Membranous Nephropathy
Abstract
1. Introduction
2. Etiology of MN
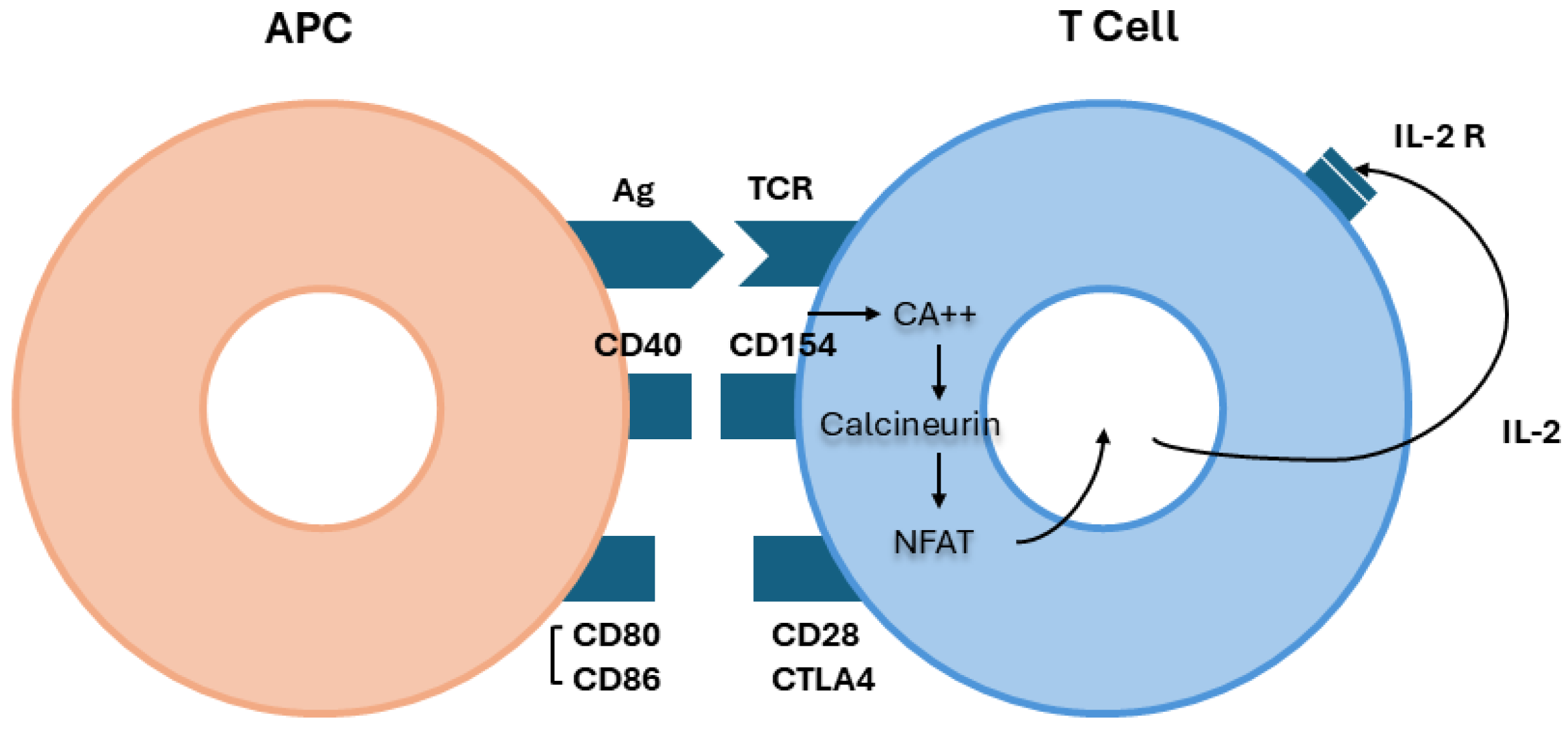
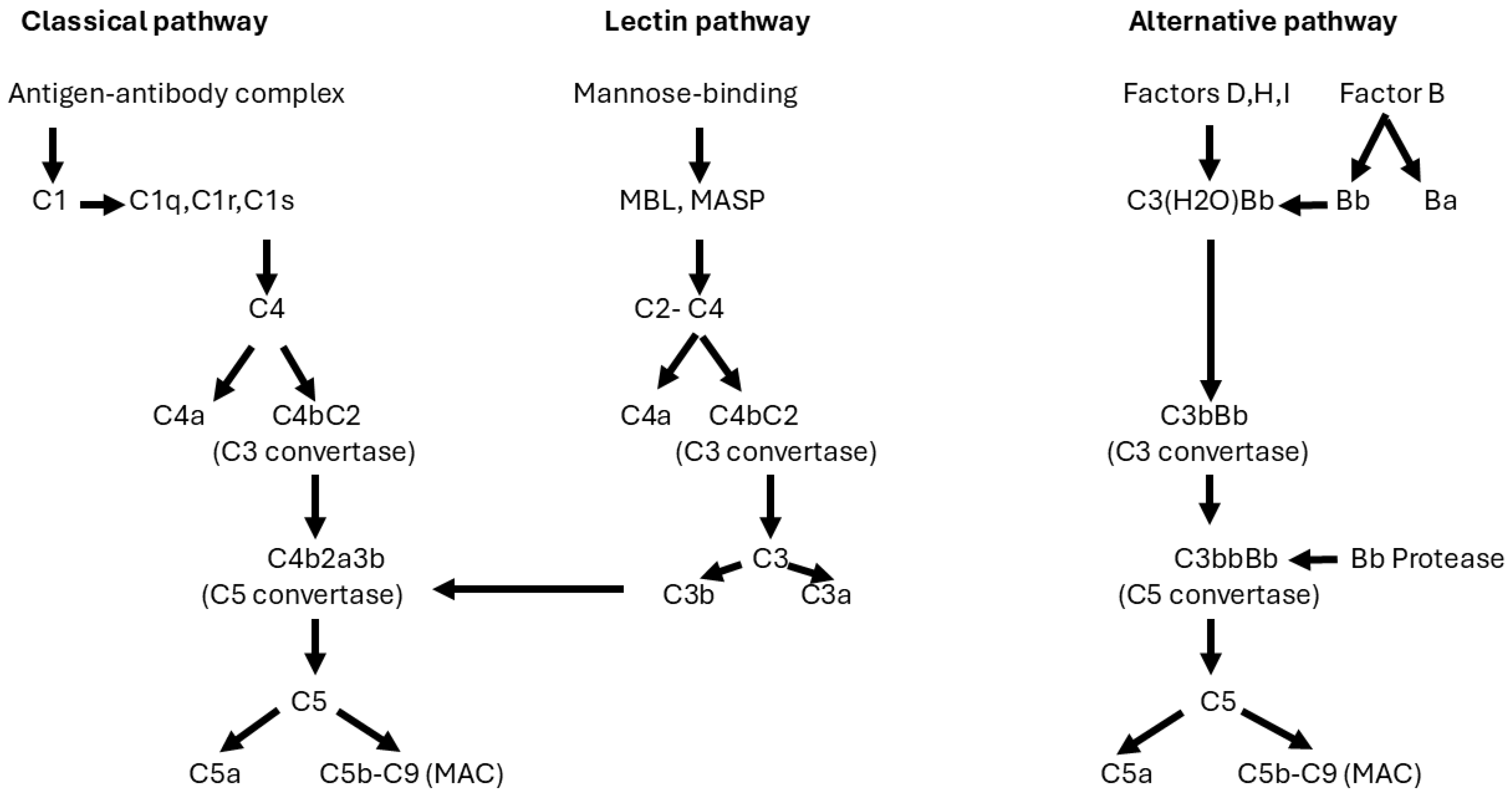
3. Physiopathology of PMN
4. Physiopathology of the Nephrotic Syndrome
4.1. Hypoalbuminemia
4.2. Dyslipidemia
4.3. Hypercoagulable State
5. The Natural Course of PMN
6. Treatment
6.1. Immunosuppressive Treatment
6.2. Symptomatic Treatment
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponticelli, C.; Glassock, R.J. Glomerular diseases: Membranous nephropathy—A modern view. Clin. J. Am. Soc. Nephrol. 2014, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G. Primary Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 7, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, G.; Chen, N.; Lu, T.; Nie, S.; Xu, G.; Zhang, P.; Luo, Y.; Wang, Y.; Wang, X.; et al. Long-Term Exposure to Air Pollution and Increased Risk of Membranous Nephropathy in China. J. Am. Soc. Nephrol. 2016, 27, 3739–3746. [Google Scholar] [CrossRef]
- Zhu, X.M.; Zhou, H.M.; Xu, W. Mendelian study on air pollution and membranous nephropathy outcomes associations. Medicine 2024, 103, e39708. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Cui, Z.; Zhao, M.-H. The Genetic and Environmental Factors of Primary Membranous Nephropathy: An Overview from China. Kidney Dis. 2018, 4, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, H.C.; Arcos-Burgos, M.; Medlar, A.; Bockenhauer, D.; Kottgen, A.; Dragomirescu, L.; Voinescu, C.; Patel, N.; Pearce, K.; Hubank, M.; et al. Risk HLA-DQA1 and PLA2R1 Alleles in Idiopathic Membranous Nephropathy. J. Med. 2011, 364, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Beggs, M.L.; Walker, P.D.; Larsen, C.P. PLA2R-associated membranous glomerulopathy is modulated by common variants in PLA2R1 and HLA-DQA1 genes. Genes Immun. 2014, 15, 556–561. [Google Scholar] [CrossRef]
- Le, W.-B.; Shi, J.-S.; Zhang, T.; Liu, L.; Qin, H.-Z.; Liang, S.; Zhang, Y.-W.; Zheng, C.-X.; Jiang, S.; Qin, W.-S.; et al. HLA-DRB1*15:01 and HLA-DRB3*02:02 in PLA2R-Related Membranous Nephropathy. J. Am. Soc. Nephrol. 2016, 28, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Köttgen, A.; Hoxha, E.; Brenchley, P.; Bockenhauer, D.; Stanescu, H.C.; Kleta, R. Genetics of membranous nephropathy. Nephrol. Dial. Transplant. 2018, 33, 1493–1502. [Google Scholar] [CrossRef]
- Kamyshova, E.S.; Bobkova, I.N.; Gorelova, I.A.; Kakhsurueva, P.A.; Filatova, E.E. Genetic determinants of the development and course of membranous nephropathy. Ter. Arkhiv 2018, 90, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, L.; Mladkova, N.; Li, Y.; Ren, H.; Wang, W.; Cui, Z.; Lin, L.; Hu, X.; Yu, X.; et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat. Commun. 2020, 11, 1600. [Google Scholar] [CrossRef]
- Wiech, T.; Reinhard, L.; Wulf, S.; Giuffrida, A.E.; Longhitano, E.; Caruso, R.; Gröne, H.-J.; Stahl, R.A.K.; Zipfel, P.F.; Kikhney, J.; et al. Bacterial infection possibly causing autoimmunity: Tropheryma whipplei and membranous nephropathy. Lancet 2022, 400, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, Y.; Zheng, B.; Zhang, X.; An, D.; Guo, N.; Wang, J.; Guo, Y.; Tang, L. Clinicopathological characteristics and prognosis of hepatitis B associated membranous nephropathy and idiopathic membranous nephropathy complicated with hepatitis B virus infection. Sci. Rep. 2021, 11, 18407. [Google Scholar] [CrossRef]
- Weng, Q.; Li, X.; Ren, H.; Xie, J.; Pan, X.; Xu, J.; Chen, N. Membranous nephropathy associated with hepatitis C virus infection treated with corticosteroids and Ledipasvir-Sofosbuvir: A case report and review of literature. Oncotarget 2017, 28, 22299–22303. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Zhang, L.; Feng, Y. Varicella-zoster virus infection and primary membranous nephropathy: A Mendelian randomization study. Sci. Rep. 2023, 13, 19212. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, X.; Xu, G. New-Onset and Relapsed Membranous Nephropathy post SARS-CoV-2 and COVID-19 Vaccination. Viruses 2022, 14, 2143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Duan, S.-B.; He, J.; Wu, X.; Wu, T. Causal effects of rheumatoid arthritis or ankylosing spondylitis on membranous nephropathy: A two-sample Mendelian randomization study. Clin. Kidney J. 2023, 16, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Moroni, G.; Fornoni, A. Lupus Membranous Nephropathy. Glomerular Dis. 2021, 2, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Tokuyama, H.; Hashiguchi, A.; Hasegawa, K.; Uchiyama, K.; Ryuzaki, M.; Yasuda, M.; Mizuno, R.; Ishidoya, S.; Wakino, S.; et al. Malignancy-associated membranous nephropathy with PLA2R double-positive for glomeruli and carcinoma. CEN Case Rep. 2021, 10, 281–286. [Google Scholar] [CrossRef]
- Juarez, A.; Galindo, L.; Ragunathan, A.; Gondal, M. Thrombospondin Type 1 Domain-Containing 7A (THSD7A)-Associated Membranous Nephropathy Leading to Metastatic Neuroendocrine Carcinoma. Cureus 2023, 15, e35277. [Google Scholar] [CrossRef]
- Aytekin, A.; Ozet, A.; Bilgetekin, I.; Ogut, B.; Ciltas, A.; Benekli, M. A case of membranous glomerulopathy associated with lung cancer and review of the literature. Mol. Clin. Oncol. 2017, 7, 241–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, P.; Huang, W.; Zheng, Q.; Tang, J.; Dong, Z.; Jiang, Y.; Liu, Y.; Liu, W. A Novel Insight into the Role of PLA2R and THSD7A in Membranous Nephropathy. J. Immunol. Res. 2021, 2021, 8163298. [Google Scholar] [CrossRef]
- Heymann, W.; Hackel, D.B.; Harwood, S.; Wilson, S.G.F.; Hunter, J.L.P. Production of Nephrotic Syndrome in Rats by Freund’s Adjuvants and Rat Kidney Suspensions. Exp. Biol. Med. 1959, 100, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Salant, D.J.; Cybulsky, A.V. Experimental glomerulonephritis. Methods Enzymol. 1988, 162, 421–461. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Farquhar, M.G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl. Acad. Sci. USA 1982, 79, 5557–5561. [Google Scholar] [CrossRef]
- Salant, D.J.; Belok, S.; Madaio, M.P.; Couser, W.G. A new role for complement in experimental membranous nephropathy in rats. J. Clin. Investig. 1980, 66, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Ochi, R.F.; Schulze, M.; Johnson, R.J.; Campbell, C.; Couser, W.G. Depletion of C6 prevents development of pro-teinuria in experimental membranous nephropathy in rats. Am. J. Pathol. 1989, 135, 185–194. [Google Scholar]
- Salant, D.J.; Quigg, R.J.; Cybulsky, A.V. Heymann nephritis: Mechanisms of renal injury. Kidney Int. 1989, 35, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Groggel, G.C.; Terreros, D.A. Role of the terminal complement pathway in accelerated autologous anti-glomerular basement membrane nephritis. Am. J. Pathol. 1990, 136, 533–540. [Google Scholar]
- Savin, V.J.; Johnson, R.J.; Couser, W.G. C5b-9 increases albumin permeability of isolated glomeruli in vitro. Kidney Int. 1994, 46, 382–387. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Neale, T.J. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J. Am. Soc. Nephrol. 1996, 7, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.B.; Jane-Wit, D.; Pober, J.S. Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets. Am. J. Pathol. 2020, 190, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Debiec, H.; Guigonis, V.; Mougenot, B.; Decobert, F.; Haymann, J.-P.; Bensman, A.; Deschênes, G.; Ronco, P.M. Antenatal Membranous Glomerulonephritis Due to Anti–Neutral Endopeptidase Antibodies. J. Med. 2002, 346, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.H., Jr.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-type phos-pholipase A2 receptor as target antigen in idiopathic membranous nephropathy. J. Med. 2009, 361, 11–21. [Google Scholar]
- Hoxha, E.; Thiele, I.; Zahner, G.; Panzer, U.; Harendza, S.; Stahl, R.A. Phospholipase A2 Receptor Autoantibodies and Clinical Outcome in Patients with Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1357–1366. [Google Scholar] [CrossRef]
- Bech, A.P.; Hofstra, J.M.; Brenchley, P.E.; Wetzels, J.F. Association of Anti-PLA2R Antibodies with Outcomes after Immunosuppressive Therapy in Idiopathic Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Radice, A.; Trezzi, B.; Maggiore, U.; Pregnolato, F.; Stellato, T.; Napodano, P.; Rolla, D.; Pesce, G.; D’Amico, M.; Santoro, D.; et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun. Rev. 2015, 15, 146–154. [Google Scholar] [CrossRef]
- van de Logt, A.-E.; Fresquet, M.; Wetzels, J.F.; Brenchley, P. The anti-PLA2R antibody in membranous nephropathy: What we know and what remains a decade after its discovery. Kidney Int. 2019, 96, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.d.O.S.; Passos, M.T.; Fernandes, D.E.; Nishida, S.K.; Andrade, L.E.C.; Kirsztajn, G.M. Autoantibodies against phospholipase A2 receptor in Brazilian patients with glomerular diseases. Int. Urol. Nephrol. 2021, 53, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Fervenza, F.C.; Induruwage, D.; Brenchley, P.E.; Rovin, B.; Hladunewich, M.A.; Reich, H.N.; Lafayette, R.; Aslam, N.; Appel, G.B.; et al. Anti-PLA2R Antibody Levels and Clinical Risk Factors for Treatment Nonresponse in Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2023, 18, 1283–1293. [Google Scholar] [CrossRef]
- Vink, C.H.; van de Logt, A.-E.; van der Molen, R.G.; Hofstra, J.M.; Wetzels, J.F. Antibody-Guided Therapy in Phospholipase A2 Receptor-Associated Membranous Nephropathy. Kidney Int. Rep. 2022, 8, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Tomas, N.M.; Beck LHJr Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.S.; Debayle, D.; et al. Thrombospondin type-1 do-main-containing 7A in idiopathic membranous nephropathy. J. Med. 2014, 371, 2277–2287. [Google Scholar]
- Iwakura, T.; Ohashi, N.; Kato, A.; Baba, S.; Yasuda, H. Prevalence of Enhanced Granular Expression of Thrombospondin Type-1 Domain-Containing 7A in the Glomeruli of Japanese Patients with Idiopathic Membranous Nephropathy. PLoS ONE 2015, 10, e0138841. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Z.; Lu, J.; Probst, C.; Zhang, Y.M.; Wang, X.; Qu, Z.; Wang, F.; Meng, L.Q.; Cheng, X.Y.; et al. Circulating Antibodies against Thrombospondin Type-I DomainContaining 7A in Chinese Patients with Idio-pathic Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Tomas, N.M.; Hoxha, E.; Reinicke, A.T.; Fester, L.; Helmchen, U.; Gerth, J.; Bachmann, F.; Budde, K.; Koch-Nolte, F.; Zahner, G.; et al. Autoantibodies against thrombospondin type 1 domain–containing 7A induce membranous nephropathy. J. Clin. Investig. 2016, 126, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Ponticelli, C. Glomerular disease: Membranous nephropathy and the Henle-Koch postulates. Nat. Rev. Nephrol. 2016, 12, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.W.; Jimenez-Lopez, J.; Geiger, X.J.; Aslam, N. Malignancy-Associated Membranous Nephropathy with Positive Anti-PLA2R Autoantibodies: Coincidence or Connection. Case Rep. Nephrol. Dial. 2021, 11, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Jhaveri, K.D.; McMahon, B.A.; Perazella, M.A. Onconephrology: The intersections between the kidney and cancer. CA A Cancer J. Clin. 2020, 71, 47–77. [Google Scholar] [CrossRef] [PubMed]
- Pani, A.; Porta, C.; Cosmai, L.; Melis, P.; Floris, M.; Piras, D.; Gallieni, M.; Rosner, M.; Ponticelli, C. Glomerular diseases and cancer: Evaluation of underlying malignancy. J. Nephrol. 2015, 29, 143–152. [Google Scholar] [CrossRef]
- Sethi, S. New ‘Antigens’ in Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabadi, L.F.; Caza, T.; Trivin-Avillach, C.; Rodan, A.R.; Andeen, N.; Hayashi, N.; Williams, B.; Revelo, M.P.; Clayton, F.; Abraham, J.; et al. Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1666–1681. [Google Scholar] [CrossRef]
- Le Quintrec, M.; Teisseyre, M.; Bec, N.; Delmont, E.; Szwarc, I.; Perrochia, H.; Machet, M.C.; Chauvin, A.; Mavroudakis, N.; Taieb, G.; et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int. 2021, 100, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Caza, T.N.; Storey, A.J.; Hassen, S.I.; Herzog, C.; Edmondson, R.D.; Arthur, J.M.; Kenan, D.J.; Larsen, C.P. Discovery of seven novel putative antigens in membranous nephropathy and membranous lupus nephritis identified by mass spectrometry. Kidney Int. 2023, 103, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Fresquet, M.; Jowitt, T.A.; Gummadova, J.; Collins, R.; O’cualain, R.; McKenzie, E.A.; Lennon, R.; Brenchley, P.E. Identification of a Major Epitope Recognized by PLA2R Autoantibodies in Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 302–313. [Google Scholar] [CrossRef]
- Kao, L.; Lam, V.; Waldman, M.; Glassock, R.J.; Zhu, Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2015, 26, 291–301. [Google Scholar] [CrossRef]
- Seitz-Polski, B.; Dolla, G.; Payré, C.; Girard, C.A.; Polidori, J.; Zorzi, K.; Birgy-Barelli, E.; Jullien, P.; Courivaud, C.; Krummel, T.; et al. Epitope Spreading of Autoantibody Response to PLA2R Associates with Poor Prognosis in Membranous Nephropathy. J. Am. Soc. Nephrol. 2015, 27, 1517–1533. [Google Scholar] [CrossRef] [PubMed]
- Seitz-Polski, B.; Debiec, H.; Rousseau, A.; Dahan, K.; Zaghrini, C.; Payré, C.; Esnault, V.L.M.; Lambeau, G.; Ronco, P. Phos-pholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J. Am. Soc. Nephrol. 2018, 29, 401–408. [Google Scholar] [CrossRef]
- Akiyama, S.; Imai, E.; Maruyama, S. Immunology of membranous nephropathy. F1000Research 2019, 8, 734. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Banu, K.; Ni, Z.; Leventhal, J.S.; Menon, M.C. Podocyte Autophagy in Homeostasis and Disease. J. Clin. Med. 2021, 10, 1184. [Google Scholar] [CrossRef]
- Hartleben, B.; Gödel, M.; Meyer-Schwesinger, C.; Liu, S.; Ulrich, T.; Köbler, S.; Wiech, T.; Grahammer, F.; Arnold, S.J.; Lindenmeyer, M.T.; et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Investig. 2010, 120, 1084–1096. [Google Scholar] [CrossRef]
- Pan, Y.; Wan, J.; Liu, Y.; Yang, Q.; Liang, W.; Singhal, P.C.; Saleem, M.A.; Ding, G. sPLA2 IB induces human podocyte apoptosis via the M-type phospholipase A2 receptor. Sci. Rep. 2014, 4, 6660. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Y.; Lin, S.; Dai, B.; Chen, H.; Tao, X.; Li, G.; Wan, J.; Pan, Y. sPLA2-IB and PLA2R mediate insufficient autophagy and contribute to podocyte injury in idiopathic membranous nephropathy by activation of the p38MAPK/mTOR/ULK1ser757 signaling pathway. FASEB J. 2021, 35, e21170. [Google Scholar] [CrossRef]
- Oh, J.E.; Lee, H.K. Pattern Recognition Receptors and Autophagy. Front. Immunol. 2014, 5, 300. [Google Scholar] [CrossRef]
- Delgado, M.A.; Deretic, V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 2009, 16, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Munz, C. Autophagy in immunity. Prog. Mol. Biol. Transl. Sci. 2020, 172, 67–85. [Google Scholar] [PubMed]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 9, 437–453. [Google Scholar] [CrossRef]
- den Haan, J.M.; Arens, R.; van Zelm, M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014, 162, 103–112. [Google Scholar] [CrossRef]
- Kistler, A.D.; Salant, D.J. Complement activation and effector pathways in membranous nephropathy. Kidney Int. 2023, 105, 473–483. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J.S.; van Swieten, P.; Aalberse, R.C. Inhibition of complement activation by IgG4 antibodies. Clin. Exp. Immunol. 1986, 64, 415–422. [Google Scholar]
- Haddad, G.; Lorenzen, J.M.; Ma, H.; de Haan, N.; Seeger, H.; Zaghrini, C.; Brandt, S.; Kölling, M.; Wegmann, U.; Kiss, B.; et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1–associated membranous nephropathy. J. Clin. Investig. 2021, 131, e140453. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, Y.; Hayashi, N.; Fujimoto, K.; Adachi, H.; Furuichi, K.; Yokoyama, H. Association between anti-complement factor H antibodies and renal outcome in primary membranous nephropathy. Clin. Nephrol. 2021, 96, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Zahner, G.; Meyer-Schwesinger, C.; Hickstein, N.; Dehde, S.; Wulf, S.; Köllner, S.M.S.; Lucas, R.; Kylies, D.; Froembling, S.; et al. The classical pathway triggers pathogenic complement activation in membranous nephropathy. Nat. Commun. 2023, 28, 473. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V.; Rennke, H.G.; Feintzeig, I.D.; Salant, D.J. Complement-induced glomerular epithelial cell injury. Role Membrane Attack Complex Rat Membranous Nephropathy. J. Clin. Investig. 1986, 77, 1096–1107. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Schulze, M.; Binder, S.; Kain, R.; Ojha, P.P.; Susani, M.; Horvat, R.; Baker, P.J.; Couser, W.G. Transcellular transport and membrane insertion of the C5b-9 membrane attack complex of complement by glomerular epithelial cells in experimental membranous nephropathy. J. Immunol. 1989, 143, 546–552. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Zhang, Y.; Qu, Z.; Wang, X.; Wang, F.; Meng, L.; Cheng, X.; Cui, Z.; Liu, G.; et al. Complement activation products in the circulation and urine of primary membranous nephropathy. BMC Nephrol. 2019, 20, 313. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Cui, Z.; Zhao, M.H. Complement C3a and C3a receptor activation mediates podocyte injuries in the mechanism of primary membranous nephropathy. J. Am. Soc. Nephrol. 2022, 33, 1742–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bin, S.; Budge, K.; Petrosyan, A.; Villani, V.; Aguiari, P.; Vink, C.; Wetzels, J.; Soloyan, H.; La Manna, G.; et al. C3aR-initiated signaling is a critical mechanism of podocyte injury in membranous nephropathy. JCI Insight 2024, 9, e172976. [Google Scholar] [CrossRef]
- Hinrichs, G.R.; Jensen, B.L.; Svenningsen, P. Mechanisms of sodium retention in nephrotic syndrome. Curr. Opin. Nephrol. Hypertens. 2020, 29, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Larionov, A.; Dahlke, E.; Kunke, M.; Rodriguez, L.Z.; Schiessl, I.M.; Magnin, J.; Kern, U.; Alli, A.A.; Mollet, G.; Schilling, O.; et al. Cathepsin B increases ENaC activity leading to hypertension early in nephrotic syndrome. J. Cell. Mol. Med. 2019, 23, 6543–6553. [Google Scholar] [CrossRef]
- Canetta, P.A.; Troost, J.P.; Mahoney, S.; Kogon, A.J.; Carlozzi, N.; Bartosh, S.M.; Cai, Y.; Davis, T.K.; Fernandez, H.; Fornoni, A.; et al. Health-related quality of life in glomerular disease. Kidney Int. 2019, 95, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Moroni, G. Nephrotic syndrome: Pathophysiology and consequences. J. Nephrol. 2023, 36, 2179–2190. [Google Scholar]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ly, N.D.K.; Tesch, G.H.; Poronnik, P.; Nikolic-Paterson, D.J. Reduced tubular degradation of glomerular filtered plasma albumin is a common feature in acute and chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 2017, 45, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, R. Bioactivity of glomerular ultrafiltrate during heavy proteinuria may contribute to renal tubulo-interstitial lesions: Evidence for a role for insulin-like growth factor I. J. Clin. Investig. 1996, 98, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Zoja, C.; Remuzzi, G. How Does Proteinuria Cause Progressive Renal Damage? J. Am. Soc. Nephrol. 2006, 17, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Zandi-Nejad, K.; Eddy, A.A.; Glassock, R.J.; Brenner, B.M. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. Suppl. 2004, 66, S76–S89. [Google Scholar] [CrossRef] [PubMed]
- Kuusniemi, A.-M.; Lapatto, R.; Holmberg, C.; Karikoski, R.; Rapola, J.; Jalanko, H. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 2005, 68, 121–132. [Google Scholar] [CrossRef]
- Sharma, S.; Smyth, B. From Proteinuria to Fibrosis: An Update on Pathophysiology and Treatment Options. Kidney Blood Press. Res. 2021, 46, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Nolin, A.C.; Mulhern, R.M.; Panchenko, M.V.; Pisarek-Horowitz, A.; Wang, Z.; Shirihai, O.; Borkan, S.C.; Havasi, A. Proteinuria causes dysfunctional autophagy in the proximal tubule. Am. J. Physiol. Physiol. 2016, 311, F1271–F1279. [Google Scholar] [CrossRef] [PubMed]
- Baines, R.J.; Brunskill, N.J. Tubular toxicity of proteinuria. Nat. Rev. Nephrol. 2010, 7, 177–180. [Google Scholar] [CrossRef]
- Liu, B.-C.; Tang, T.-T.; Lv, L.-L.; Lan, H.-Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Isaka, Y.; Kimura, T.; Takabatake, Y. The protective role of autophagy against aging and acute ischemic injury in kidney proximal tubular cells. Autophagy 2011, 7, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.; Gifford, C.C.; Pandey, R.P.; Raj, V.S.; Sabbisetti, V.S.; Ajay, A.K. Autophagy as a Therapeutic Target for Chronic Kidney Disease and the Roles of TGF-β1 in Autophagy and Kidney Fibrosis. Cells 2023, 12, 412. [Google Scholar] [CrossRef]
- You, D.; Nie, K.; Wu, X.; Weng, M.; Yang, L.; Chen, Y.; Cui, J.; Wan, J. C3a/C3aR synergies with TGF-beta to promote epithelial-mesenchymal transition of renal tubular epithelial cells via the activation of the NLRP3 inflammasome. J. Transl. Med. 2023, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Zucchelli, P.; Passerini, P.; Cesana, B.; Locatelli, F.; Pasquali, S.; Sasdelli, M.; Redaelli, B.; Grassi, C.; Pozzi, C.; et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995, 48, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Ganguli, A.; Saha, T.K.; Kohli, H.S.; Sud, K.; Gupta, K.L.; Joshi, K.; Sakhuja, V. A Randomized, Controlled Trial of Steroids and Cyclophosphamide in Adults with Nephrotic Syndrome Caused by Idiopathic Membranous Nephropathy. J. Am. Soc. Nephrol. 2007, 18, 1899–1904. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef]
- Ponticelli, C.; Arnaboldi, L.; Moroni, G.; Fornoni, A. How We Treat Dyslipidemia in Prolonged Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2024, 19, 1327–1329. [Google Scholar] [CrossRef]
- Artunc, F. Kidney-derived PCSK9-a new driver of hyperlipidemia in nephrotic syndrome? Kidney Int. 2020, 98, 1393–1395. [Google Scholar] [CrossRef]
- Parks, J.S.; Kim, S.; Kim, C.H.; Vaziri, N.D.; Dang, B.; Zhan, C.-D.; Liang, K. Acquired lecithin-cholesterol acyltransferase deficiency in nephrotic syndrome. Am. J. Physiol. Ren. Physiol. 2001, 280, F823–F828. [Google Scholar] [CrossRef][Green Version]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2017, 14, 57–70. [Google Scholar] [CrossRef]
- Ishibashi, R.; Takemoto, M.; Tsurutani, Y.; Kuroda, M.; Ogawa, M.; Wakabayashi, H.; Uesugi, N.; Nagata, M.; Imai, N.; Hattori, A.; et al. Immune-mediated acquired lecithin-cholesterol acyltransferase deficiency: A case report and literature review. J. Clin. Lipidol. 2018, 12, 888–897. [Google Scholar] [CrossRef]
- Bisgaard, L.S.; Christoffersen, C. The apoM/S1P Complex-A Mediator in Kidney Biology and Disease? Front. Med. 2021, 8, 754490. [Google Scholar] [CrossRef]
- Go, A.S.; Tan, T.C.; Chertow, G.M.; Ordonez, J.D.; Fan, D.; Law, D.; Yankulin, L.; Wojcicki, J.M.; Zheng, S.; Chen, K.K.; et al. Primary Nephrotic Syndrome and Risks of ESKD, Cardiovascular Events, and Death: The Kaiser Permanente Nephrotic Syndrome Study. J. Am. Soc. Nephrol. 2021, 32, 2303–2314. [Google Scholar] [CrossRef]
- Izquierdo-Lahuerta, A.; Martínez-García, C.; Medina-Gómez, G. Lipotoxicity as a trigger factor of renal disease. J. Nephrol. 2016, 29, 603–610. [Google Scholar] [CrossRef]
- Wahl, P.; Ducasa, G.M.; Fornoni, A. Systemic and renal lipids in kidney disease development and progression. Am. J. Physiol. Ren. Physiol. 2016, 310, F433–F445. [Google Scholar] [CrossRef]
- Fornoni, A.; Merscher, S. Lipid Metabolism Gets in a JAML during Kidney Disease. Cell Metab. 2020, 32, 903–905. [Google Scholar] [CrossRef]
- Simon, N.; Hertig, A. Alteration of Fatty Acid Oxidation in Tubular Epithelial Cells: From Acute Kidney Injury to Renal Fibrogenesis. Front. Med. 2015, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Stæhr, M.; Buhl, K.B.; Andersen, R.F.; Svenningsen, P.; Nielsen, F.; Hinrichs, G.R.; Bistrup, C.; Jensen, B.L. Aberrant glomerular filtration of urokinase-type plasminogen activator in nephrotic syndrome leads to amiloride-sensitive plasminogen activation in urine. Am. J. Physiol. Ren. Physiol. 2015, 309, F235–F241. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P. Hypercoagulability and Reduced Fibrinolysis in Hyperlipidemia: Relationship to the Metabolic Cardiovascular Syndrome. J. Cardiovasc. Pharmacol. 1992, 20, S29–S31. [Google Scholar] [CrossRef]
- Abdelghani, E.; Waller, A.P.; Wolfgang, K.J.; Stanek, J.R.; Parikh, S.V.; Rovin, B.H.; Smoyer, W.E.; Kerlin, B.A. The Neptune Investigators Exploring the Role of Antithrombin in Nephrotic Syndrome–Associated Hypercoagulopathy: A Multi-Cohort Study and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2023, 18, 234–244. [Google Scholar] [CrossRef]
- Gigante, A.; Barbano, B.; Sardo, L.; Martina, P.; Gasperini, M.L.; Labbadia, R.; Liberatori, M.; Amoroso, A.; Cianci, R. Hypercoagulability and Nephrotic Syndrome. Curr. Vasc. Pharmacol. 2014, 12, 512–517. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Wang, S.; Jiang, L.; Chen, X.; Zhou, Y.; Li, J.; Shi, J.; Liu, P.; Shu, Z.; et al. Critical role of peroxisome proliferator-activated receptor α in promoting platelet hyperreactivity and thrombosis under hyperlipidemia. Haematologica 2021, 107, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; Carallo, C.; Scavelli, F.; Esposito, T.; De Franceschi, M.S.; Tripolino, C.; Gnasso, A. Influence of blood lipids on plasma and blood viscosity. Clin. Hemorheol. Microcirc. 2014, 57, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Mogielnicki, A.; Chabielska, E.; Pawlak, R.; Szemraj, J.; Buczko, W. Angiotensin II enhances thrombosis development in renovascular hypertensive rats. Thromb. Haemost. 2005, 93, 1069–1076. [Google Scholar] [CrossRef]
- Lu, X.; Kan, C.; Zhang, R. Phospholipase A2 receptor is associated with hypercoagulable status in membranous nephropathy: A narrative review. Ann. Transl. Med. 2022, 10, 938. [Google Scholar] [CrossRef]
- Parker, K.; Ragy, O.; Hamilton, P.; Thachil, J.; Kanigicherla, D. Thromboembolism in nephrotic syndrome: Controversies and uncertainties. Res. Pract. Thromb. Haemost. 2023, 7, 102162. [Google Scholar] [CrossRef]
- Barbour, S.J.; Greenwald, A.; Djurdjev, O.; Levin, A.; Hladunewich, M.A.; Nachman, P.H.; Hogan, S.L.; Cattran, D.C.; Reich, H.N. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012, 81, 190–195. [Google Scholar] [CrossRef]
- Kimura, Y.; Miura, N.; Debiec, H.; Morita, H.; Yamada, H.; Banno, S.; Ronco, P.; Imai, H. Circulating antibodies to α-enolase and phospholipase A2 receptor and composition of glomerular deposits in Japanese patients with primary or secondary membranous nephropathy. Clin. Exp. Nephrol. 2016, 21, 117–126. [Google Scholar] [CrossRef]
- Godier, A.; Hunt, B.J. Plasminogen receptors and their role in the pathogenesis of inflammatory, autoimmune and malignant disease. J. Thromb. Haemost. 2013, 11, 26–34. [Google Scholar] [CrossRef]
- Hladunewich, M.A.; Troyanov, S.; Calafati, J.; Metropolitan Toronto Glomerulonephritis Registry; Cattran, D.C. The Natural History of the Non-Nephrotic Membranous Nephropathy Patient. Clin. J. Am. Soc. Nephrol. 2009, 4, 1417–1422. [Google Scholar] [CrossRef]
- Cattran, D. Management of membranous nephropathy: When and what for treatment. J. Am. Soc. Nephrol. 2005, 16, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Ponticelli, C.; Zucchelli, P.; Imbasciati, E.; Cagnoli, L.; Pozzi, C.; Passerini, P.; Grassi, C.; Limido, D.; Pasquali, S.; Volpini, T.; et al. Controlled Trial of Methylprednisolone and Chlorambucil in Idiopathic Membranous Nephropathy. J. Med. 1984, 310, 946–950. [Google Scholar] [CrossRef]
- Ponticelli, C.; Zucchelli, P.; Passerini, P.; Cagnoli, L.; Cesana, B.; Pozzi, C.; Pasquali, S.; Imbasciati, E.; Grassi, C.; Redaelli, B.; et al. A Randomized Trial of Methylprednisolone and Chlorambucil in Idiopathic Membranous Nephropathy. J. Med. 1989, 320, 8–13. [Google Scholar] [CrossRef]
- Ponticelli, C.; Zucchelli, P.; Passerini, P.; Cesana, B. Methylprednisolone plus chlorambucil as compared with methylpredni-solone alone for the treatment of idiopathic membranous nephropathy. The Italian Idiopathic Membranous Nephropathy Treatment Study Group. J. Med. 1992, 327, 599–603. [Google Scholar]
- Ponticelli, C.; Altieri, P.; Scolari, F.; Passerini, P.; Roccatello, D.; Cesana, B.; Melis, P.; Valzorio, B.; Sasdelli, M.; Pasquali, S.; et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 1998, 9, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Glassock, R.J.; Passerini, P. Membranous Nephropathy. In Treatment of Primary Glo-Merulonephritis, 3rd ed.; Ponticelli, C., Glassock, R.J., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 283–346. [Google Scholar]
- Praga, M.; For the GRUPO ESPAÑOL DE ESTUDIO DE LA NEFROPATÍA MEMBRANOSA (Members of the Group listed at the end of the paper); Barrio, V.; Juárez, G.F.; Luño, J. Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int. 2007, 71, 924–930. [Google Scholar] [CrossRef]
- Ponticelli, C. Membranous nephropathy; An endless story. J. Nephrol. 2023, 36, 563–574. [Google Scholar]
- Dahan, K.; Debiec, H.; Plaisier, E.; Cachanado, M.; Rousseau, A.; Wakselman, L.; Michel, P.A.; Mihout, F.; Dussol, B.; Matignon, M.; et al. GEMRITUX Study Group. Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J. Am. Soc. Nephrol. 2017, 28, 348–358. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Jefferson, J.A.; Gipson, P.E.; Rizk, D.V.; Sedor, J.R.; et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. J. Med. 2019, 381, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kadatz, M.; Klarenbach, S.; So, H.; Fervenza, F.C.; Cattran, D.C.; Barbour, S.J. Rituximab or cyclosporine A for the treatment of membranous nephropathy: Economic evaluation of the MENTOR trial. Nephrol. Dial. Transpl. 2024, 39, 2058–2066. [Google Scholar] [CrossRef]
- Fernández-Juárez, G.; Rojas-Rivera, J.; van de Logt, A.-E.; Justino, J.; Sevillano, A.; Rabasco, C.; Cabello, V.; Varela, A.; Martín-Reyes, G.; Diezhandino, M.G.; et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2020, 99, 986–998. [Google Scholar] [CrossRef]
- Scolari, F.; Delbarba, E.; Santoro, D.; Gesualdo, L.; Pani, A.; Dallera, N.; Mani, L.-Y.; Santostefano, M.; Feriozzi, S.; Quaglia, M.; et al. Rituximab or Cyclophosphamide in the Treatment of Membranous Nephropathy: The RI-CYCLO Randomized Trial. J. Am. Soc. Nephrol. 2021, 32, 972–982. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Yang, X.; Bai, S.; Zang, X.; Liao, L.; Wang, Y.; Lv, Z.; Zhang, T.; Zhuang, S.; et al. A multicenter retrospective study on comparing the efficacy and safety of the therapy of intermittent cyclophosphamide and corticosteroids versus rituximab for primary membranous nephropathy. Ren. Fail. 2024, 46, 2409353. [Google Scholar] [CrossRef]
- Ramachandran, R.; Prabakaran, R.; Priya, G.; Nayak, S.; Kumar, P.; Kumar, A.; Kumar, V.; Agrawal, N.; Rathi, M.; Kohli, H.S.; et al. Immunosuppressive Therapy in Primary Membranous Nephropathy with Compromised Renal Function. Nephron 2021, 146, 138–145. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Leaf, D.E.; Owens, C.T.; Laliberte, K.; Pendergraft, W.F., 3rd; Niles, J.L. Combination therapy with rituximab, low-dose cyclophosphamide, and prednisone for idiopathic membranous nephropathy: A case series. BMC Nephrol. 2017, 18, 44. [Google Scholar] [CrossRef]
- Zonozi, R.; Laliberte, K.; Huizenga, N.R.; Rosenthal, J.K.; Jeyabalan, A.; Collins, A.B.; Cortazar, F.B.; Niles, J.L. Combination of Rituximab, Low-Dose Cyclophosphamide, and Prednisone for Primary Membranous Nephropathy: A Case Series with Extended Follow Up. Am. J. Kidney Dis. 2021, 78, 793–803. [Google Scholar] [CrossRef]
- Gauckler, P.; Shin, J.I.; Alberici, F.; Audard, V.; Bruchfeld, A.; Busch, M.; Cheung, C.K.; Crnogorac, M.; Delbarba, E.; Eller, K.; et al. Rituximab in Membranous Nephropathy. Kidney Int. Rep. 2021, 6, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Rheault, M.N.; Alpers, C.E.; Barratt, J.; Bieler, S.; Canetta, P.; Chae, D.-W.; Coppock, G.; Diva, U.; Gesualdo, L.; Heerspink, H.J.; et al. Sparsentan versus Irbesartan in Focal Segmental Glomerulosclerosis. J. Med. 2023, 389, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Kalay, Z.; Sahin, O.E.; Copur, S.; Danacı, S.; Ortiz, A.; Yau, K.; Cherney, D.Z.I.; Kanbay, M. SGLT-2 inhibitors in nephrotic-range proteinuria: Emerging clinical evidence. Clin. Kidney J. 2022, 16, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yebyo, H.G.; Aschmann, H.E.; Kaufmann, M.; Puhan, M.A. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis and network meta-analysis of randomized trials with 94,283 participants. Am. Heart J. 2019, 210, 18–28. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, O.; Maxwell, A.P. Diagnosis and management of nephrotic syndrome. Practitioner 2017, 261, 11–15. [Google Scholar] [PubMed]
- Sarasin, F.P.; Schifferli, J.A. Prophylactic oral anticoagulation in nephrotic patients with idiopathic membranous nephropathy. Kidney Int. 1994, 45, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Biddle, A.K.; Lionaki, S.; Derebail, V.K.; Barbour, S.J.; Tannous, S.; Hladunewich, M.A.; Hu, Y.; Poulton, C.J.; Mahoney, S.L.; et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014, 85, 1412–1420. [Google Scholar] [CrossRef]
- Kelddal, S.; Hvas, A.-M.; Grove, E.L.; Birn, H. Safety and effectiveness of direct oral anticoagulants in patients with nephrotic syndrome: A report of 21 cases. BMC Nephrol. 2022, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Tijani, A.; Coons, E.M.; Mizuki, B.; Dermady, M.; Stanilova, K.; Casey, A.L.; Alqudsi, M.; Gastanaduy, M.; Elmayan, A.; Bamnolker, A.; et al. Direct Oral Anticoagulants Versus Warfarin for Venous Thromboembolism Prophylaxis in Patients with Nephrotic Syndrome: A Retrospective Cohort Study. Ann. Pharmacother. 2022, 57, 787–794. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticelli, C. Membranous Nephropathy. J. Clin. Med. 2025, 14, 761. https://doi.org/10.3390/jcm14030761
Ponticelli C. Membranous Nephropathy. Journal of Clinical Medicine. 2025; 14(3):761. https://doi.org/10.3390/jcm14030761
Chicago/Turabian StylePonticelli, Claudio. 2025. "Membranous Nephropathy" Journal of Clinical Medicine 14, no. 3: 761. https://doi.org/10.3390/jcm14030761
APA StylePonticelli, C. (2025). Membranous Nephropathy. Journal of Clinical Medicine, 14(3), 761. https://doi.org/10.3390/jcm14030761






