Gender-Based Differences in Stroke Types and Risk Factors Among Young Adults: A Comparative Retrospective Analysis
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics of the Study Population
3.2. Characteristics and Symptoms by Gender in Young Stroke Patients
3.3. Comparative Analysis of Clinical, Laboratory Characteristics, and Symptoms by Stroke Type in Young Patients
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, D.A.; Krishnamurthi, R.V.; Barker-Collo, S.; Forouzanfar, M.H.; Naghavi, M.; Connor, M.; Lawes, C.M.; Moran, A.E.; Anderson, L.M.; Roth, G.A.; et al. The global burden of ischemic stroke: Findings of the GBD 2010 study. Glob. Heart 2014, 9, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.U.; Kashem, M.A.; Biswas, S.; Hoque, M.M. Risk factors in young stroke. J. Med. 2020, 21, 26–30. [Google Scholar] [CrossRef]
- Aho, K.; Harmsen, P.; Hatano, S.; Marquardsen, J.; Smirnov, V.E.; Strasser, T. Cerebrovascular disease in the community: Results of a WHO collaborative study. Bull. World Health Organ. 1980, 58, 113–130. [Google Scholar]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hemorrhagic Stroke. Available online: https://www.stroke.org/en/about-stroke/types-of-stroke/hemorrhagic-strokes-bleeds (accessed on 21 May 2024).
- Reeves, M.J.; Bushnell, C.D.; Howard, G.; Gargano, J.W.; Duncan, P.W.; Lynch, G.; Khatiwoda, A.; Lisabeth, L. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008, 7, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. (1985) 2006, 101, 1252–1261. [Google Scholar] [CrossRef]
- Haast, R.A.; Gustafson, D.R.; Kiliaan, A.J. Sex differences in stroke. J. Cereb. Blood Flow Metab. 2012, 32, 2100–2107. [Google Scholar] [CrossRef]
- Bukhari, S.; Yaghi, S.; Bashir, Z. Stroke in Young Adults. J. Clin. Med. 2023, 12, 4999. [Google Scholar] [CrossRef]
- Hathidara, M.Y.; Saini, V.; Malik, A.M. Stroke in the Young: A Global Update. Curr. Neurol. Neurosci. Rep. 2019, 19, 91. [Google Scholar] [CrossRef]
- Mehndiratta, P.; Wasay, M.; Mehndiratta, M.M. Implications of female sex on stroke risk factors, care, outcome and rehabilitation: An Asian perspective. Cerebrovasc. Dis. 2015, 39, 302–308. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Anderson, L.M.; Goyal, A.; Keenan, N.L. Stroke in South Asia: A systematic review of epidemiologic literature from 1980 to 2010. Neuroepidemiology 2012, 38, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Dangel, W.J.; Ostroff, S.M.; Kiani, A.G.; Glenn, S.D.; Abbas, J.; Afzal, M.S.; Afzal, S.; Ahmad, S.; Ahmed, A.; et al. The state of health in Pakistan and its provinces and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Glob. Health 2023, 11, e229–e243. [Google Scholar] [CrossRef] [PubMed]
- Jafar, T.H. Blood pressure, diabetes, and increased dietary salt associated with stroke: Results from a community-based study in Pakistan. J. Hum. Hypertens. 2006, 20, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.K.; Itrat, A.; Murtaza, M.; Khan, M.; Rasheed, A.; Ali, A.; Akber, A.; Akber, Z.; Iqbal, N.; Shoukat, S.; et al. The burden of stroke and transient ischemic attack in Pakistan: A community-based prevalence study. BMC Neurol. 2009, 9, 58. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Owolabi, M.O.; Akarolo-Anthony, S.; Akinyemi, R.; Arnett, D.; Gebregziabher, M.; Jenkins, C.; Tiwari, H.; Arulogun, O.; Akpalu, A.; Sarfo, F.S.; et al. The burden of stroke in Africa: A glance at the present and a glimpse into the future. Cardiovasc. J. Afr. 2015, 26 (Suppl. S1), S27–S38. [Google Scholar] [CrossRef]
- Hashmi, M.; Khan, M.; Wasay, M. Growing burden of stroke in Pakistan: A review of progress and limitations. Int. J. Stroke 2013, 8, 575–581. [Google Scholar] [CrossRef]
- Namaganda, P.; Nakibuuka, J.; Kaddumukasa, M.; Katabira, E. Stroke in young adults, stroke types and risk factors: A case control study. BMC Neurol. 2022, 22, 335. [Google Scholar] [CrossRef]
- Chraa, M.; Louhab, N.; Kissani, N. Stroke in young adults: About 128 cases. Pan Afr. Med. J. 2014, 17, 37. [Google Scholar] [CrossRef]
- Khan, S.; Ali, U.; Ahmad, N.; Khan, M. Gender Differences in Ischemic Stroke Patients: A Six-Month Study at Mayo Hospital, Lahore. Pak. J. Med. Sci. 2018, 34, 1394–1398. [Google Scholar]
- Hedau, V.N.; Patil, T. Mounting Stroke Crisis in India: A Systematic Review. Cureus 2024, 16, e57058. [Google Scholar] [CrossRef] [PubMed]
- Marzona, I.; Proietti, M.; Farcomeni, A.; Romiti, G.F.; Romanazzi, I.; Raparelli, V.; Basili, S.; Lip, G.Y.; Nobili, A.; Roncaglioni, M.C. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: A systematic review and meta-analysis of 993,600 patients. Int. J. Cardiol. 2018, 269, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.W.; Liu, Y.K.; Zhang, Y.Q.; Chen, X.L.; Jiang, T.; Hou, J.K.; Shi, H.C.; Lu, M.; Zhou, F.; Wang, W.; et al. Low triglyceride to high-density lipoprotein cholesterol ratio predicts hemorrhagic transformation in large atherosclerotic infarction of acute ischemic stroke. Aging 2019, 11, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Newman-Toker, D.E.; Della Santina, C.C.; Blitz, A.M. Vertigo and hearing loss. Handb. Clin. Neurol. 2016, 136, 905–921. [Google Scholar] [CrossRef]
- Willmot, M.; Leonardi-Bee, J.; Bath, P.M. High blood pressure in acute stroke and subsequent outcome: A systematic review. Hypertension 2004, 43, 18–24. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Cairella, G.; Garbagnati, F.; Cappuccio, F.P.; Scalfi, L. Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke 2010, 41, e418–e426. [Google Scholar] [CrossRef]
- O'Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Castelli, W.P.; Doyle, J.T.; Gordon, T.; Hames, C.G.; Hjortland, M.C.; Hulley, S.B.; Kagan, A.; Zukel, W.J. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977, 55, 767–772. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef]
- Tirschwell, D.L.; Smith, N.L.; Heckbert, S.R.; Lemaitre, R.N.; Longstreth, W.T.; Psaty, B.M. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology 2004, 63, 1868–1875. [Google Scholar] [CrossRef]
- Amarenco, P.; Labreuche, J.; Touboul, P.J. High-density lipoprotein cholesterol and risk of stroke. Stroke 2006, 37, 1365–1370. [Google Scholar]
- Salvadori, E.; Papi, G.; Insalata, G.; Rinnoci, V.; Donnini, I.; Martini, M.; Falsini, C.; Hakiki, B.; Romoli, A.; Barbato, C.; et al. Comparison between Ischemic and Hemorrhagic Strokes in Functional Outcome at Discharge from an Intensive Rehabilitation Hospital. Diagnostics 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral haemorrhage. Lancet 2009, 373, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á. Role of Inflammation in Stroke and Atherothrombosis. Cerebrovasc. Dis. 2004, 17 (Suppl. S3), 1–5. [Google Scholar] [CrossRef]
| Demographics | All n (%) | Gender | p-Value | |
|---|---|---|---|---|
| All | Male n (%) | Female n (%) | ||
| 185 | 122 (65.9) | 63 (34.1) | ||
| Age in years: mean + (SD) | M: 39.1 SD: 5.5 | M: 39.3 SD: 5.3 | M: 38.7 SD: 5.9 | |
| Stay in hospital (in days) | M:15.9 SD:37.6 | M: 13.3 SD 34.8 | M: 15.2 SD 35.5 | |
| Age | 185 | 122 (65.9) | 63 (34.1) | |
| 18–25 | 2 (1.0) | 4 (2.1) | ||
| 26–30 | 5 (2.7) | 1 (0.5) | ||
| 31–35 | 19 (1.02) | 5 (2.7) | 0.21 | |
| 36–40 | 36 (19.4) | 21 (11.3) | ||
| 41–45 | 60 (32.4) | 32 (17.2) | ||
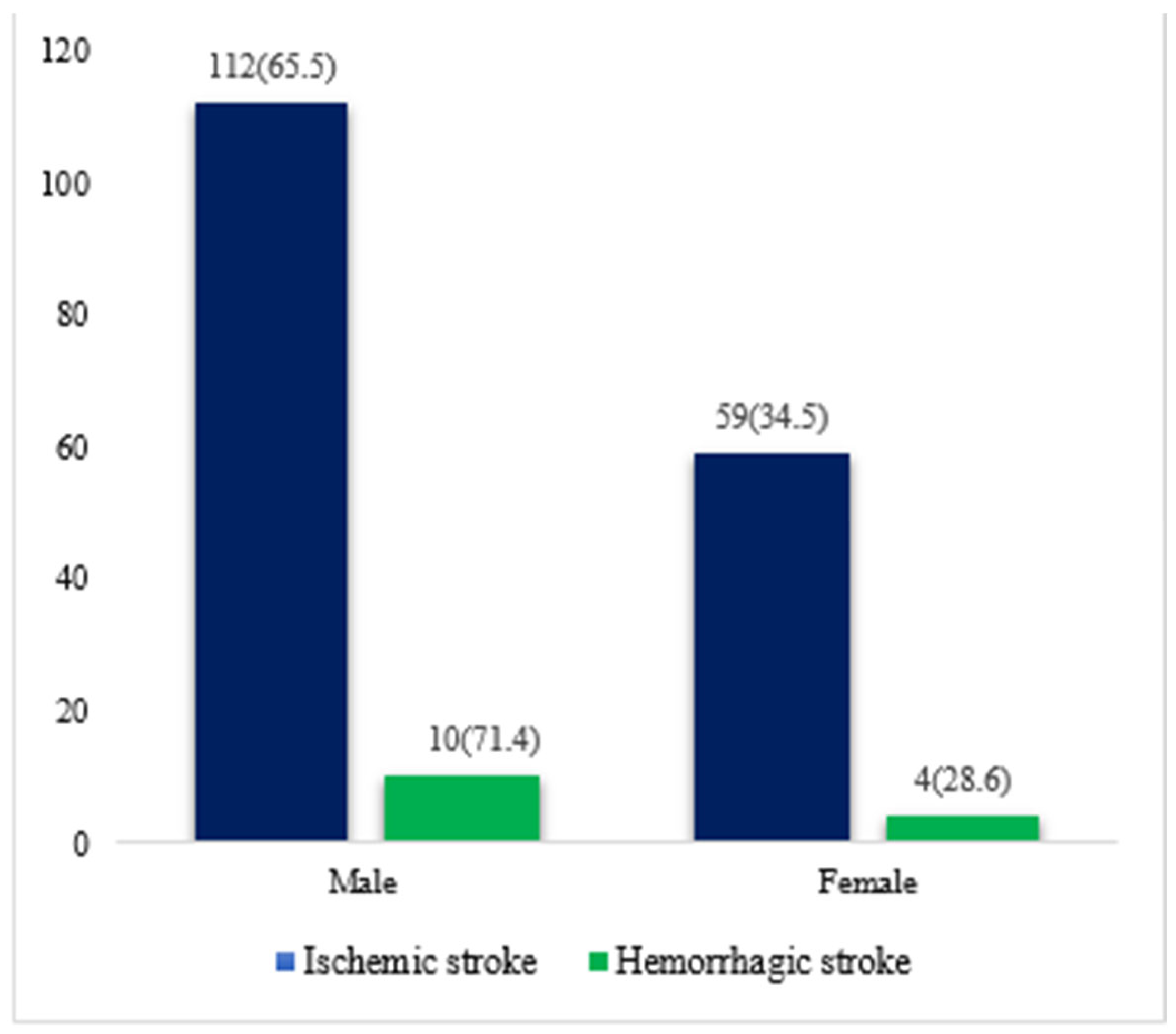
| Ischemic stroke (TOAST) | 171 (92.4) | 112 (65.5) | 59 (34.5) | |
| Large-artery atherosclerosis (LAA) | 124 (67.0) | 82 (44.3) | 42 (22.7) | |
| Cardioembolism (CA) | 1 (0.5) | 0 | 1 (0.5) | 0.39 |
| Small-vessel occlusion (SVD) | 25 (13.5) | 16(8.6) | 9 (4.8) | |
| Stroke of determined cause | 9 (4.9) | 4(2.1) | 5 (2.7) | |
| Stroke of undetermined cause | 12 (6.5) | 10(5.4) | 2 (1.0) | |
| Hemorrhagic stroke | 14 (7.6) | 10(71.4) | 4 (28.6) | |
| Intracerebral hemorrhage | 7 (3.8) | 6 (3.2) | 1 (0.5) | 0.23 |
| Subarachnoid hemorrhage | 7 (3.8) | 4 (2.1) | 3 (1.6) | |
| Male n (%) | Female n (%) | p-Value | |
|---|---|---|---|
| Hypertensive | |||
| Yes | 47 (25.4) | 27 (14.6) | 0.5 |
| No | 75 (40.5) | 36 (19.5) | |
| Diabetes | |||
| Normal | 57 (30.8) | 24 (13) | |
| Pre-diabetic | 14 (7.6) | 12 (6.5) | 0.23 |
| Diabetic | 32 (17.3) | 21 (11.4) | |
| Unknown | 19 (10.2) | 6 (3.2) | |
| Body mass index (kg/m2) | |||
| Underweight: BMI < 18.5 kg/m2 | 39 (21.1) | 20 (10.8) | |
| Normal weight: BMI 18.5–24.99 kg/m2 | 40 (21.6) | 23 (12.4) | 0.60 |
| Overweight: BMI 25–29.99 kg/m2 | 36 (19.5) | 19 (10.3) | |
| Obese: BMI ≥ 30 kg/m2 | 7 (3.8) | 1 (0.5) | |
| Smoking | |||
| Yes | 15 (8.1) | 107 (57.8) | 0.014 * |
| No | 1 (0.5) | 62 (33.5) |
| Laboratory Characteristics | Male n (%) | Female n (%) | p-Value |
|---|---|---|---|
| LDL (mg/dL) | |||
| Optimal (<100 mg/dL) | 32 (17.3) | 21 (11.3) | |
| High (>160 mg/dL) | 12 (6.5) | 5 (2.7) | |
| Borderline (130–160 mg/dL) | 25 (13.5) | 5 (2.7) | 0.22 |
| Above optimal (<130 mg/dL) | 26 (14.1) | 14 (7.6) | |
| Unknown | 27 (14.6) | 18 (9.7) | |
| HDL (mg/dL) | |||
| Major risk factor for heart diseases (<40 mg/dL) | 70 (37.8) | 23 (12.4) | |
| Negative risk for heart disease (≥ 60 mg/dL) | 47 (25.4) | 38 (20.5) | 0.018 * |
| Unknown | 5 (2.7) | 2 (1.1) | |
| Cholesterol (mg/dL) | |||
| Desirable (<200 mg/dL) | 69 (37.2) | 38 (20.5) | |
| Borderline (200–239 mg/dL) | 17 (9.1) | 6 (3.2) | 0.50 |
| High (>240 mg/dL) | 8 (4.3) | 2 (1.1) | |
| Unknown | 25 (13.5) | 17 (9.1) | |
| Triglycerides (mg/dL) | |||
| Normal (<150 mg/dL) | 57 (30.8) | 29 (15.7) | |
| Borderline (150–190 mg/dL) | 9 (4.9) | 6 (3.2) | 0.60 |
| High (200–499 mg/dL) | 24 (13) | 8 (4.3) | |
| Unknown | 32 (17.3) | 20 (10.8) |
| Clinical Characteristics | Ischemic Stroke | Hemorrhagic Stroke | p-Value |
|---|---|---|---|
| Hypertensive | 0.427 | ||
| Yes | 67 (36.2) | 7 (3.8) | |
| No | 104 (56.2) | 7 (3.8) | |
| Diabetes | |||
| Normal | 74 (40) | 7 (3.8) | 0.397 |
| Pre-diabetic | 26 (14.0) | 0 | |
| Diabetic | 49 (26.5) | 4 (2.1) | |
| Unknown | 22 (11.9) | 3 (1.6) | |
| Body mass index (kg/m2) | |||
| Underweight: BMI < 18.5 kg/m2 | 8 (4.3) | 0 | 0.70 |
| Normal weight: BMI 18.5–24.99 kg/m2 | 56 (30.2) | 3 (1.6) | |
| Overweight: BMI 25–29.99 kg/m2 | 50 (27.0) | 5 (2.7) | |
| Obese: BMI ≥ 30 kg/m2 | 57 (30.8) | 6 (3.2) | |
| Smoking | |||
| Yes | 16 (8.6) | 0 | 0.77 |
| No | 155 (83.8) | 14 (17.6) |
| Laboratory Characteristics | Ischemic Stroke | Hemorrhagic Stroke | p-Value |
|---|---|---|---|
| LDL(mg/dL) | |||
| Optimal (<100 mg/dL) | 51 (27.6) | 2 (1.1) | 0.15 |
| High (>160 mg/dL) | 17 (9.2) | 0 | |
| Borderline (130–160 mg/dL) | 28 (15.3) | 2 (1.1) | |
| Above optimal (<130 mg/dL) | 37 (20) | 3 (1.6) | |
| Unknown | 38 (20.5) | 7(3.8) | |
| HDL (mg/dL) | |||
| Major risk factor for heart diseases (<40 mg/dL) | 89 (48.1) | 4 (2.2) | 0.127 |
| Negative risk for heart disease | 75 (40.5) | 10 | |
| Unknown | 7 (3.8) | 0 | |
| Cholesterol (mg/dL) | |||
| Desirable (<200 mg/dL) | 102 (55.1) | 5 (2.7) | 0.87 |
| Borderline (200–239 mg/dL) | 22 (11.9) | 1 (0.5) | |
| High (>240 mg/dL) | 9 (4.9) | 1 (0.5) | |
| Unknown | 35 (19) | 7 (3.8) | |
| Triglycerides (mg/dL) | |||
| Normal (<150 mg/dL) | 80 (43.2) | 6 (3.2) | 0.191 |
| Borderline (150–190 mg/dL) | 15 (8.1) | 0 | |
| High (200–499 mg/dL) | 31 (16.7) | 1 (0.5) | |
| Unknown | 45 (24.3 | 7 (3.8) |
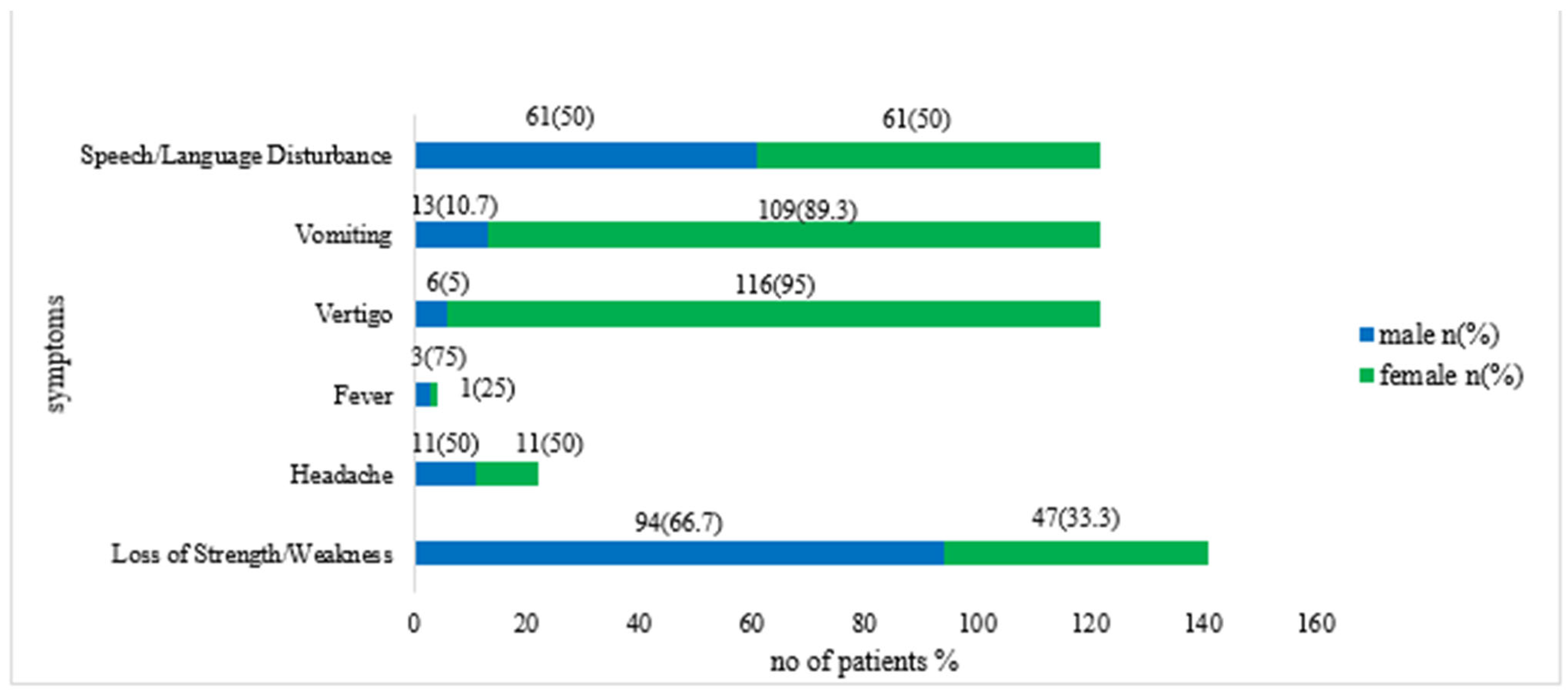
| Symptoms | Ischemic Stroke n (%) | Hemorrhagic Stroke n (%) | p-Value |
|---|---|---|---|
| Loss of Strength/Weakness | |||
| Yes | 135 (73) | 6 (3.2) | 0.002 * |
| No | 36 (19.4) | 8 (4.3) | |
| Headache | |||
| Yes | 14 (7.6) | 8 (4.3) | 0.00001 * |
| No | 157 (84.9) | 6 (3.2) | |
| Fever | |||
| Yes | 4 (2.1) | 0 | <0.00001* |
| No | 167 (90.2) | 14 (7.6) | |
| Vertigo | |||
| Yes | 89 (48.1) | 5 (2.7) | 0.08 |
| No | 82 (44.3) | 9 (4.9) | |
| Vomiting | |||
| Yes | 18 (9.7) | 3 (1.6) | 0.21 |
| No | 153 (82.7) | 11 (5.9) | |
| Speech/Language Disturbance | |||
| Yes | 89 (48.1) | 5 (2.7) | 0.24 |
| No | 82 (44.3) | 9 (4.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulzar, S.; Kiani, B.H.; Akram, R.W.; Hussein, A.M.; Alamri, A. Gender-Based Differences in Stroke Types and Risk Factors Among Young Adults: A Comparative Retrospective Analysis. J. Clin. Med. 2025, 14, 663. https://doi.org/10.3390/jcm14030663
Gulzar S, Kiani BH, Akram RW, Hussein AM, Alamri A. Gender-Based Differences in Stroke Types and Risk Factors Among Young Adults: A Comparative Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(3):663. https://doi.org/10.3390/jcm14030663
Chicago/Turabian StyleGulzar, Sumaira, Bushra Hafeez Kiani, Raja Waseem Akram, Ahmed M. Hussein, and Abdulaziz Alamri. 2025. "Gender-Based Differences in Stroke Types and Risk Factors Among Young Adults: A Comparative Retrospective Analysis" Journal of Clinical Medicine 14, no. 3: 663. https://doi.org/10.3390/jcm14030663
APA StyleGulzar, S., Kiani, B. H., Akram, R. W., Hussein, A. M., & Alamri, A. (2025). Gender-Based Differences in Stroke Types and Risk Factors Among Young Adults: A Comparative Retrospective Analysis. Journal of Clinical Medicine, 14(3), 663. https://doi.org/10.3390/jcm14030663







