Interrelationships Between Plasma Levels of Brain Natriuretic Peptide and Prolonged Symptoms Due to Long COVID
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Clinical Evaluation of Long COVID
2.2. Inclusion of Patients
2.3. Evaluated Factors
2.4. Measurements of BNP and Laboratory Markers
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ely, E.W.; Brown, L.M.; Fineberg, H.V. Long COVID Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Santamaría, R.; Fernández-Solana, J.; Méndez-López, F.; Domínguez-García, M.; González-Bernal, J.J.; Magallón-Botaya, R.; Oliván-Blázquez, B.; González-Santos, J.; Santamaría-Peláez, M. Functionality, physical activity, fatigue and quality of life in patients with acute COVID-19 and Long COVID infection. Sci. Rep. 2023, 13, 19907. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Sakurada, Y.; Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Sunada, N.; Honda, H.; Hasegawa, T.; Takase, R.; Omura, D.; et al. Changes in Working Situations of Employed Long COVID Patients: Retrospective Study in Japanese Outpatient Clinic. J. Clin. Med. 2024, 13, 3809. [Google Scholar] [CrossRef]
- Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Honda, H.; Sakurada, Y.; Sunada, N.; Omura, D.; Hasegawa, K.; Hagiya, H.; Obika, M.; et al. Clinical Characteristics of Japanese Patients Who Visited a COVID-19 Aftercare Clinic for Post-Acute Sequelae of COVID-19/Long COVID. Cureus 2021, 13, e18568. [Google Scholar] [CrossRef]
- Yong, S.J.; Halim, A.; Halim, M.; Liu, S.; Aljeldah, M.; Al Shammari, B.R.; Alwarthan, S.; Alhajri, M.; Alawfi, A.; Alshengeti, A.; et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: A systematic review and meta-analysis of over 20 biomarkers. Rev. Med. Virol. 2023, 33, e2424. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Christodoulatos, G.S.; Papavasileiou, G.; Petropoulou, D.; Magkos, F.; Dalamaga, M. Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges. Int. J. Mol. Sci. 2023, 24, 10458. [Google Scholar] [CrossRef]
- Collins, E.; Philippe, E.; Gravel, C.A.; Hawken, S.; Langlois, M.; Little, J. Serological markers and long COVID—A rapid systematic review. Eur. J. Clin. Investig. 2024, 54, e14149. [Google Scholar] [CrossRef]
- Sunada, N.; Hanayama, Y.; Yamamoto, K.; Nakano, Y.; Nada, T.; Honda, H.; Hasegawa, K.; Hagiya, H.; Otsuka, F. Clinical utility of urinary levels of catecholamines and their fraction ratios related to heart rate and thyroid function. Endocr. J. 2022, 69, 417–425. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Topol, E. Solving the puzzle of Long COVID. Science 2024, 383, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Otsuka, Y.; Tokumasu, K.; Sunada, N.; Nakano, Y.; Honda, H.; Sakurada, Y.; Hasegawa, T.; Hagiya, H.; Otsuka, F. Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID. J. Clin. Med. 2023, 12, 4737. [Google Scholar] [CrossRef]
- Hagiya, H.; Tokumasu, K.; Otsuka, Y.; Sunada, N.; Nakano, Y.; Honda, H.; Furukawa, M.; Otsuka, F. Relevance of complement immunity with brain fog in patients with long COVID. J. Infect. Chemother. 2023, 30, 236–241. [Google Scholar] [CrossRef]
- Hall, C. Essential biochemistry and physiology of (NT-pro)BNP. Eur. J. Heart Fail. 2004, 6, 257–260. [Google Scholar] [CrossRef]
- Roberts, E.; Ludman, A.J.; Dworzynski, K.; Al-Mohammad, A.; Cowie, M.R.; McMurray, J.J.V.; Mant, J. The diagnostic accuracy of the natriuretic peptides in heart failure: Systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015, 350, h910. [Google Scholar] [CrossRef]
- Maisel, A.; Hollander, J.E.; Guss, D.; McCullough, P.; Nowak, R.; Green, G.; Saltzberg, M.; Ellison, S.R.; Bhalla, M.A.; Bhalla, V.; et al. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J. Am. Coll. Cardiol. 2004, 44, 1328–1333. [Google Scholar] [CrossRef]
- Cruz, I.E.O.Y.; García, V.J.; Gil Velázquez, I.N.; Curi, P.J.C. Brain natriuretic peptide as a prognostic factor in COVID-19. Med. Clínica Práctica 2023, 6, 100385. [Google Scholar] [CrossRef]
- Vakhshoori, M.; Heidarpour, M.; Shafie, D.; Taheri, M.; Rezaei, N.; Sarrafzadegan, N. Acute Cardiac Injury in COVID-19: A Systematic Review and Meta-analysis. Arch. Iran. Med. 2020, 23, 801–812. [Google Scholar] [CrossRef]
- Majidi, F.; Ranj, A.M.D.; Jameie, M.; Jameie, M.; Mansouri, P.; Varpaei, H.A.B.; Shirani, S. The relationship between cardiological parameters and PCR in patients with coronavirus infection: A cross-sectional study. Medicine 2022, 101, e31935. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Tokumasu, K.; Otsuka, Y.; Honda, H.; Nakano, Y.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Soejima, Y.; Ueda, K.; et al. Phase-dependent trends in the prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) related to long COVID: A criteria-based retrospective study in Japan. PLoS ONE 2024, 19, e0315385. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Case Management of COVID-19 (Secondary Version). JMA J. 2021, 4, 191–197. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Tsutsui, H.; Isobe, M.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; Komuro, I.; et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure—Digest Version. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef]
- Ohsawa, M.; Itai, K.; Tanno, K.; Onoda, T.; Ogawa, A.; Nakamura, M.; Kuribayashi, T.; Yoshida, Y.; Kawamura, K.; Sasaki, S.; et al. Cardiovascular risk factors in the Japanese northeastern rural population. Int. J. Cardiol. 2009, 137, 226–235. [Google Scholar] [CrossRef]
- Moneim, A.A.; Radwan, M.A.; Yousef, A.I. COVID-19 and cardiovascular disease: Manifestations, pathophysiology, vaccination, and long-term implication. Curr. Med. Res. Opin. 2022, 38, 1071–1079. [Google Scholar] [CrossRef]
- Vosko, I.; Zirlik, A.; Bugger, H. Impact of COVID-19 on Cardiovascular Disease. Viruses 2023, 15, 508. [Google Scholar] [CrossRef]
- Roca-Fernandez, A.; Wamil, M.; Telford, A.; Carapella, V.; Borlotti, A.; Monteiro, D.; Thomaides-Brears, H.; Kelly, M.; Dennis, A.; Banerjee, R.; et al. Cardiac abnormalities in Long COVID 1-year post-SARS-CoV-2 infection. Open Heart 2023, 10, e002241. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Liu, S.-H.; Manachevakul, S.; Lee, T.-A.; Kuo, C.-T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N.; Daniels, L.B.; Reilly, M.P.; Lima, J.A.; Wang, T.J.; et al. Inflammation and Circulating Natriuretic Peptide Levels. Circ. Heart Fail. 2020, 13, e006570. [Google Scholar] [CrossRef] [PubMed]
- Mottram, P.M.; Leano, R.; Marwick, T.H. Usefulness of B-type natriuretic peptide in hypertensive patients with exertional dyspnea and normal left ventricular ejection fraction and correlation with new echocardiographic indexes of systolic and diastolic function. Am. J. Cardiol. 2003, 92, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hwang, S.J.; Sung, K.C.; Kim, B.S.; Kang, J.H.; Lee, M.H.; Park, J.R. Assessment of factors affecting plasma BNP levels in patients with chronic atrial fibrillation and preserved left ventricular systolic function. Int. J. Cardiol. 2007, 118, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-Y.; Wang, A.-M.; Chang, S.-H.; Hung, C.-L.; Chen, C.-Y.; Shih, B.-F.; Yeh, H.-I. Responses of cardiac natriuretic peptides after paroxysmal supraventricular tachycardia: ANP surges faster than BNP and CNP. Am. J. Physiol. Circ. Physiol. 2016, 310, H725–H731. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakagawa, K.; Otsuka, F. Idiopathic ventricular tachycardia detected after coronavirus disease 2019. J. Gen. Fam. Med. 2024, 25, 164–165. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Bende, F.; Fofiu, R.; Enache, A.; Pescariu, S.A.; Novacescu, D. The Impact of Metabolic Syndrome and Obesity on the Evolution of Diastolic Dysfunction in Apparently Healthy Patients Suffering from Post-COVID-19 Syndrome. Biomedicines 2022, 10, 1519. [Google Scholar] [CrossRef]
- Mojón-Álvarez, D.; Giralt, T.; Carreras-Mora, J.; Calvo-Fernández, A.; Izquierdo, A.; Soler, C.; Cabero, P.; Pérez-Fernández, S.; Vaquerizo, B.; Barquet, N.R. Baseline NT-proBNP levels as a predictor of short-and long-term prognosis in COVID-19 patients: A prospective observational study. BMC Infect. Dis. 2024, 24, 58. [Google Scholar] [CrossRef]
- Ogungbe, O.; Kumbe, B.; Fadodun, O.A.; Latha, T.; Meyer, D.; Asala, A.F.; Davidson, P.M.; Himmelfarb, C.R.D.; Post, W.S.; Commodore-Mensah, Y. Subclinical myocardial injury, coagulopathy, and inflammation in COVID-19: A meta-analysis of 41,013 hospitalized patients. Int. J. Cardiol. 2022, 40, 100950. [Google Scholar] [CrossRef]
- Cersosimo, A.; Cimino, G.; Amore, L.; Calvi, E.; Pascariello, G.; Inciardi, R.M.; Lombardi, C.M.; Vizzardi, E.; Metra, M. Cardiac biomarkers and mortality in COVID-19 infection: A review. Monaldi Arch. Chest Dis. 2022, 93, 2276. [Google Scholar] [CrossRef]
- Chakraborty, A.; Johnson, J.N.; Spagnoli, J.; Amin, N.; Mccoy, M.; Swaminathan, N.; Yohannan, T.; Philip, R. Long-Term Cardiovascular Outcomes of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 Using an Institution Based Algorithm. Pediatr. Cardiol. 2022, 44, 367–380. [Google Scholar] [CrossRef]
- Kato, A.; Tokumasu, K.; Yamamoto, K.; Otsuka, Y.; Nakano, Y.; Honda, H.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; et al. Clinical and endocrine features of orthostatic intolerance detected in patients with long COVID. Sci. Rep. 2024, 14, 17025. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, R.; Ma, H.; Zhang, W. The Pathogenesis of COVID-19–Related Taste Disorder and Treatments. J. Dent. Res. 2023, 102, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Karamali, K.; Elliott, M.; Hopkins, C. COVID-19 related olfactory dysfunction. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 30, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.K.; Kandasamy, M. SARS-CoV-2-Mediated Neuropathogenesis, Deterioration of Hippocampal Neurogenesis and Dementia. Am. J. Alzheimer’s Dis. Other Dement. 2022, 37, 15333175221078418. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
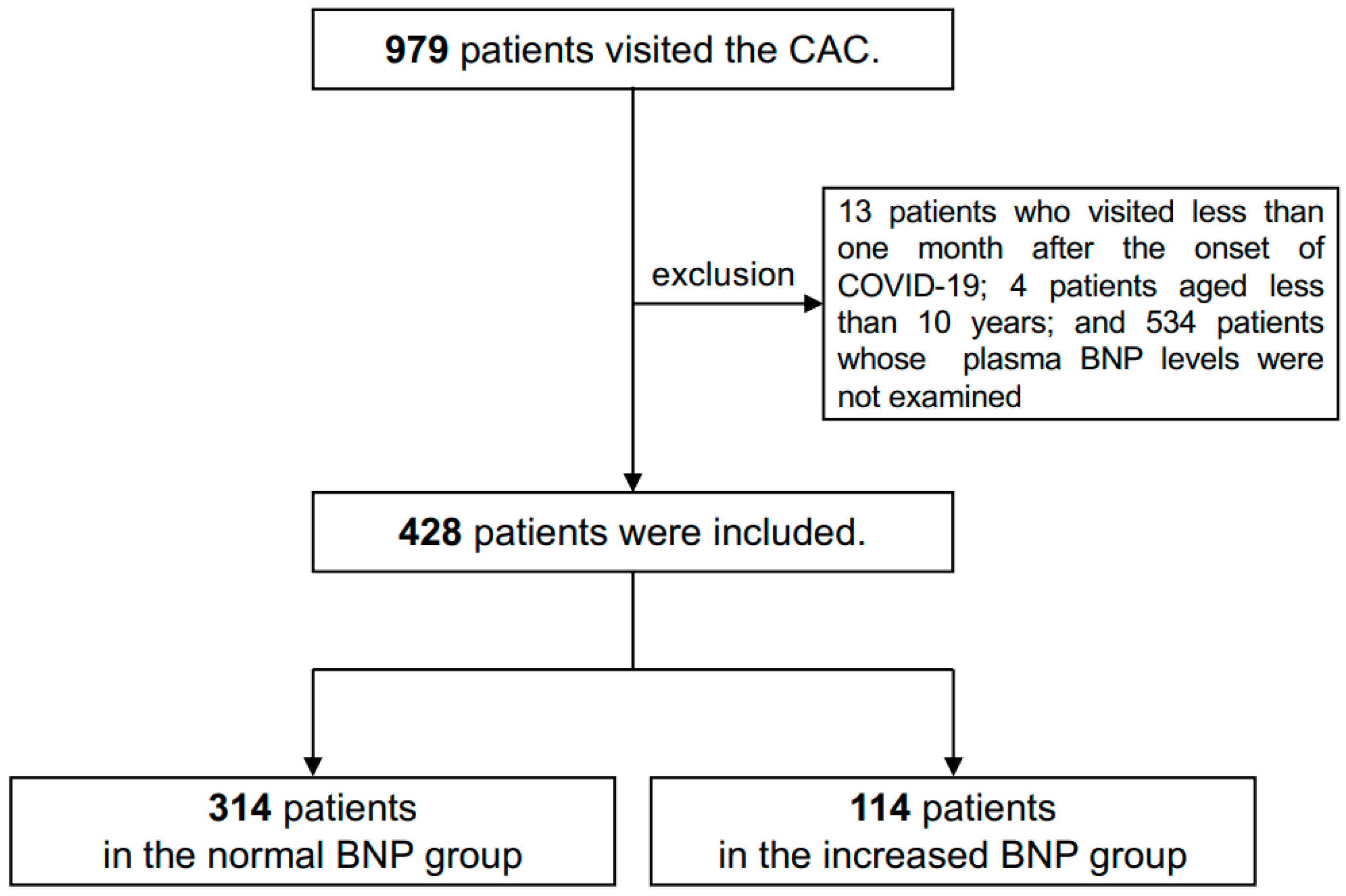
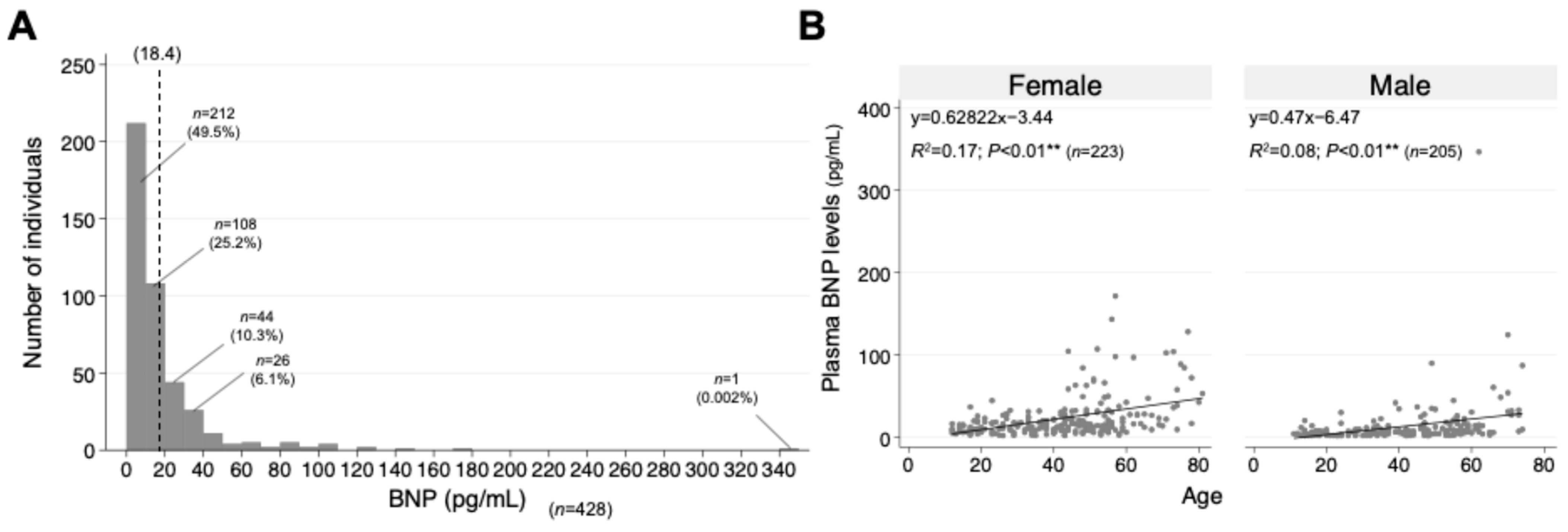
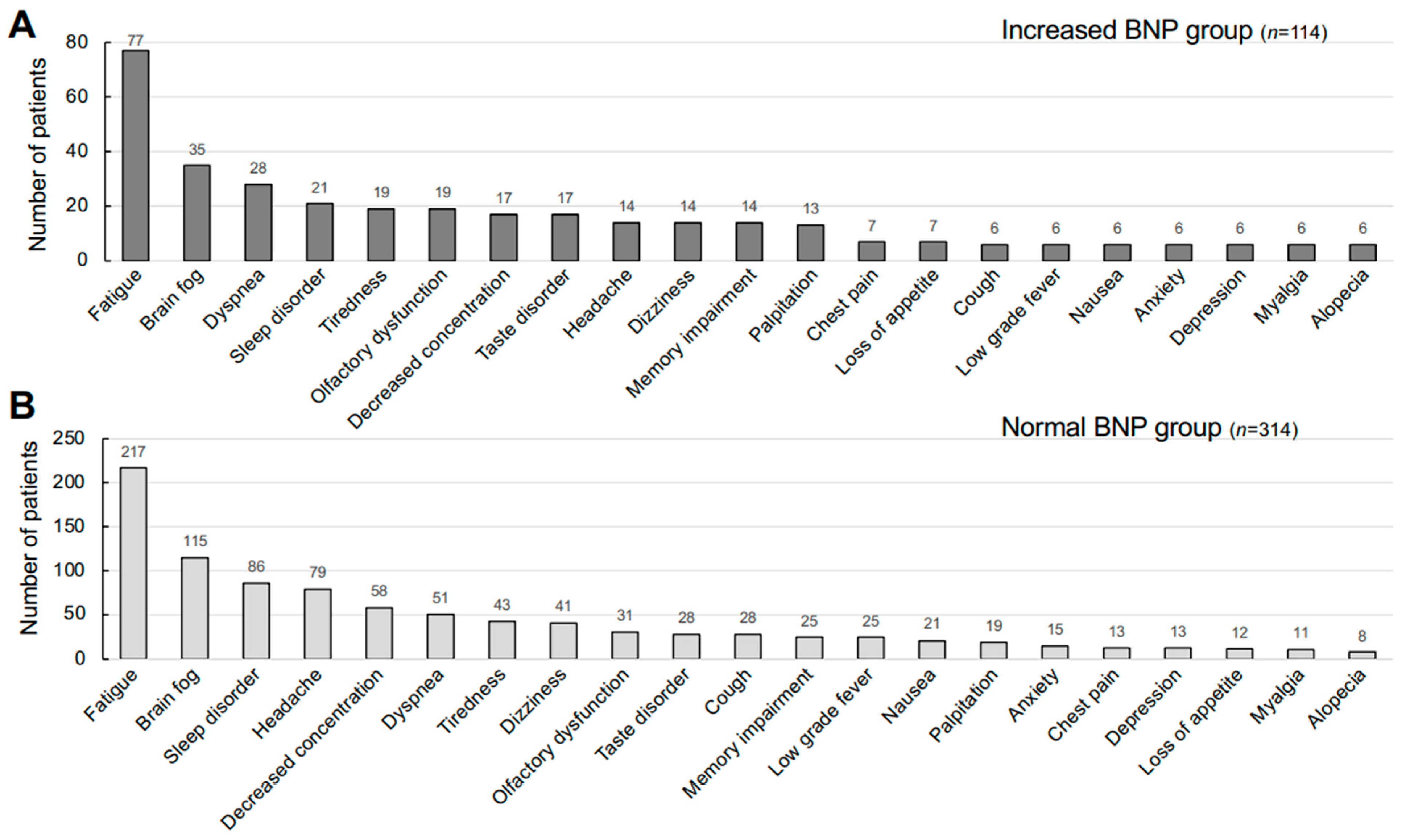
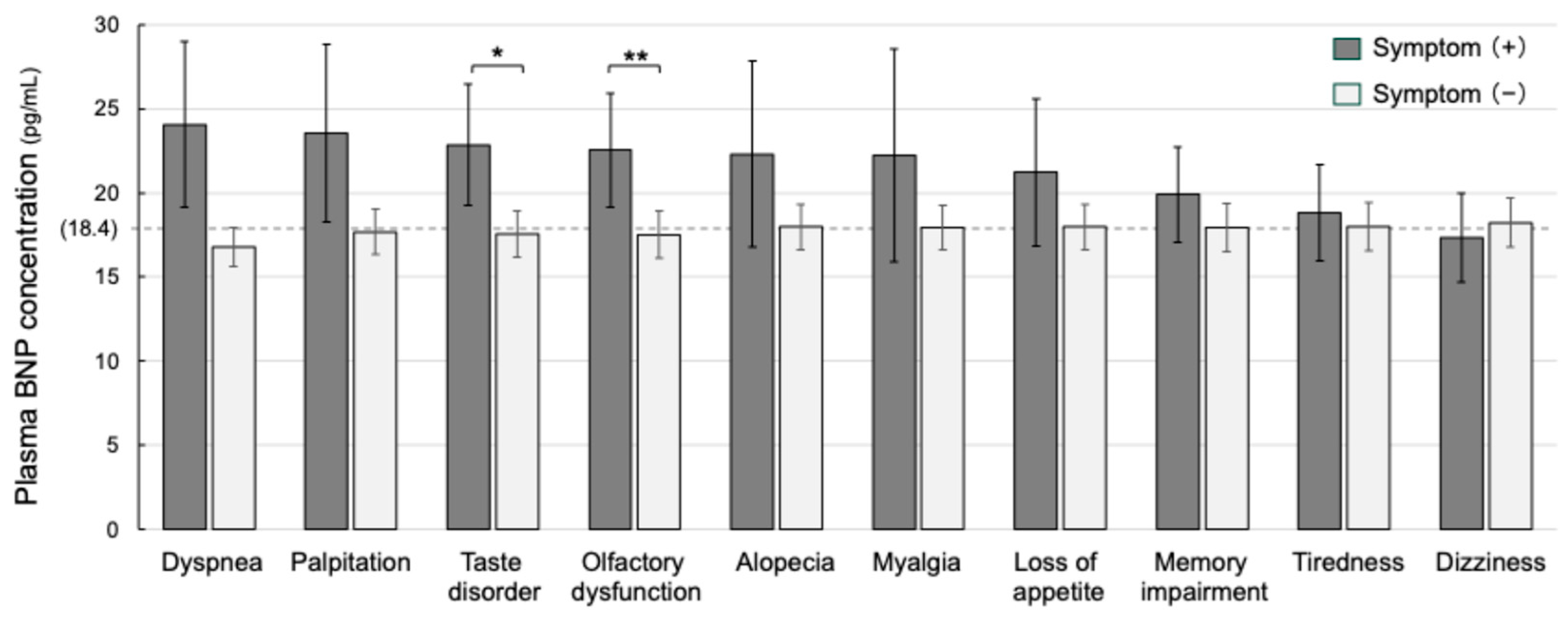
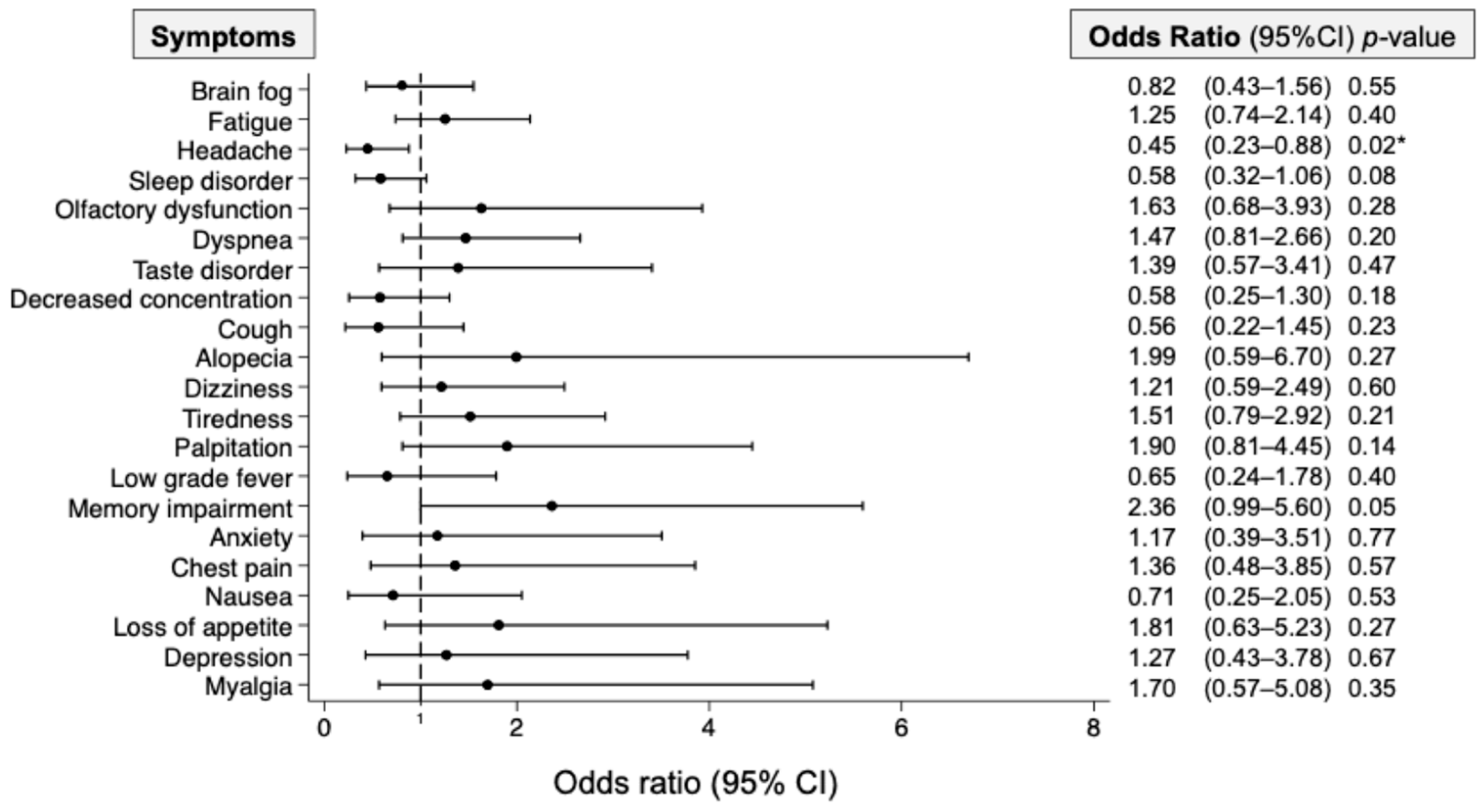
| Patients’ Groups | Normal BNP (n = 314, 73.4%) | Increased BNP (n = 114, 26.6%) | p-Value |
|---|---|---|---|
| Patients’ profile | |||
| Sex: male/female (%female) | 175/139 (44.3%) | 30/84 (73.7%) | <0.01 ** (a) |
| Age (years), median [IQR] | 38 [24–50.8] | 51 [42.3–62] | <0.01 ** (c) |
| BMI: (kg/m2), median [IQR] | 22.1 [19.9–25.9] | 22 [19.5–24.6] | 0.45 (c) |
| SBP: median [IQR] | 121 [112–134] | 128 [113–143] | <0.05 * (c) |
| DBP: median [IQR] | 73 [66.8–83] | 73 [65.8–83] | 0.09 (c) |
| PR: median [IQR] | 82 [74.5–91] | 81 [74–88] | 0.10 (c) |
| Patient’s lifestyle | |||
| Smoking habit, n (%) | 74 (23.6%) | 33 (28.9%) | 0.25 (a) |
| Alcohol drinking, n (%) | 61 (19.4%) | 30 (26.3%) | 0.11 (a) |
| Clinical management in acute phase of COVID-19 | |||
| Hospital admission, n (%) | 13 (4.14%) | 11 (9.65%) | <0.05 * (a) |
| Oxygen therapy, n (%) | 1 (0.32%) | 3 (2.63%) | 0.06 (b) |
| Steroid therapy, n (%) | 1 (0.32%) | 2 (1.75%) | 0.19 (a) |
| Mild condition, n (%) | 308 (98.1%) | 106 (93%) | 0.97 (b) |
| Moderate to severe condition, n (%) | 4 (1.27%) | 7 (6.14%) | |
| COVID-19 vaccination status | |||
| <2 doses, n (%) | 75 (23.9%) | 28 (24.6%) | 0.67 (a) |
| ≥2 doses, n (%) | 236 (75.2%) | 82 (71.9%) | |
| Duration from the onset to the first visit | |||
| Days: median [IQR] | 108 [73.3–202] | 135 [78–207] | 0.16 (c) |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.06 | 1.04–1.07 | <0.01 ** |
| Female | 3.80 | 2.22–6.50 | <0.01 ** |
| BMI (kg/m2) | 0.98 | 0.94–1.04 | 0.61 |
| Severity | 1.65 | 0.80–3.40 | 0.18 |
| Vaccination | 0.72 | 0.40–1.28 | 0.2 |
| Patients’ Groups | Normal BNP (n = 314) | Increased BNP (n = 114) | Sample Defects | p-Value |
|---|---|---|---|---|
| Hb (g/dL) | 14.7 (1.43) | 13.5 (1.48) | (0; 0) | <0.01 ** |
| Alb (g/dL) | 4.52 (0.34) | 4.24 (0.39) | (−5; 0) | <0.01 ** |
| AST (U/L) | 23.4 (24.1) | 20.1 (7.23) | (−1; 0) | 0.2 |
| ALT (U/L) | 27.4 (26.2) | 21 (13.9) | (−1; 0) | 0.17 |
| ALP (U/L) | 82.2 (44.8) | 71.7 (20.7) | (−2; 0) | 0.08 |
| eGFR (mL/min/1.73 m2) | 83.7 (17.4) | 81.1 (20.3) | (−50: −3) | 0.57 |
| CRP (mg/dL) | 0.13 (0.42) | 0.21 (0.72) | (0; 0) | 0.07 |
| ESR (mm/h) | 8.57 (8.04) | 14.93 (20.84) | (−6; −1) | <0.01 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, Y.; Otsuka, Y.; Tokumasu, K.; Honda, H.; Sakurada, Y.; Matsuda, Y.; Nakano, Y.; Takase, R.; Omura, D.; Hasegawa, T.; et al. Interrelationships Between Plasma Levels of Brain Natriuretic Peptide and Prolonged Symptoms Due to Long COVID. J. Clin. Med. 2025, 14, 817. https://doi.org/10.3390/jcm14030817
Masuda Y, Otsuka Y, Tokumasu K, Honda H, Sakurada Y, Matsuda Y, Nakano Y, Takase R, Omura D, Hasegawa T, et al. Interrelationships Between Plasma Levels of Brain Natriuretic Peptide and Prolonged Symptoms Due to Long COVID. Journal of Clinical Medicine. 2025; 14(3):817. https://doi.org/10.3390/jcm14030817
Chicago/Turabian StyleMasuda, Yohei, Yuki Otsuka, Kazuki Tokumasu, Hiroyuki Honda, Yasue Sakurada, Yui Matsuda, Yasuhiro Nakano, Ryosuke Takase, Daisuke Omura, Toru Hasegawa, and et al. 2025. "Interrelationships Between Plasma Levels of Brain Natriuretic Peptide and Prolonged Symptoms Due to Long COVID" Journal of Clinical Medicine 14, no. 3: 817. https://doi.org/10.3390/jcm14030817
APA StyleMasuda, Y., Otsuka, Y., Tokumasu, K., Honda, H., Sakurada, Y., Matsuda, Y., Nakano, Y., Takase, R., Omura, D., Hasegawa, T., Ueda, K., & Otsuka, F. (2025). Interrelationships Between Plasma Levels of Brain Natriuretic Peptide and Prolonged Symptoms Due to Long COVID. Journal of Clinical Medicine, 14(3), 817. https://doi.org/10.3390/jcm14030817





