12 (54.5) | ||
| Surgical resection | 6 (13.6) | 4 (18.2) | 2 (9.1) | |||
| All | Cystic-Type | Diffuse-Type | p-Value | ||
|---|---|---|---|---|---|
| (n = 44) | (n = 22) | (n = 22) | |||
| 5% EO | Total volume, median (min, max) mL | 9.00 (2.00, 22.1) | 8.35 (2.00, 22.0) | 12.2 (2.60, 22.1) | 0.17 |
| Amount used per lesion, median (min, max) mL/cm3 | 0.26 (0.04, 0.40) | 0.25 (0.04, 0.40) | 0.31 (0.05, 0.40) | 0.15 | |
| Amount used per body weight, median (min, max) mL/kg | 0.27 (0.04, 1.78) | 0.31 (0.10, 1.16) | 0.27 (0.04, 1.78) | 0.27 |
| No. of Patients | Achieving a Reduction of at Least 20% | |||
|---|---|---|---|---|
| No. of Achievers | Percentage | 95% Confidence Intervals | ||
| All | 44 | 26 | 59.1 | 44.41–72.31 |
| Cystic type | 22 | 16 | 72.7 | 51.85–86.85 |
| Diffuse type | 22 | 10 | 45.5 | 26.92–65.34 |
| All (n = 44) | Venous Malformations | p-Value | |||
|---|---|---|---|---|---|
| Achieved Group | Non-Achieved Group | ||||
| (n = 26) | (n = 18) | ||||
| Type of lesion, n (%) | 0.12 | ||||
| Cystic type | 22 | 16 (72.7) | 6 (27.3) | ||
| Diffuse type | 22 | 10 (45.5) | 12 (54.5) | ||
| Pre-treatment lesion size, median (IQR) cm3 | 27.9 (12.6, 35.0) | 79.2 (34.3, 129.3) | 0.003 * | ||
| 5% EO | Total volume, mean (±SD) | 8.9 (±5.0 mL) | 11.5 (±5.5 mL) | 0.10 | |
| Amount used per lesion, mean (±SD) | 0.42 (±0.29 mL/cm3) | 0.37 (±0.43 mL/cm3) | 0.40 | ||
| Amount used per weight, mean (±SD) | 0.27 (±0.22 mL/kg) | 0.31 (±0.10 mL/kg) | 0.31 | ||
| D-dimer elevation value, median (IQR) μg/mL † | 1.20 (0.33, 4.2) | 3.2 (1.7, 13.8) | 0.03 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, T.; Ozaki, M.; Osuga, K.; Kurita, M.; Hayashi, A.; Yuzuriha, S.; Aramaki-Hattori, N.; Hikosaka, M.; Nozaki, T.; Ozeki, M.; et al. Post-Hoc Analysis of a Multicenter Clinical Trial: Correlation of Coagulation Factor Changes and MRI-Defined Treatment Outcomes After Sclerotherapy for Venous Malformations. J. Clin. Med. 2025, 14, 905. https://doi.org/10.3390/jcm14030905
Nomura T, Ozaki M, Osuga K, Kurita M, Hayashi A, Yuzuriha S, Aramaki-Hattori N, Hikosaka M, Nozaki T, Ozeki M, et al. Post-Hoc Analysis of a Multicenter Clinical Trial: Correlation of Coagulation Factor Changes and MRI-Defined Treatment Outcomes After Sclerotherapy for Venous Malformations. Journal of Clinical Medicine. 2025; 14(3):905. https://doi.org/10.3390/jcm14030905
Chicago/Turabian StyleNomura, Tadashi, Mine Ozaki, Keigo Osuga, Masakazu Kurita, Ayato Hayashi, Shunsuke Yuzuriha, Noriko Aramaki-Hattori, Makoto Hikosaka, Taiki Nozaki, Michio Ozeki, and et al. 2025. "Post-Hoc Analysis of a Multicenter Clinical Trial: Correlation of Coagulation Factor Changes and MRI-Defined Treatment Outcomes After Sclerotherapy for Venous Malformations" Journal of Clinical Medicine 14, no. 3: 905. https://doi.org/10.3390/jcm14030905
APA StyleNomura, T., Ozaki, M., Osuga, K., Kurita, M., Hayashi, A., Yuzuriha, S., Aramaki-Hattori, N., Hikosaka, M., Nozaki, T., Ozeki, M., Ochi, J., Akiyama, S., Kakei, Y., Miyakoda, K., Kashiwagi, N., Yasuda, T., Iwashina, Y., Kaneko, T., Terashi, H., & Harii, K. (2025). Post-Hoc Analysis of a Multicenter Clinical Trial: Correlation of Coagulation Factor Changes and MRI-Defined Treatment Outcomes After Sclerotherapy for Venous Malformations. Journal of Clinical Medicine, 14(3), 905. https://doi.org/10.3390/jcm14030905





