Contrast Sensitivity and Stereopsis Outcomes Following LASIK Presbyopia Correction Based on the Corneal Aberration Modulation or Corneal Multifocality Induction Methods: A Systematic Review
Abstract
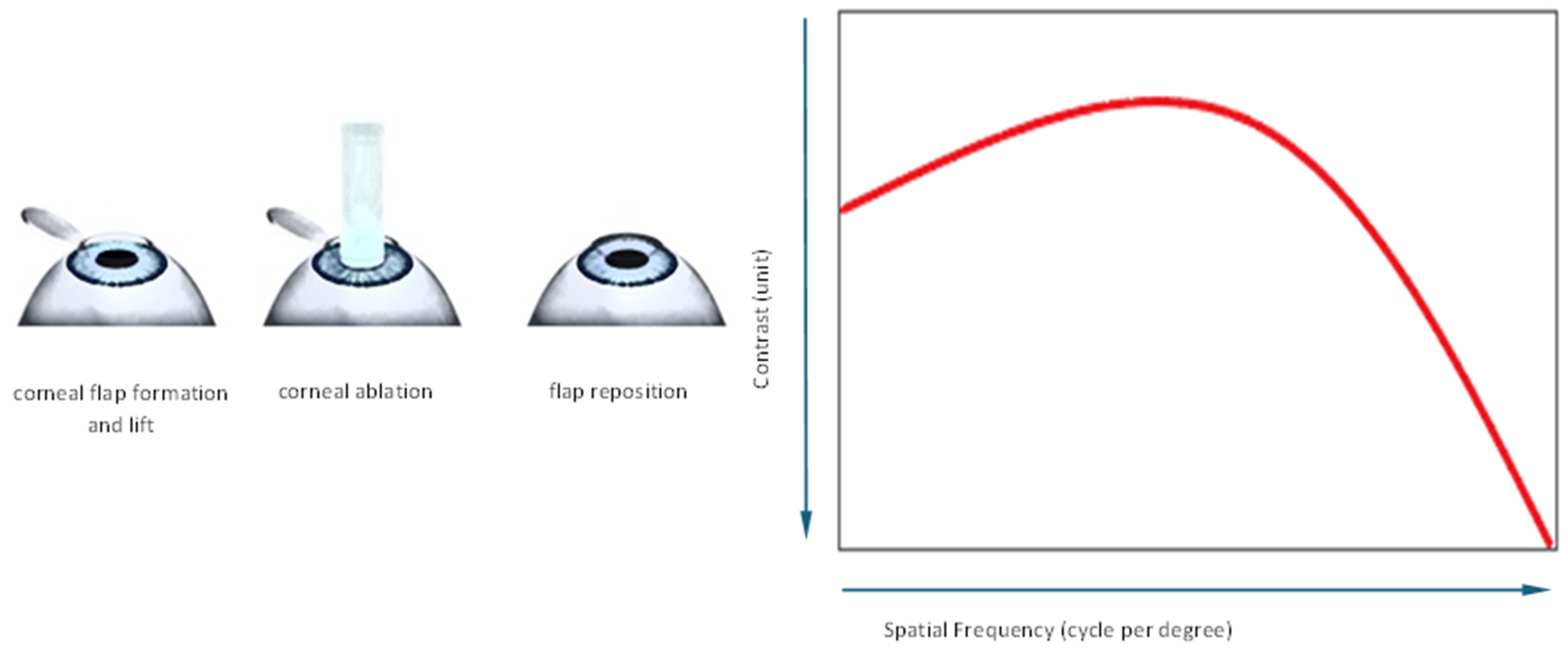
1. Introduction
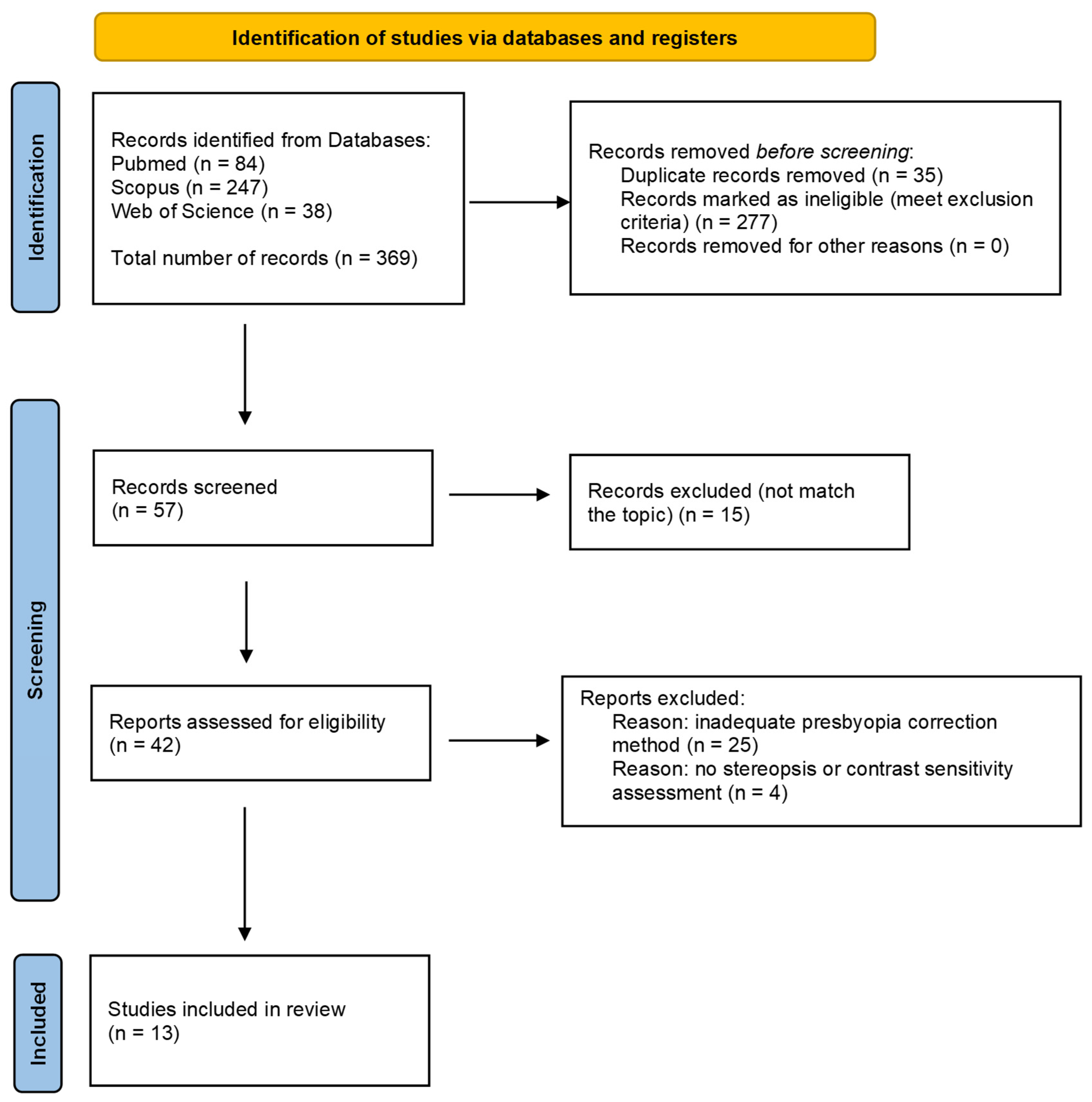
2. Materials and Methods
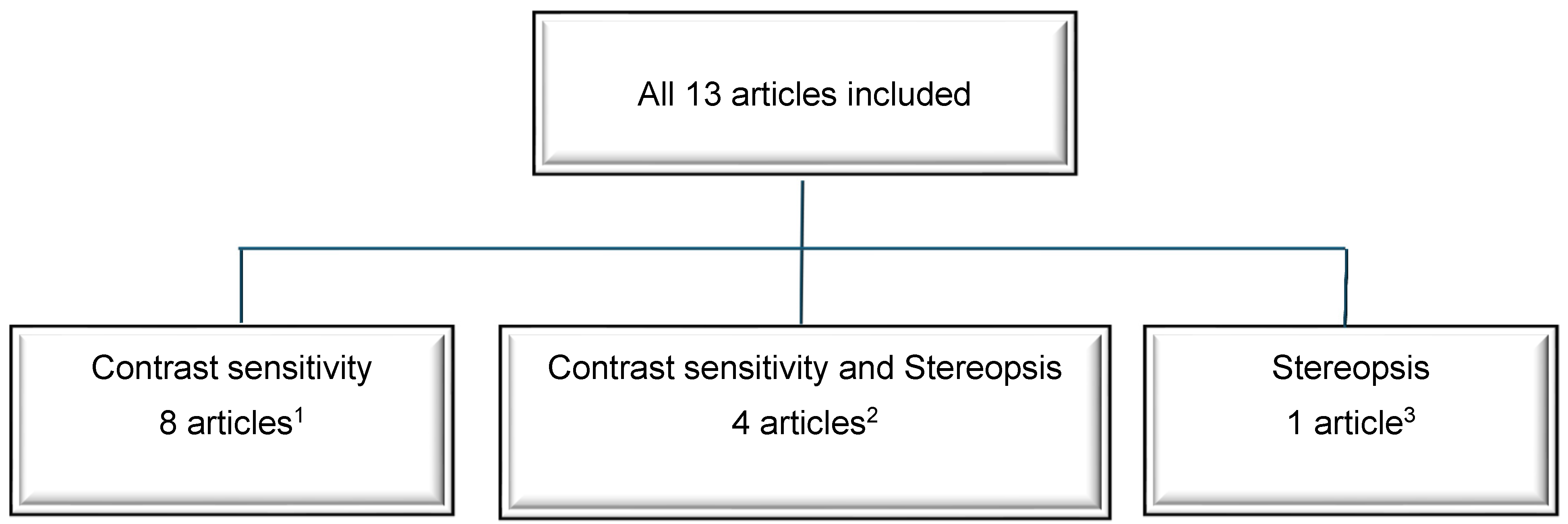
3. Results
3.1. Contrast Sensitivity
3.2. Stereopsis
4. Discussion
4.1. Contrast Sensitivity
4.2. Stereopsis
4.3. Limitations of the Study
4.4. Future Prospects
5. Conclusions
- Extending the DoF by inducing the controlled amount of SA in both eyes and the creation of micro-monovision to −1.50 D in the non-dominant eye is a promising binocular laser corneal approach to overcoming presbyopia.
- All studies on Presbyond® LBV included in this review indicated that this aspheric micro-monovision protocol was a safe procedure in terms of the preservation of contrast sensitivity for treating myopic, emmetropic and hyperopic presbyopes.
- Three out of four analyzed studies reported an unfavorable impact of multifocal or wavefront-guided presbyopic LASIK on CS.
- In terms of the preservation of stereopsis, the results of this review are inconclusive. Several studies assessing the effect of Presbyond® on stereopsis reported conflicting results, with the near stereopsis being reduced, unchanged or increased. A significant decrease in stereopsis was reported after aspheric monovision LASIK.
- Preoperative screening is of great importance when making a decision concerning laser surgery with micro-monovision. Quantitative stereopsis tests, which provide key information on the highest level of binocular function, are valuable tools to use in clinic preoperatively and after each LASIK presbyopia treatment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glasser, A.; Campbell, M.C. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999, 39, 1991–2015. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vision 2020. In Global Initiative for the Elimination of Avoidable Blindness; Fact Sheet No 1213; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Vargas-Fragoso, V.; Alió, J.L. Corneal compensation of presbyopia: PresbyLASIK: An updated review. Eye Vis. 2017, 4, 11. [Google Scholar] [CrossRef]
- Datta, S.; Foss, A.J.; Grainge, M.J.; Gregson, R.M.; Zaman, A.; Masud, T.; Osborn, F.; Harwood, R.H. The importance of acuity, stereopsis, and contrast sensitivity for health-related quality of life in elderly women with cataracts. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1–6. [Google Scholar] [CrossRef]
- Luger, M.H.; McAlinden, C.; Buckhurst, P.J.; Wolffsohn, J.S.; Verma, S.; Arba Mosquera, S. Presbyopic LASIK using hybrid bi-aspheric micro-monovision ablation profile for presbyopic corneal treatments. Am. J. Ophthalmol. 2015, 160, 493–505. [Google Scholar] [CrossRef]
- Shetty, R.; Brar, S.; Sharma, M.; Dadachanji, Z.; Lalgudi, V.G. PresbyLASIK: A review of PresbyMAX, Supracor, and laser blended vision: Principles, planning, and outcomes. Indian J. Ophthalmol. 2020, 68, 2723–2731. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Couch, D.G.; Archer, T.J. LASIK for hyperopic astigmatism and presbyopia using micro-monovision with the Carl Zeiss Meditec MEL80 platform. J. Refract. Surg. 2009, 25, 37–58. [Google Scholar] [CrossRef]
- Artola, A.; Patel, S.; Schimchak, P.; Ayala, M.J.; Ruiz-Moreno, J.M.; Alió, J.L. Evidence for delayed presbyopia after photorefractive keratectomy for myopia. Ophthalmology 2006, 113, 735–741. [Google Scholar] [CrossRef]
- Rocha, K.M.; Vabre, L.; Chateau, N.; Krueger, R.R. Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. J. Cataract Refract. Surg. 2009, 35, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Molina-Martín, A.; Rocha-de-Lossada, C.; Rodríguez-Vallejo, M.; Piñero, D.P. Clinical outcomes of presbyopia correction with the latest techniques of presbyLASIK: A systematic review. Eye 2023, 37, 587–596. [Google Scholar] [CrossRef]
- Ryan, D.S.; Sia, R.K.; Rabin, J.; Rivers, B.A.; Stutzman, R.D.; Pasternak, J.F.; Eaddy, J.B.; Logan, L.A.; Bower, K.S. Contrast Sensitivity After Wavefront-Guided and Wavefront-Optimized PRK and LASIK for Myopia and Myopic Astigmatism. J. Refract. Surg. 2018, 34, 590–596. [Google Scholar] [CrossRef]
- Ohlsson, J.; Villarreal, G.; Abrahamsson, M.; Cavazos, H.; Sjöström, A.; Sjöstrand, J. Screening merits of the Lang II, Frisby, Randot, Titmus and TNO stereo tests. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2001, 5, 316–322. [Google Scholar] [CrossRef]
- O’Cnnor, A.R.; Birch, E.E.; Anderson, S.; Draper, H.; the FSOS Research Group. The Functional Significance of Stereopsis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2019–2023. [Google Scholar] [CrossRef]
- Reilly, C.D.; Lee, W.B.; Alvarenga, L.; Caspar, J.; Garcia-Ferrer, F.; Mannis, M.J. Surgical monovision and monovision reversal in LASIK. Cornea 2006, 25, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Levinger, E.; Trivizki, O.; Pokroy, R.; Levartovsky, S.; Sholohov, G.; Levinger, S. Monovision surgery in myopic presbyopes: Visual function and satisfaction. Optom. Vis. Sci. 2013, 90, 1092–1097. [Google Scholar] [CrossRef]
- Uthoff, D.; Pölzl, M.; Hepper, D.; Holland, D. A new method of cornea modulation with excimer laser for simultaneous correction of presbyopia and ametropia. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Ortiz, D.; Simonetto, A.; Bacchi, C.; Sala, E.; Alió, J.L. Correction of presbyopia in hyperopia with a center-distance, paracentral-near technique using the Technolas 217z platform. J. Refract. Surg. 2008, 24, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T. Bifocal profiles and strategies of presbyLASIK for pseudoaccommodation. J. Refract. Surg. 2006, 22, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. LASIK for Myopic Astigmatism and Presbyopia Using Non-Linear Aspheric Micro-Monovision with the Carl Zeiss Meditec MEL 80 Platform. J. Refract. Surg. 2011, 27, 23–37. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Carp, G.I.; Archer, T.J.; Gobbe, M. LASIK for presbyopia correction in emmetropic patients using aspheric ablation profiles and a micro-monovision protocol with the Carl Zeiss Meditec MEL 80 and VisuMax. J. Refract. Surg. 2012, 28, 531–541. [Google Scholar] [CrossRef]
- Gifford, P.; Kang, P.; Swarbrick, H.; Versace, P. Changes to corneal aberrations and vision after Presbylasik refractive surgery using the MEL 80 platform. J. Refract. Surg. 2014, 30, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Falcon, C.; Norero Martinez, M.; Sancho Miralles, Y. Laser blended vision (vision combinée) pour la correction de la presbytie: Résultats à 3 ans. J. Fr. Ophthal. 2015, 38, 431–439. [Google Scholar] [CrossRef]
- Brar, S.; Sute, S.S.; Bagare, S.N.; Ganesh, S. Functional Outcomes and Reading Speeds following PRESBYOND LBV Using Nonlinear Aspheric Ablation Profiles Combined with Micro-Monovision. J. Ophthalmol. 2021, 2021, 2957443. [Google Scholar] [CrossRef]
- Romero, M.; Castillo, A.; Carmona, D.; Palomino, C. Visual quality after presbyopia correction with excimer laser ablation using micromonovision and modulation of spherical aberration. J. Cataract Refract. Surg. 2019, 45, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Chung, E.S.; Kim, M.J.; Chung, T.Y. Visual quality assessment after presbyopic laser in-situ keratomileusis. Int. J. Ophthalmol. 2018, 11, 462–469. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Ivory, E.; Chorley, A.; Archer, T.J.; Vida, R.S.; Gupta, R.; Lewis, T.; Carp, G.I.; Fonseca, A.; Parbhoo, M. PRESBYOND Laser Blended Vision LASIK in Commercial and Military Pilots Requiring Class 1 Medical Certification. J. Refract. Surg. 2023, 39, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, Y.; Weng, S.; Liu, M.; Zhou, Y.; Yang, X.; Stojanovic, A.; Liu, Q. Aspheric Micro-monovision LASIK in Correction of Presbyopia and Myopic Astigmatism: Early Clinical Outcomes in a Chinese Population. J. Refract. Surg. 2016, 32, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Reinstein, D.Z.; Filini, O.; Archer, T.J.; Boldini, A.; Cardin, G.; Festa, G.; Morescalchi, F.; Salvalai, C.; Semeraro, F. Visual and Refractive Outcomes Following Laser Blended Vision With Non-linear Aspheric Micro-anisometropia (PRESBYOND) in Myopic and Hyperopic Patients. J. Refract. Surg. 2022, 38, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Alió, J.L.; Chaubard, J.J.; Caliz, A.; Sala, E.; Patel, S. Correction of presbyopia by technovision central multifocal LASIK (presbyLASIK). J. Refract. Surg. 2006, 22, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.B.; Tuan, K.M.; Mintsioulis, G. Aspheric wavefront-guided LASIK to treat hyperopic presbyopia: 12-month results with the VISX platform. J. Refract. Surg. 2011, 27, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, A.; Anera, R.G.; Villa, C.; Jiménez del Barco, L.; Gutierrez, R. Visual quality after monovision correction by laser in situ keratomileusis in presbyopic patients. J. Cataract Refract. Surg. 2011, 37, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santonja, J.J.; Sakla, H.F.; Alió, J.L. Contrast sensitivity after laser in situ keratomileusis. J. Cataract Refract. Surg. 1998, 24, 183–189. [Google Scholar] [CrossRef]
- NHLBI. Study Quality Assessment Tools|National Heart, Lung, and Blood Institute (NHLBI). Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 May 2020).
- Campbell, F.; Green, D. Monocular versus binocular visual acuity. Nature 1965, 208, 191–192. [Google Scholar] [CrossRef]
- Pardhan, S.; Gilchrist, J. The effect of monocular defocus on binocular contrast sensitivity. Ophthalmic Physiol. Opt. 1990, 10, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Zheleznyak, L.; Sabesan, R.; Oh, J.S.; MacRae, S.; Yoon, G. Modified monovision with spherical aberration to improve presbyopic through-focus visual performance. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3157–3165. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Aspheric ablation profile to presbyopic corneal treatment using the MEL 80 and CRS Master Laser Blended Vision module. J. Emmetropia 2011, 2, 161–175. [Google Scholar]
- Nio, Y.K.; Jansonius, N.M.; Fidler, V.; Geraghty, E.; Norrby, S.; Kooijman, A.C. Spherical and irregular aberrations are important for the optimal performance of the human eye. Ophthalmic Physiol. Opt. 2002, 22, 103–112. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Marcos, S.; Prieto, P.M.; Burns, S.A. Imperfect optics may be the eye’s defence against chromatic blur. Nature 2002, 417, 174–176. [Google Scholar] [CrossRef]
- Bakaraju, R.C.; Ehrmann, K.; Papas, E.B.; Ho, A. Depth-of-focus and its association with the spherical aberration sign. A ray-tracing analysis. J. Optom. 2010, 3, 51–59. [Google Scholar] [CrossRef]
- Ganesh, S.; Brar, S.; Gautam, M.; Sriprakash, K. Visual and refractive outcomes following laser blended vision using non-linear aspheric micro-monovision. J. Refract. Surg. 2020, 36, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Goode, A.; Brown, B. Distance visual acuity and monovision. Optom. Vis. Sci. 1993, 70, 723–728. [Google Scholar] [CrossRef]
- Zheleznyak, L.; Alarcon, A.; Dieter, K.C.; Tadin, D.; Yoon, G. The role of sensory ocular dominance on through-focus visual performance in monovision presbyopia corrections. J. Vis. 2015, 15, 17. [Google Scholar] [CrossRef]
- Luger, M.H.; Ewering, T.; Arba-Mosquera, S. One-year experience in presbyopia correction with biaspheric multifocal central presbyopia laser in situ keratomileusis. Cornea 2013, 32, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.W.; Guemes, A.; Kapadia, M.S.; Wilson, S.E. Binocular function and patient satisfaction after monovision induced by myopic photorefractive keratectomy. J. Cataract Refract. Surg. 1999, 25, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, S.L.; Herman, W.K.; Alfieri, C.D.; Castleberry, K.A.; Parks, M.M.; Birch, E.E. Stereoacuity and foveal fusion in adults with long-standing surgical monovision. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2001, 5, 342–347. [Google Scholar] [CrossRef]
- Schor, C.; Landsman, L.; Erickson, P. Ocular dominance and the interocular suppression of blur in monovision. Am. J. Optom. Physiol. Opt. 1987, 64, 723–730. [Google Scholar] [CrossRef]
- Weakley, D.R. The association between anisometropia, amblyopia, and binocularity in the absence of strabismus. Trans. Am. Ophthalmol. Soc. 1999, 97, 987–1021. [Google Scholar]
- García-Montero, M.; Diego, C.A.; Garzón-Jiménez, N.; Pérez-Cambrodí, R.J.; López-Artero, E.; Ondategui-Parra, J.C. Binocular vision alterations after refractive and cataract surgery: A review. Acta Ophthalmol. 2019, 97, e145–e155. [Google Scholar] [CrossRef]
- Jiang, R.; Meng, M. Integration and suppression interact in binocular vision. J. Vis. 2023, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Meng, M.; Blake, R. Neural bases of binocular rivalry. Trends Cogn. Sci. 2006, 10, 502–511. [Google Scholar] [CrossRef]
- Razmjoo, H.; Akhlaghi, M.R.; Dehghani, A.R.; Peyman, A.R.; Sari-Mohammadli, M.; Ghatreh-Samani, H. Stereoacuity following LASIK. J. Ophthalmic Vis. Res. 2008, 3, 28. [Google Scholar] [PubMed]
- Zarei-Ghanavati, S.; Gharaee, H.; Eslampour, A.; Ehsaei, A.; Abrishami, M. Stereoacuity after photorefractive keratectomy in myopia. J. Curr. Ophthalmol. 2016, 28, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Jabbarvand, M.; Hashemian, H.; Khodaparast, M.; Anvari, P. Changes in stereopsis after photorefractive keratectomy. J. Cataract Refract. Surg. 2016, 42, 899–903. [Google Scholar] [CrossRef]
- Mravicic, I.; Bohac, M.; Lukacevic, S.; Jagaric, K.; Maja, M.; Patel, S. The relationship between clinical measures of aniseikonia and stereoacuity before and after LASIK. J. Optom. 2020, 13, 59–68. [Google Scholar] [CrossRef]
- Karimian, F.; Ownagh, V.; Amiri, M.A.; Tabatabaee, S.M.; Dadbin, N. Stereoacuity after wavefront-guided photorefractive keratectomy in anisometropia. J. Ophthalmic Vis. Res. 2017, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Keeffe, J.E.; Garoufalis, P.; Islam, F.M.; Dirani, M.; Couper, T.A.; Taylor, H.R.; Baird, P.N. Vision-related quality of life comparison for emmetropes, myopes after refractive surgery, and myopes wearing spectacles or contact lenses. J. Refract. Surg. 2007, 23, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Rutstein, R.P.; Corliss, D. Relationship between anisometropia, amblyopia, and binocularity. Optom. Vis. Sci. 1999, 76, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Weakley, D.R., Jr. The association between non strabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology 2001, 108, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Carp, G.I. Non-linear aspheric ablation profile for presbyopic corneal treatment using with the MEL 80/90 and CRS master. Pesbyond module. PRESBYOND LBV White paper.
- Alpern, M. Accommodation and convergence with contact lenses. Am. J. Optom. Arch Am. Acad. Optom. 1949, 26, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ou, Y.; Chin, M.P.; Zhao, D.; Zhang, R. Transient change in the binocular visual function after femtosecond laser-assisted in situ keratomileusis for myopia patients. Indian J. Ophthalmol. 2023, 71, 481–485. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, W.K.; Lee, K.; Moon, N.J. Diminished ciliary muscle movement on accommodation in myopia. Exp. Eye Res. 2012, 105, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hong, S.; Lee, S.; Kim, J.K.; Lee, H.K.; Han, S.H. Changes in fusional vergence amplitudes after laser refractive surgery for moderate myopia. J. Cataract Refract. Surg. 2014, 40, 1670–1675. [Google Scholar] [CrossRef]
- Bohac, M.; Jagic, M.; Biscevic, A.; Lukacevic, S.; Mravicic, I.; Popovic Suic, S.; Dekaris, I. Stereoacuity and Multifocal Intraocular Lenses—a Systematic Review. Acta Inform. Med. 2023, 31, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Pourazizi, M.; Akhlaghi, M.; Feizi, A.; Rahimi, A.; Soltani, E. Stereopsis after corneal refractive surgeries: A systematic review and meta-analysis. Int. Ophthalmol. 2022, 42, 2273–2288. [Google Scholar] [CrossRef]
- Martino, F.; Castro-Torres, J.J.; Casares-Lopez, M.; Ortiz-Peregrina, S.; Granados-Delgado, P.; Jimenez, J.R. Influence of Interocular Differences and Alcohol Consumption on Binocular Visual Performance. Int. J. Environ. Res. Public Health 2023, 20, 1751. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.Y.; Vivas, P.R. Visual outcomes in hyperopic myopic and emmetropic patients with customized aspheric ablation (Q factor) and micro-monovision. Int. Ophthalmol. 2021, 41, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
| Author and Date | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
|---|---|---|---|---|---|---|---|
| Reinstein et al. (2009) [7] | yes | yes | no | no | yes | yes | yes |
| Reinstein et al. (2011) [19] | yes | yes | no | no | yes | yes | yes |
| Reinstein et al. (2012) [20] | yes | yes | no | n/a | yes | yes | yes |
| Zhang et al. (2016) [27] | yes | yes | no | yes | yes | no | yes |
| Lim et al. (2018) [25] | yes | yes | yes | no | yes | yes | yes |
| Romero et al. (2019) [24] | yes | no | n/a | no | yes | yes | yes |
| Brar et al. (2021) [23] | yes | yes | yes | no | yes | yes | yes |
| Russo et al. (2022) [28] | yes | yes | yes | yes | yes | yes | yes |
| Reinstein et al. (2023) [26] | yes | yes | no | yes | yes | yes | yes |
| Luger et al. (2015) [5] | yes | yes | yes | yes | yes | yes | yes |
| Alió et al. (2006) [29] | yes | yes | yes | yes | yes | yes | yes |
| Alarcón et al. (2011) [31] | yes | yes | yes | yes | yes | no | yes |
| Jackson et al. (2011) [30] | yes | yes | yes | no | yes | yes | yes |
| Study | Presbyopia Correction Method | Study Size | Female/ Male % (No. of Cases) | Age (Years) Mean ± SD or Median (Range) | SE Pre-op (D) Mean ± SD (Range) | Target in Non-Dominant Eye (D) SE (Range) | Contrast Sensitivity | Follow-Up (Months) Mean or Median | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Eyes | No. of Patients | Method/ Device | Pre-op (log) | Post-op (log) | |||||||
| Studies which reported no change in post-op contrast sensitivity | |||||||||||
| D.Z. Reinstein et al., 2011 [19] | Presbyond® LBV | 272 | 136 | 57/43 (77/59) | median 49 (43 to 63) | Dominant eye −4.24 ± 1.69 (−0.75 to −8.38) Non-dominant eye −4.21 ± 1.64 (−1.38 to −8.38) | −1.27 ± 0.31 (−0.75 to −2.00) | CSV-1000 (VectorVision, Greenville, OH, USA) | 3 cpd 0.98 6 cpd 0.96 12 cpd 0.96 18 cpd 0.93 | 3 cpd 0.97 6 cpd 0.96 12 cpd 0.98 18 cpd 0.95 | median 12.5 |
| D.H. Lim et al., 2018 [25] | Presbyond® LBV | 54 | 27 | 44/56 (12/15) | 50.2 ± 7.5 | −2.14 ± 2.91 (−7.50 to +3.25) | −1.44 ± 0.21 (−1.00 to −1.50) | CSV-1000E and CSV-1000- (VectorVision, Greenville, OH, USA) | Far 3 cpd 1.826 6 cpd 1.866 12 cpd 1.390 18 cpd 0.800 Near 3 cpd 1.601 6 cpd 1.567 12 cpd 1.116 18 cpd 0.337 | Far 3 cpd 1.781 6 cpd 1.863 12 cpd 1.426 18 cpd 0.855 Near 3 cpd 1.649 6 cpd 1.697 12 cpd 1.313 18 cpd 0.729 | mean 22.3 |
| M. Romero et al., 2019 [24] | Presbyond® LBV | 100 | 50 | n/a | 46.8 ± 4.2 | Group 1 +1.71 ± 0.62 (+0.50 to +3.00) Group 2 −2.11 ± 0.85 (−1.00 to −3.00) Group 3 −3.93 ± 0.87 (−3.0 to −6.00) | (−0.75 to −1.50) | CSV-1000 (VectorVision, Greenville, OH, USA) | Group 1 3 cpd 1.54 6 cpd 1.62 12 cpd 1.29 18 cpd 0.83 Group 2 3 cpd 1.47 6 cpd 1.56 12 cpd 1.34 18 cpd 1.05 Group 3 3 cpd 1.42 6 cpd 1.60 12 cpd 1.25 18 cpd 1.10 | Group 1 3 cpd 1.49 6 cpd 1.56 12 cpd 1.31 18 cpd 0.84 Group 2 3 cpd 1.38 6 cpd 1.52 12 cpd 1.27 18 cpd 0.91 Group 3 3 cpd 1.49 6 cpd 1.51 12 cpd 1.18 18 cpd 0.92 | mean 6 |
| S. Brar et al., 2021 [23] | Presbyond® LBV | 60 | 30 | (16/14) | 50.5 ± 6.4 | Hyperopic eyes +1.28 ± 1.38 Myopic eyes −2.84 ± 1.86 | −1.26 ± 0.40 (−2.25 to −0.75) | CSV-1000 (VectorVision, Greenville, OH, USA) | 1.5 cpd 1.5 3 cpd 1.67 6 cpd 1.58 12 cpd 1.2 18 cpd 0.72 | 1.5 cpd 1.48 3 cpd 1.63 6 cpd 1.54 12 cpd 1.13 18 cpd 0.63 | mean 6.0 |
| T. Zhang et al., 2016 [27] | Presbyond® LBV | 80 | 40 | n/a | 43.4 ± 4.9 (38 to 63) | −5.68 ± 1.98 (−1.25 to −11.13) | −1.41 ± 0.28 (−0.75 to −2.25) | CSV-1000 (VectorVision, Greenville, OH, USA) | change (p > 0.05) for 3cpd,6cpd, 12cpd, 18 cpd change (p > 0.05) for mesopic AULCSF pre-op 1.38 post-op 1.41 photopic AULCSF pre-op 1.42 post-op 1.43 | mean 3 | |
| Studies which reported a significant (p < 0.05) increase in post-op contrast sensitivity | |||||||||||
| D.Z. Reinstein et al., 2009 [7] | Presbyond® LBV | 258 | 129 | 66/34 | median 56 (44 to 66) | +2.54 ± 1.16 (+0.25 to +5.75) | (−1.00 to −1.50) | CSV-1000 (VectorVision, Greenville, OH, USA) | 3 cpd 0.96 6 cpd 0.94 12 cpd 0.95 18 cpd 0.90 | 3 cpd 0.99 6 cpd 0.96 12 cpd 0.97 18 cpd 0.92 | median 12.5 |
| D.Z. Reinstein et al., 2012 [20] | Presbyond® LBV | 296 | 148 | 59/41 | median 55 (44 to 65) | Dominant eye +0.25 ± 0.43 (−0.88 to +1.00) Non-dominant eye +0.24 ± 0.48 (−0.88 to +1.00) | Intended: −1.52 ± 0.09 (−1.88 to −1.00) Obtained: −1.46 ± −0.42 (−2.50 to −0.38) | CSV-1000 (VectorVision, Greenville, OH, USA) | 3 cpd 0.95 6 cpd 0.95 12 cpd 0.97 18 cpd 0.94 | 3 cpd 0.96 6 cpd 0.95 12 cpd 0.96 18 cpd 0.92 | median 12.9 |
| Studies which reported a significant (p < 0.05) decrease in post-op contrast sensitivity | |||||||||||
| J.L. Alió et al., 2006 [29] | PresbyLASIK | 50 | 25 | (10/15) | 58 (51 to 68) | +1.92 ± 0.68 (+0.50 to +3.50) | 0 | VSRC CST 1800 (Vision Science Research, San Ramon, CA, USA) | A significant reduction (p < 0.05) at 3, 6, 12 and 18 cpd and no change at 1.5 cpd. | 6 | |
| A. Alarcon et al., 2011 [31] | aspheric monovision LASIK | 50 | 25 | n/a | 49.3 ± 4.5 | −1.93 ± 2.57 (+2.75 to −6.5) | PR-704 Spectroradiometer (Photo Research) and Vision Works software (Vision Research Graphics) | In dominant eyes, a significant reduction at 3 cpd (p < 0.05), 5.9 cpd (p < 0.05) and 9.9 cpd (p < 0.05). In non-dominant eyes, a significant reduction at all spatial frequencies, except for 18.5 cpd and 21.2 cpd. Under binocular conditions, a significant reduction at all spatial frequencies except for 14.8 cpd and 21.2 cpd. | 3 | ||
| W.B. Jackson et al., 2011 [30] | aspheric non- monovision LASIK | 66 | 33 | 66/34 | 55.1 ± 4.6 | +1.97 ± 0.59 (+0.75 to +3.63) | 0 | CSV-1000 (VectorVision, Greenville, OH, USA) | A significant reduction under mesopic conditions (3 cd/m2) at 6 cpd, 12 cpd and 18 cpd (p < 0.05). | 12 | |
| Study | Presbyopia Correction Method | Study Size | Female/ Male % (No. of Cases) | Age (Years) Mean ± SD or Median (Range) | SE Preop. (D) Mean ± SD (Range) | Target in Non-Dominant Eye (D) SE (Range) | Stereopsis | Follow-Up (Months) Mean or Median | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Eyes | No. of Patients | Method/ Device | Pre-op (arc sec) Mean ± SD (Range) | Post-op (arc sec) Mean ± SD (Range) | |||||||
| T. Zhang et al., 2016 [27] | Presbyond® LBV | 80 | 40 | n/a | 43.4 ± 4.9 (38 to 63) | −5.68 ± 1.98 (−1.25 to −11.13) | −1.41 ± 0.28 (−0.75 to −2.25) | Random dot test for near (40 cm) and distance (3 m) | Near median 50 Distance median 100 | Near median 45 Distance median nil | median 3 |
| M. Romero et al., 2019 [24] | Presbyond® LBV | 100 | 50 | n/a | 46.84 ± 4.17 | Group 1 +1.71 ± 0.62 (+0.50 to +3.00) Group 2 −2.11 ± 0.85 (−1.0 to −3.00) Group 3 −3.93 ± 0.87 (−3.0 to −6.00) | (−0.75 to −1.50) | TNO stereo test (Lameris Ootech BV) | Group 1 181.6 ± 73.8 (30 to 240) Group 2 143.6 ± 80.0 (30 to 240) Group 3 215.3 ± 99.6 (60 to 480) | Group 1 183.2 ± 70.6 (60 to 240) Gorup 2 145.7 ± 77.0 (60 to 240) Group 3 169.4 ± 71.1 (60 to 240) | mean 6 |
| S. Brar et al., 2021 [23] | Presbyond® LBV | 60 | 30 | (16)/(14) | 50.47 ± 6.43 | Hyperopic eyes +1.28 ± 1.38 Myopic −2.84 ± 1.86 | −1.26 ± 0.40 (−2.25 to −0.75) | Titmus-C circles (Stereo Optical Co., Chicago, USA) | 50.7 ± 17.2 | 89.7 ± 36.0 | 6.0 ± 1.2 |
| A. Russo et al., 2022 [28] | Presbyond® LBV | 278 | 139 | n/a | 53.13 ± 5.84 (42 to 70) | Hyperopic +1.61 ± 0.98 (−1.25 to +4.63) Myopic −3.40 ± 1.83 (−0.50 to −8.25) | −0.90 ± 0.44 (−0.13 to−2.25) | Titmus Stereo Test | Hyperopic 50.5 ± 16.6 Myopic 56.3 ± 20.7 | Hyperopic 90.7 ± 32.7 Myopic 95.6 ± 33.5 | 6 |
| A. Alarcón et al., 2011 [31] | aspheric monovision LASIK | 50 | 25 | n/a | 49.3 ± 4.5 | −1.93 ± 2.57 (−6.5 to +2.75) | −1.25 | Stereo Test circles (Stereo Optical Co., Inc.) | 165.6 ± 138.3 | 451.7 ± 287.0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, J.; Pniakowska, Z.; Roszkowska, A.M. Contrast Sensitivity and Stereopsis Outcomes Following LASIK Presbyopia Correction Based on the Corneal Aberration Modulation or Corneal Multifocality Induction Methods: A Systematic Review. J. Clin. Med. 2025, 14, 871. https://doi.org/10.3390/jcm14030871
Wierzbowska J, Pniakowska Z, Roszkowska AM. Contrast Sensitivity and Stereopsis Outcomes Following LASIK Presbyopia Correction Based on the Corneal Aberration Modulation or Corneal Multifocality Induction Methods: A Systematic Review. Journal of Clinical Medicine. 2025; 14(3):871. https://doi.org/10.3390/jcm14030871
Chicago/Turabian StyleWierzbowska, Joanna, Zofia Pniakowska, and Anna M. Roszkowska. 2025. "Contrast Sensitivity and Stereopsis Outcomes Following LASIK Presbyopia Correction Based on the Corneal Aberration Modulation or Corneal Multifocality Induction Methods: A Systematic Review" Journal of Clinical Medicine 14, no. 3: 871. https://doi.org/10.3390/jcm14030871
APA StyleWierzbowska, J., Pniakowska, Z., & Roszkowska, A. M. (2025). Contrast Sensitivity and Stereopsis Outcomes Following LASIK Presbyopia Correction Based on the Corneal Aberration Modulation or Corneal Multifocality Induction Methods: A Systematic Review. Journal of Clinical Medicine, 14(3), 871. https://doi.org/10.3390/jcm14030871





