Three-Dimensional Echocardiographic Assessment of Right Ventricular Global Myocardial Work and Ventricular–Pulmonary Coupling in ATTR Cardiac Amyloidosis
Abstract
1. Background
2. Methods
3. Results
3.1. ATTR-CM vs. Healthy Controls
3.2. ATTR-CM vs. PAH
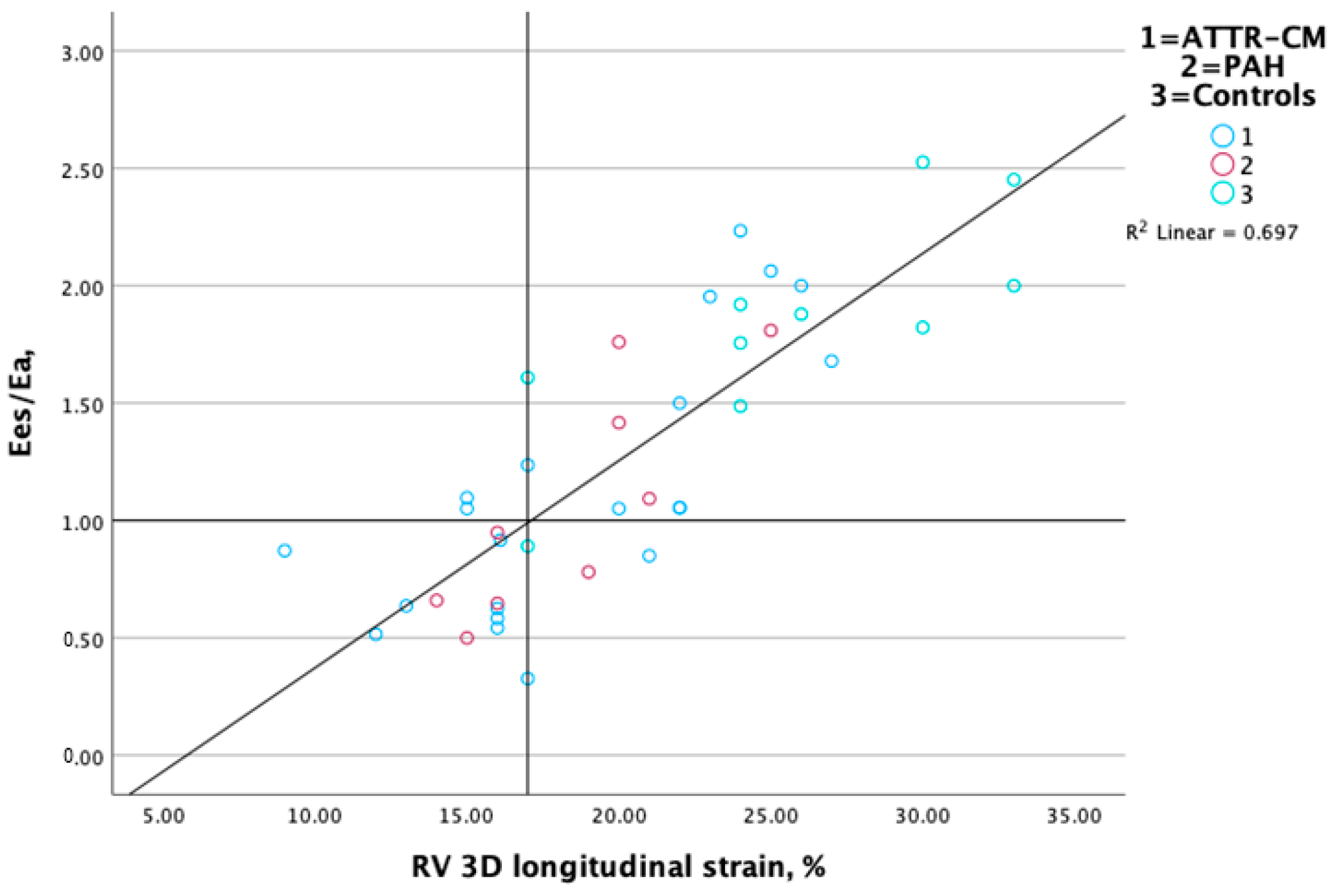
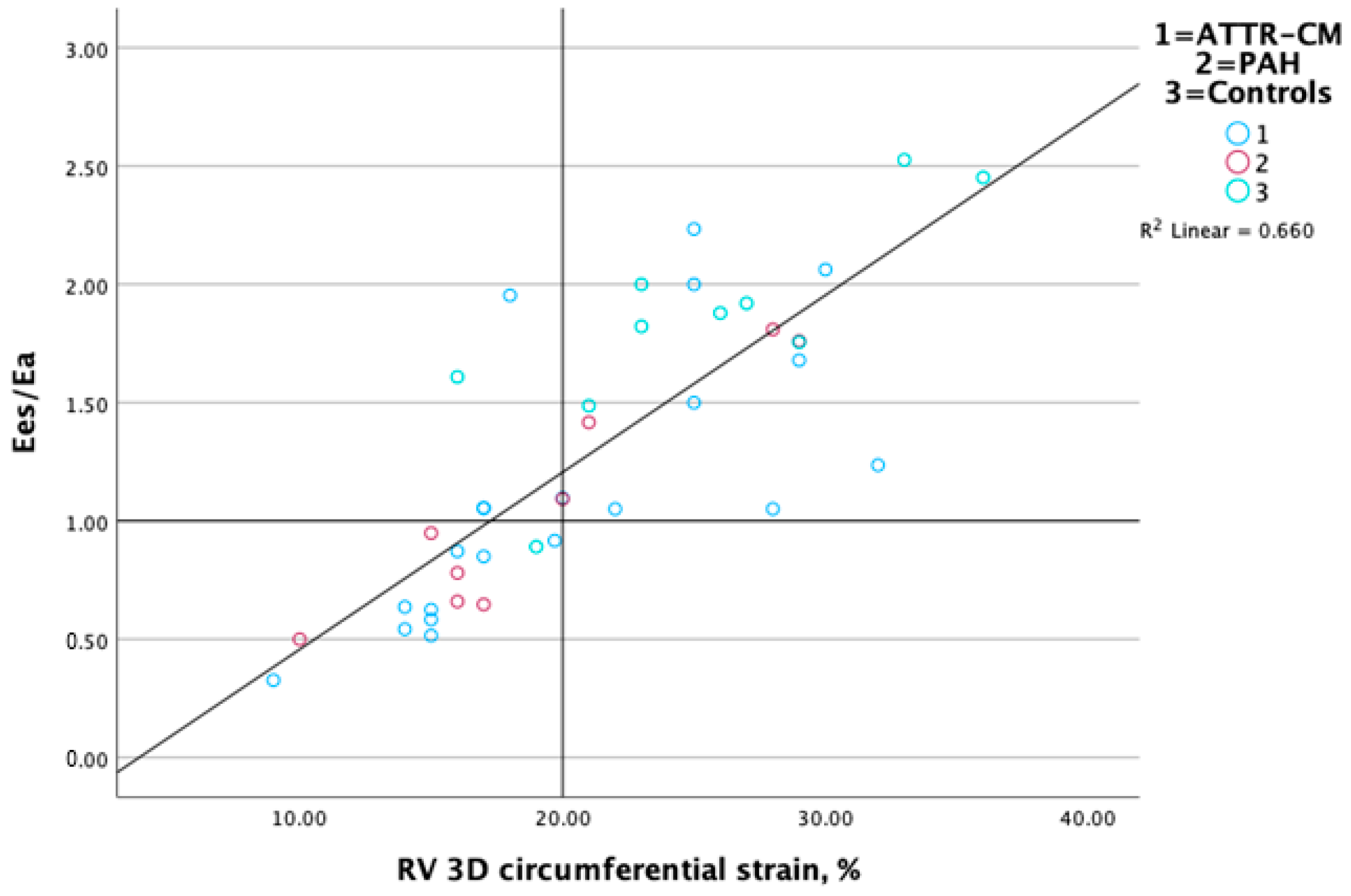
3.3. Association Between RV-PA Coupling and RV Performance Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATTR-CM | Transthyretin amyloid cardiomyopathy |
| Ea | RV afterload |
| Ees | RV elastance |
| Ees/Ea | RV–PA coupling |
| EF | Ejection fraction |
| GCW | RV constructive work |
| GLS | RV global longitudinal strain |
| GWI | RV global myocardial work index |
| HF | Heart failure |
| IVST | Interventricular septal thickness in diastole |
| PA | Pulmonary artery |
| PAH | Pulmonary arterial hypertension |
| PWT | Posterior wall thickness in diastole |
| RA | Right atrial |
| RV | Right ventricular |
| TAPSE | Tricuspid annular plane systolic excursion |
References
- Kim, J.; Di Franco, A.; Seoane, T.; Srinivasan, A.; Kampaktsis, P.N.; Geevarghese, A.; Goldburg, S.R.; Khan, S.A.; Szulc, M.; Ratcliffe, M.B. Right ventricular dysfunction impairs effort tolerance independent of left ventricular function among patients undergoing exercise stress myocardial perfusion imaging. Circ. Cardiovasc. Imaging 2016, 9, e005115. [Google Scholar] [CrossRef] [PubMed]
- Field, M.E.; Solomon, S.D.; Lewis, E.F.; Kramer, D.B.; Baughman, K.L.; Stevenson, L.W.; Tedrow, U.B. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J. Card. Fail. 2006, 12, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Meluzin, J.; Spinarová, L.; Hude, P.; Krejcí, J.; Kincl, V.; Panovský, R.; Dusek, L. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J. Am. Soc. Echocardiogr. 2005, 18, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Lella, L.K.; Sales, V.L.; Goldsmith, Y.; Chan, J.; Iskandir, M.; Gulkarov, I.; Tortolani, A.; Brener, S.J.; Sacchi, T.J.; Heitner, J.F. Reduced right ventricular function predicts long-term cardiac re-hospitalization after cardiac surgery. PLoS ONE 2015, 10, e0132808. [Google Scholar] [CrossRef] [PubMed]
- Usuku, H.; Takashio, S.; Yamamoto, E.; Yamada, T.; Egashira, K.; Morioka, M.; Nishi, M.; Komorita, T.; Oike, F.; Tabata, N. Prognostic value of right ventricular global longitudinal strain in transthyretin amyloid cardiomyopathy. J. Cardiol. 2022, 80, 56–63. [Google Scholar] [CrossRef]
- Fine, N.M.; White, J.A.; Jimenez-Zepeda, V.; Howlett, J.G. Determinants and prognostic significance of serial right heart function changes in patients with cardiac amyloidosis. Can. J. Cardiol. 2020, 36, 432–440. [Google Scholar] [CrossRef]
- He, Q.; Lin, Y.; Zhu, Y.; Gao, L.; Ji, M.; Zhang, L.; Xie, M.; Li, Y. Clinical usefulness of right ventricle–pulmonary artery coupling in cardiovascular disease. J. Clin. Med. 2023, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, T.; Helmke, S.; Patel, A.R.; Ruberg, F.L.; Packman, J.; Cheung, K.; Grogan, D.; Maurer, M.S. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circ. Heart Fail. 2011, 4, 121–128. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Eiskjær, H.; Ladefoged, B.; Mikkelsen, F.; Sørensen, J.; Granstam, S.-O.; Rosengren, S.; Flachskampf, F.A.; Poulsen, S.H. Prognostic implications of left ventricular myocardial work indices in cardiac amyloidosis. Eur. Heart J.-Cardiovasc. Imaging 2021, 22, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Giblin, G.T.; Cuddy, S.A.; González-López, E.; Sewell, A.; Murphy, A.; Dorbala, S.; Falk, R.H. Effect of tafamidis on global longitudinal strain and myocardial work in transthyretin cardiac amyloidosis. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, 1029–1039. [Google Scholar] [CrossRef]
- Lindqvist, P.; Waldenström, A.; Henein, M.; Mörner, S.; Kazzam, E. Regional and global right ventricular function in healthy individuals aged 20–90 years: A pulsed Doppler Tissue Imaging Study Umeå General Population Heart Study. Echocardiogr. J. Cardiovasc. Ultrasound Allied Tech. 2005, 22, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Venkateshvaran, A.; Seidova, N.; Tureli, H.O.; Kjellström, B.; Lund, L.H.; Tossavainen, E.; Lindquist, P. Accuracy of echocardiographic estimates of pulmonary artery pressures in pulmonary hypertension: Insights from the KARUM hemodynamic database. Int. J. Cardiovasc. Imaging 2021, 37, 2637–2645. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2012, 13, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Surkova, E.; Kovács, A.; Tokodi, M.; Lakatos, B.K.; Merkely, B.; Muraru, D.; Ruocco, A.; Parati, G.; Badano, L.P. Contraction patterns of the right ventricle associated with different degrees of left ventricular systolic dysfunction. Circ. Cardiovasc. Imaging 2021, 14, e012774. [Google Scholar] [CrossRef]
- Tokodi, M.; Staub, L.; Budai, Á.; Lakatos, B.K.; Csákvári, M.; Suhai, F.I.; Szabó, L.; Fábián, A.; Vágó, H.; Tősér, Z. Partitioning the right ventricle into 15 segments and decomposing its motion using 3D echocardiography-based models: The updated ReVISION method. Front. Cardiovasc. Med. 2021, 8, 622118. [Google Scholar] [CrossRef] [PubMed]
- Szijarto, A.; Nicoara, A.; Podgoreanu, M.; Tokodi, M.; Fabian, A.; Merkely, B.; Sarkany, A.; Toser, Z.; Lakatos, B.K.; Kovacs, A. Artificial intelligence-enabled reconstruction of the right ventricular pressure curve using the peak pressure value: A proof-of-concept study. Eur. Heart J.-Imaging Methods Pract. 2024, 2, qyae099. [Google Scholar] [CrossRef]
- Lakatos, B.K.; Rako, Z.; Szijártó, Á.; da Rocha, B.R.B.; Richter, M.J.; Fábián, A.; Gall, H.; Ghofrani, H.A.; Kremer, N.; Seeger, W.; et al. Right ventricular pressure-strain relationship-derived myocardial work reflects contractility: Validation with invasive pressure-volume analysis. J. Heart Lung Transplant. 2024, 43, 1183–1187. [Google Scholar] [CrossRef]
- Naeije, R.; Richter, M.J.; Rubin, L.J. The physiological basis of pulmonary arterial hypertension. Eur. Respir. J. 2022, 59, 2102334. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Rueter, P.; Ng, M.; Chandramohan, S.; Hibbert, T.; O’Sullivan, J.F.; Kaye, D.; Lal, S. Echocardiographic predictors of cardiovascular outcome in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2024, 26, 1778–1787. [Google Scholar] [CrossRef]
- Bay, K.; Gustafsson, F.; Maiborg, M.; Bagger-Bahnsen, A.; Strand, A.M.; Pilgaard, T.; Poulsen, S.H. Suspicion, screening, and diagnosis of wild-type transthyretin amyloid cardiomyopathy: A systematic literature review. ESC Heart Fail. 2022, 9, 1524–1541. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Trimarchi, G.; Faro, D.C.; Poleggi, C.; Teresi, L.; De Gaetano, F.; Zito, C.; Lofrumento, F.; Koniari, I.; Licordari, R.; et al. Systemic Vascular Resistance and Myocardial Work Analysis in Hypertrophic Cardi-omyopathy and Transthyretin Cardiac Amyloidosis with Preserved Left Ventricular Ejection Fraction. J. Clin. Med. 2024, 13, 1671. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Moral, J.A.; Gangadharamurthy, D.; Comenzo, R.L.; Pandian, N.G.; Patel, A.R. Three-dimensional speckle tracking echocardiography in light chain cardiac amyloidosis: Examination of left and right ventricular myocardial mechanics parameters. Rev. Española Cardiol. (Engl. Ed.) 2015, 68, 657–664. [Google Scholar] [CrossRef]
- Vitarelli, A.; Lai, S.; Petrucci, M.T.; Gaudio, C.; Capotosto, L.; Mangieri, E.; Ricci, S.; Germanò, G.; De Sio, S.; Truscelli, G.; et al. Biventricular assessment of light-chain amyloidosis using 3D speckle tracking echocardiography: Differentiation from other forms of myocardial hypertrophy. Int. J. Cardiol. 2018, 271, 371–377. [Google Scholar] [CrossRef]
- Mishra, T.; Pahuja, M.; Abidov, A. Increasingly Recognized Role of Right Ventricle Assessment in Cardiac Amyloidosis. JACC Heart Fail. 2019, 7, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Uzan, C.; Lairez, O.; Raud-Raynier, P.; Garcia, R.; Degand, B.; Christiaens, L.P.; Rehman, M.B. Right ventricular longitudinal strain: A tool for diagnosis and prognosis in light-chain amyloidosis. Amyloid 2018, 25, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Licordari, R.; Minutoli, F.; Recupero, A.; Campisi, M.; Donato, R.; Mazzeo, A.; Dattilo, G.; Baldari, S.; Vita, G.; Zito, C. Early impairment of right ventricular morphology and function in transthyretin-related cardiac amyloidosis. J. Cardiovasc. Echogr. 2021, 31, 17–22. [Google Scholar] [CrossRef]
- Arvidsson, S.; Henein, M.Y.; Wikström, G.; Suhr, O.B.; Lindqvist, P. Right ventricular involvement in transthyretin amyloidosis. Amyloid 2018, 25, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Perlini, S.; Palladini, G.; Marsan, N.A.; Faggiano, G.; Vezzoli, M.; Klersy, C.; Campana, C.; Merlini, G.; Tavazzi, L. Importance of the echocardiographic evaluation of right ventricular function in patients with AL amyloidosis. Eur. J. Heart Fail. 2007, 9, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Bodez, D.; Ternacle, J.; Guellich, A.; Galat, A.; Lim, P.; Radu, C.; Guendouz, S.; Bergoend, E.; Couetil, J.-P.; Hittinger, L. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid 2016, 23, 158–167. [Google Scholar] [CrossRef]
- Wan, K.; Lin, J.; Guo, X.; Song, R.; Wang, J.; Xu, Y.; Li, W.; Cheng, W.; Sun, J.; Zhang, Q. Prognostic value of right ventricular dysfunction in patients with AL amyloidosis: Comparison of different techniques by cardiac magnetic resonance. J. Magn. Reson. Imaging 2020, 52, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, S.; Baldasseroni, S.; Fattirolli, F.; Silverii, M.V.; Piccioli, L.; Perfetto, F.; Marchionni, N.; Di Mario, C.; Martone, R.; Taborchi, G.; et al. Poor right ventricular function is associated with impaired exercise capacity and ventilatory efficiency in transthyretin cardiac amyloid patients. Intern. Emerg. Med. 2021, 16, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Kurata, A.; Mizuno, H.; Nashiro, T.; Hangaishi, A.; Kuroda, M.; Usuki, K.; Horiuchi, H. Pulmonary arterial hypertension due to pulmonary vascular amyloid deposition in a patient with multiple myeloma. Int. J. Clin. Exp. Pathol. 2015, 8, 15391. [Google Scholar] [PubMed]
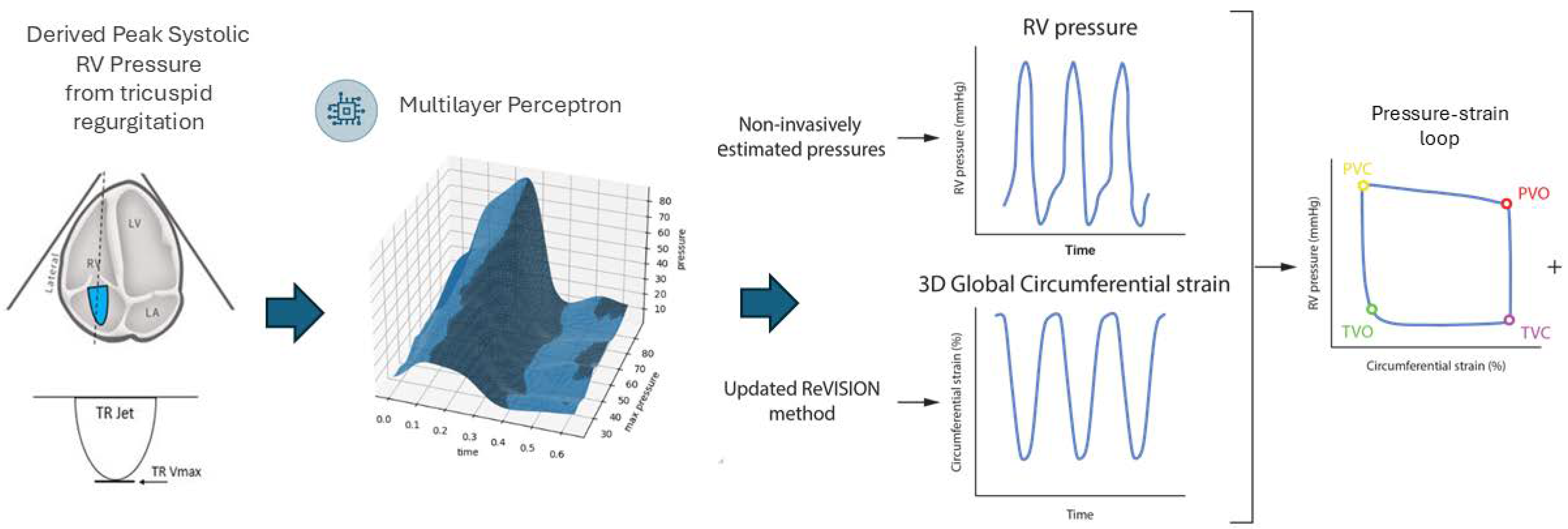

| Healthy Controls (n = 12) | Pulmonary Hypertension (n = 9) | ATTR-CM (n = 21) | p Value ATTR-CM vs. Controls | p Value ATTR-CM vs. PH | |
|---|---|---|---|---|---|
| Age, years | 56 (51–66) | 64 (45–74) | 69 (63–73) | 0.001 | ns |
| Weight, kg | 63 (60–78) | 77 (62–78) | 78 (69–84) | 0.051 | 0.044 |
| Height, cm | 172 (167–183) | 164 (160–171) | 176 (169–183) | ns | 0.004 |
| SBP, mmHg | - | 125 (116–140) | 130 (113–1148) | - | ns |
| DBP, mmHg | - | 75 (69–80) | 80 (65–81) | - | ns |
| HR, bpm | 61 (54–69) | 74 (67–80) | 73 (60–80) | 0.045 | ns |
| NT-proBNP, pg/mL | - | 256 (61–479) | 1418 (438–3372) | - | 0.001 |
| Troponin, ng/mL | - | 9 (5–29) | 39 (29–56) | - | 0.004 |
| 2D/Doppler echocardiography | |||||
| Stroke volume, mL | 76 (70–80) | 75 (61–97) | 63 (55–87) | ns | ns |
| Cardiac output, L/min | 4.5 (4.2–5.0) | 6.0 (4.5–7.5) | 5.1 (3.6–6.7) | ns | ns |
| LVDd, mm | 45 (42–52) | 47 (42–50) | 41 (39–50) | ns | ns |
| IVSTd, mm | 9 (8–10) | 10 (9–11) | 20 (16–23) | <0.001 | <0.001 |
| PWTd, mm | 7 (7–7) | 8 (8–9) | 14 (12–16) | <0.001 | <0.001 |
| LVEF, % | 55 (50–60) | 55 (53–55) | 50 (45–55) | 0.027 | ns |
| sPAP, mmHg | 25 (20–28) | 47 (37–77) | 32 (28–40) | <0.001 | 0.002 |
| TAPSE, mm | 24 (20–27) | 21 (19–23) | 17 (12–20) | <0.001 | 0.018 |
| TAPSE/sPAP, mm/mmHg | 0.96 (0.78–1.22) | 0.49 (0.27–0.61) | 0.48 (0.35–0.66) | <0.001 | ns |
| 3D RV variables | |||||
| RVDV, mL | 100 (76–124) | 90 (69–149) | 131 (109–143) | 0.022 | ns |
| RVEF,% | 64 (57–67) | 49 (39–60) | 50 (38–62) | 0.022 | ns |
| RV stroke volume, mL | 60 (48–78) | 47 (35–67) | 60 (48–38) | ns | ns |
| RV strain circ., % | 25 (20–29) | 17 (16–25) | 18 (15–26) | 0.053 | ns |
| RV strain long., % | 24 (18–30) | 19 (15–20) | 17 (15–22) | 0.017 | ns |
| GWI, mmHg/% | 280 (199–377) | 554 (504–973) | 446 (336–524) | 0.015 | 0.022 |
| GCW, mmHg/% | 270 (210–346) | 562 (504–955) | 448 (312–518 | 0.017 | 0.019 |
| GWW, mmHg/% | 11 (6–14) | 24 (8–38) | 18 (6–32) | ns | ns |
| RV Ea, mmHg/mL | 0.4 (0.4–0.4) | 0.9 (0.6–1.5) | 0.5 (0.4–0.8) | 0.04 | 0.006 |
| RV Ees, mmHg/mL | 0.7 (0.5–0.8) | 1.1 (0.6–1.6) | 0.5 (0.4–0.6) | 0.04 | 0.005 |
| RV Ees/Ea | 1.8 (1.6–2.1) | 0.9 (0.7–1.6) | 1.1 (0.6–1.8) | 0.005 | ns |
| RV Ees | RV Ees/Ea | TAPSE/sPAP (Coupling) | RV GMWI | |
|---|---|---|---|---|
| RVEF, % | R = 0.41, p = 0.08 | R = 0.94, p < 0.001 | R = 0.61, p < 0.001 | R = −0.14, p = 0.36 |
| RV strain C, % | R = 0.26, p = 0.10 | R = 0.83, p > 0.001 | R = 0.54, p < 0.001 | R = −0.16, p = 0.32 |
| RV strain L, % | R = 0.24, p = 0.13 | R = 0.84, p < 0.001 | R = 0.53, p < 0.001 | R = −0.08, p = 0.62 |
| TAPSE, mm | R = −0.36, p = 0.33 | R = 0.73, p = 0.02 | - | R = −0.61, p = 0.07 |
| RV GWI, mmHg/% | R = 0.27, p = 0.09 | R = −0.16, p = 0.32 | R = 0.51, p < 0.001 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkateshvaran, A.; Edbom, F.; Arvidsson, S.; Kovacs, A.; Lindqvist, P. Three-Dimensional Echocardiographic Assessment of Right Ventricular Global Myocardial Work and Ventricular–Pulmonary Coupling in ATTR Cardiac Amyloidosis. J. Clin. Med. 2025, 14, 668. https://doi.org/10.3390/jcm14030668
Venkateshvaran A, Edbom F, Arvidsson S, Kovacs A, Lindqvist P. Three-Dimensional Echocardiographic Assessment of Right Ventricular Global Myocardial Work and Ventricular–Pulmonary Coupling in ATTR Cardiac Amyloidosis. Journal of Clinical Medicine. 2025; 14(3):668. https://doi.org/10.3390/jcm14030668
Chicago/Turabian StyleVenkateshvaran, Ashwin, Fredrik Edbom, Sandra Arvidsson, Attila Kovacs, and Per Lindqvist. 2025. "Three-Dimensional Echocardiographic Assessment of Right Ventricular Global Myocardial Work and Ventricular–Pulmonary Coupling in ATTR Cardiac Amyloidosis" Journal of Clinical Medicine 14, no. 3: 668. https://doi.org/10.3390/jcm14030668
APA StyleVenkateshvaran, A., Edbom, F., Arvidsson, S., Kovacs, A., & Lindqvist, P. (2025). Three-Dimensional Echocardiographic Assessment of Right Ventricular Global Myocardial Work and Ventricular–Pulmonary Coupling in ATTR Cardiac Amyloidosis. Journal of Clinical Medicine, 14(3), 668. https://doi.org/10.3390/jcm14030668






