Early Sacral Neuromodulation: A Promising Opportunity or an Overload for Patients with a Recent Spinal Cord Injury? A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaires
2.3. Statistics
- Dichotomous variable vs. dichotomous variable: phi coefficient.
- Dichotomous variable vs. categorical (non-dichotomous) variable: Cramer’s V.
- Dichotomous variable vs. ordinal variable or continuous (not normally distributed) variable: point-biserial Spearman’s rank correlation coefficient.
- Continuous variable vs. ordinal variable or continuous (not normally distributed) variable: Spearman’s rank correlation coefficient.
3. Results
3.1. Participants
3.2. Neuro-Urological Follow-Up
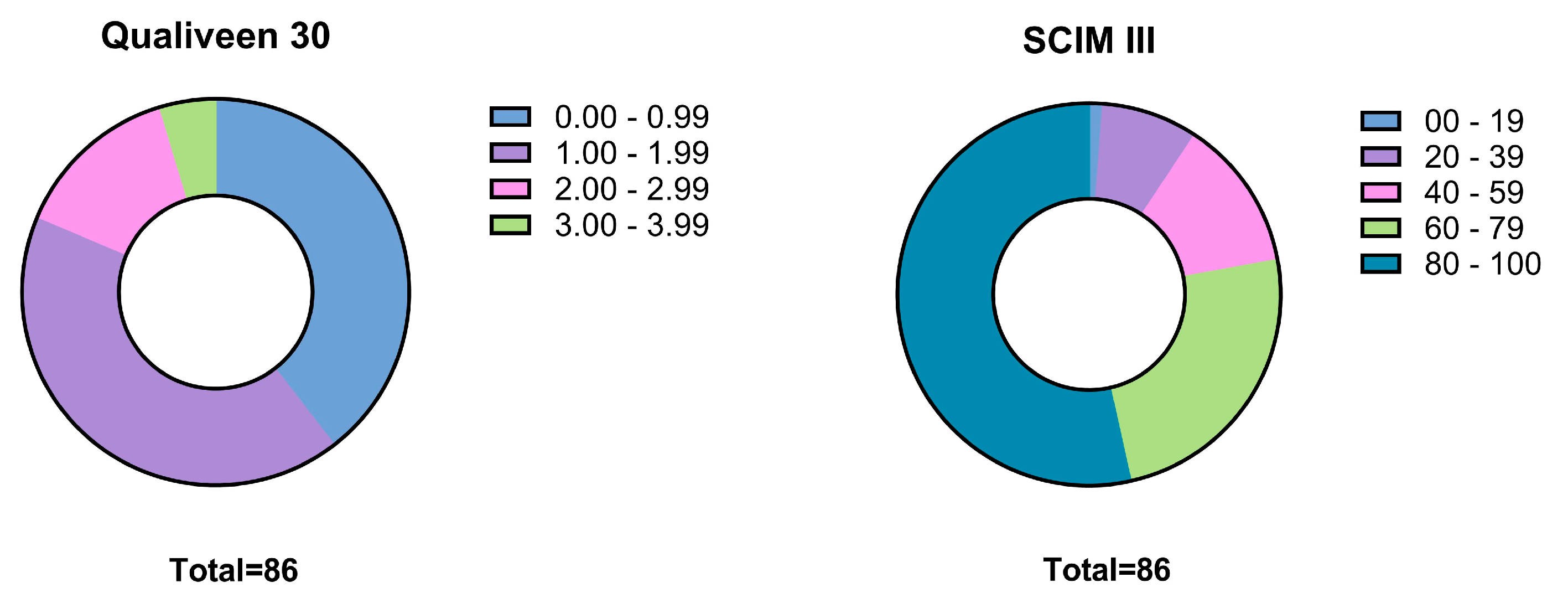
3.3. Specific Questionnaire
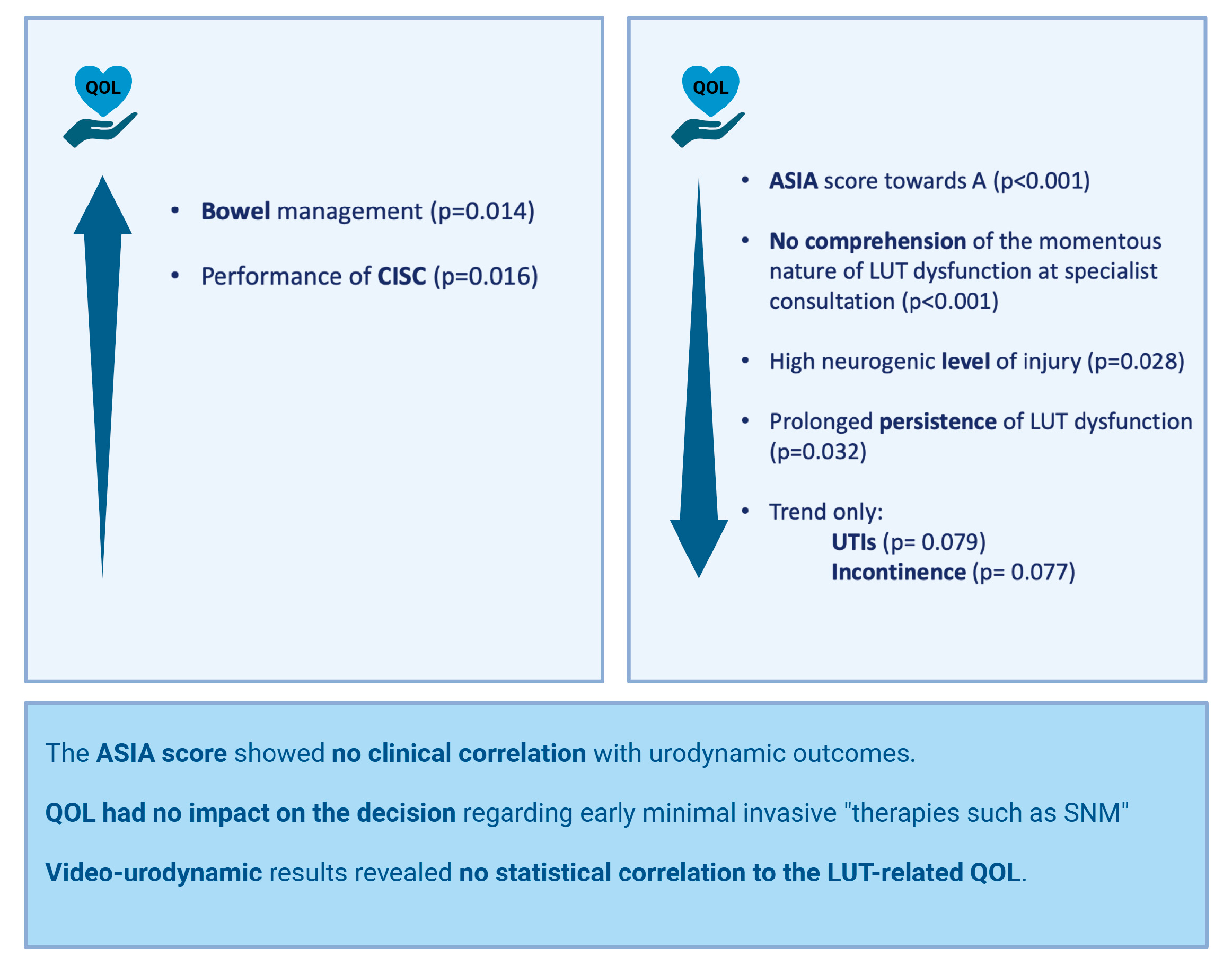
3.4. Correlation Analysis
4. Discussion
4.1. Patient Population
4.2. Quality of Life
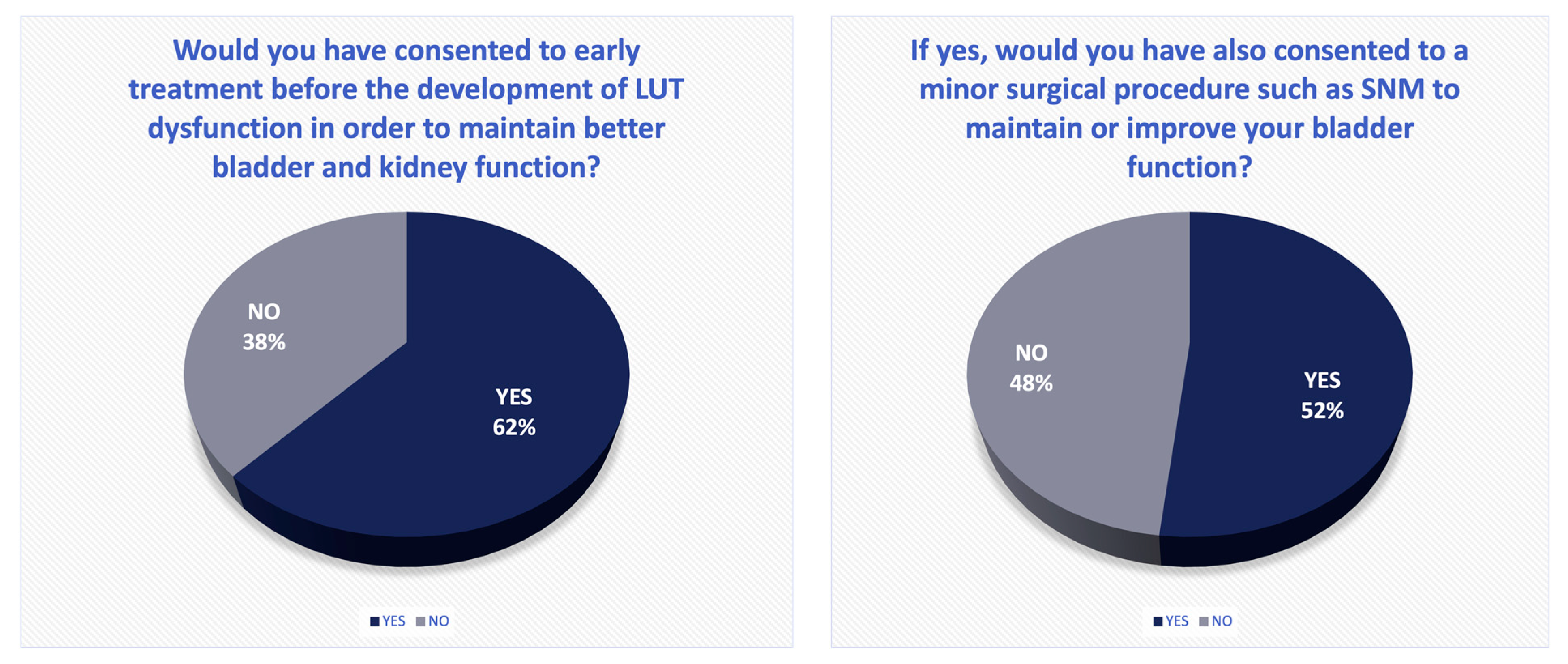
4.3. Sacral Neuromodulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | American Spinal Injury Association (ASIA) Impairment Scale |
| ASIA | American Spinal Injury Association |
| CISC | Clean intermittent self-catheterisation |
| DSD | Detrusor sphincter dyssynergia |
| EMSCI | European Multicentre Study on Spinal Cord Injury |
| EAU | European Association for Urology |
| LUT | Lower urinary tract |
| LUTD | Lower urinary tract dysfunction |
| LUTSs | Lower urinary tract symptoms |
| nLUTD | Neurogenic lower urinary tract dysfunction |
| NDO | Neurogenic detrusor overactivity |
| QOL | Quality of life |
| SCI | Spinal cord injury |
| SCIM | Spinal cord independence measure |
| SD | Standard deviation |
| SNM | Sacral neuromodulation |
| UTI | Urinary tract infection |
| VUD | Video-urodynamics |
References
- Frankel, H.L.; Coll, J.R.; Charlifue, S.W.; Whiteneck, G.G.; Gardner, B.P.; Jamous, M.A.; Krishnan, K.R.; Nuseibeh, I.; Savic, G.; Sett, P. Long-term survival in spinal cord injury: A fifty year investigation. Spinal Cord 1998, 36, 266–274. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Madersbacher, H. Neurourology—Theory and Practice, 1st ed.; Springer: Dordrecht, The Netherlands, 2019; p. 583. (In English) [Google Scholar]
- Groen, J.; Pannek, J.; Diaz, D.C.; Del Popolo, G.; Gross, T.; Hamid, R.; Karsenty, G.; Kessler, T.M.; Schneider, M.; Hoen, L.; et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur. Urol. 2016, 69, 324–333. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.M.; La Framboise, D.; Trelle, S.; Fowler, C.J.; Kiss, G.; Pannek, J.; Schurch, B.; Sievert, K.-D.; Engeler, D.S. Sacral neuromodulation for neurogenic lower urinary tract dysfunction: Systematic review and meta-analysis. Eur. Urol. 2010, 58, 865–874. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.; Amend, B.; Gakis, G.; Toomey, P.; Badke, A.; Kaps, H.; Stenzl, A. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann. Neurol. 2010, 67, 74–84. (In English) [Google Scholar] [CrossRef] [PubMed]
- Keller, E.E.; Patras, I.; Hutu, I.; Roider, K.; Sievert, K.; Aigner, L.; Janetschek, G.; Lusuardi, L.; Zimmermann, R.; Bauer, S. Early sacral neuromodulation ameliorates urinary bladder function and structure in complete spinal cord injury minipigs. Neurourol. Urodyn. 2020, 39, 586–593. (In English) [Google Scholar] [CrossRef] [PubMed]
- Pannek, J.; Kullik, B. Does optimizing bladder management equal optimizing quality of life? Correlation between health-related quality of life and urodynamic parameters in patients with spinal cord lesions. Urology 2009, 74, 263–266. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hicken, B.L.; Putzke, J.D.; Richards, J.S. Bladder management and quality of life after spinal cord injury. Am. J. Phys. Med. Rehabil. 2001, 80, 916–922. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.W.; Jee, S.H.; Kim, J.C.; Choi, J.B.; Cho, S.Y.; Kim, J.H.; Korea Spinal Cord Injury Association. Factors Affecting Quality of Life Among Spinal Cord Injury Patients in Korea. Int. Neurourol. J. 2016, 20, 316–320. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.D.; van der Lely, S.; Knüpfer, S.C.; Abt, D.; Kiss, B.; Leitner, L.; Mordasini, L.; Tornic, J.; Wöllner, J.; Mehnert, U.; et al. Sacral Neuromodulation for Neurogenic Lower Urinary Tract Dysfunction. NEJM Evid. 2022, 1, EVIDoa2200071. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gaunt, R.A.; Prochazka, A. Control of urinary bladder function with devices: Successes and failures. Prog. Brain Res. 2006, 152, 163–194. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hohenfellner, M.; Humke, J.; Hampel, C.; Dahms, S.; Matzel, K.; Roth, S.; Thüroff, J.W.; Schultz-Lampel, D. Chronic sacral neuromodulation for treatment of neurogenic bladder dysfunction: Long-term results with unilateral implants. Urology 2001, 58, 887–892. (In English) [Google Scholar] [CrossRef] [PubMed]
- Foditsch, E.E.; Roider, K.; Patras, I.; Hutu, I.; Bauer, S.; Janetschek, G.; Zimmermann, R. Structural Changes of the Urinary Bladder After Chronic Complete Spinal Cord Injury in Minipigs. Int. Neurourol. J. 2017, 21, 12–19. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, W.; Guo, D.; Shi, B.; Li, Y. Early Sacral Neuromodulation Prevented Detrusor Overactivity in Rats with Spinal Cord Injury. Neuromodulation 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, J.D.; Lenherr, S.M.; Elliott, S.P.; Stoffel, J.T.; Rosenbluth, J.P.; Presson, A.P.; Myers, J.B.; Neurogenic Bladder Research Group (NBRG.org). Protocol for a randomized clinical trial investigating early sacral nerve stimulation as an adjunct to standard neurogenic bladder management following acute spinal cord injury. BMC Urol. 2018, 18, 72. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, Y.; Hou, J. Effectiveness and safety of sacral neuromodulation for neurogenic bladder. Neurol. Res. 2023, 45, 520–529. (In English) [Google Scholar] [CrossRef] [PubMed]
- De Wachter, S.; Vaganee, D.; Kessler, T.M. Kessler Sacral Neuromodulation: Mechanism of Action. Eur. Urol. Focus 2020, 6, 823–825. (In English) [Google Scholar] [CrossRef] [PubMed]
- Curt, A.; Rodic, B.; Schurch, B.; Dietz, V. Recovery of bladder function in patients with acute spinal cord injury: Significance of ASIA scores and somatosensory evoked potentials. Spinal Cord. 1997, 35, 368–373. (In English) [Google Scholar] [CrossRef] [PubMed][Green Version]

| Time point of the last neuro-urological evaluation | |
| Sex (male n (%)) | 74 (86.0) |
| Age at the time of the questionnaire in years (median + IQR) | 47.5 (±15.69) |
| Cause of SCI (traumatic n (%)) | 69 (81.2) |
| Neurogenic level of injury (paraplegia n (%)) | 83 (96.5) |
| Cervical n (%) | 5 (6.2) |
| Thoracic n (%) | 63 (77.8) |
| Lumbar n (%) | 13 (16.0) |
| ASIA Impairment Scale (AIS, n, (%)) | |
| A n (%) | 34 (40.0) |
| B n (%) | 14 (16.5) |
| C n (%) | 10 (11.8) |
| D n (%) | 27 (31.8) |
| Neuro-urological diagnosis n (%) | |
| Neurogenic detrusor overactivity (NDO) n (%) | 12 (17.1) |
| NDO with DSD n (%) | 31 (44.3) |
| Incontinence n (%) | 19 (32.8) |
| Recurrent urinary tract infection n (%) | 30 (46.2) |
| Anticholinergic therapy n (%) | 24 (35.3) |
| Botulinum toxin therapy n (%) | 12 (17.9) |
| Method of bladder emptying | |
| Clean intermittent self-catheterisation n (%) | 50 (72.5) |
| Suprapubic catheter n (%) | 13 (19.1) |
| Indwelling catheter n (%) | 6 (9.0) |
| Aids required for bowel management n (%) | 45 (93.8) |
| None | 3 (6.3) |
| Laxatives | 11 (22.9) |
| Suppositories | 16 (33.3) |
| Digital manoeuvres | 13 (27.1) |
| Irrigation | 1 (2.1) |
| Combinations | 4 (8.3) |
| Sexual function | |
| Erection in males possible n (%) | 26 (56.6) |
| Ejaculation in males possible n (%) | 12 (27.3) |
| Sensation of orgasm possible n (%) | 12 (26.1) |
| Maximal bladder capacity (mL) (mean ± SD) | 526.75 (±170.35) |
| Detrusor pressure (cm H2O) (mean ± SD) | 28.22 (±24.06) |
| Bladder compliance (cm H2O/mL) (median, min.–max.) | 42 (7–∞) |
| Detrusor sphincter dyssynergia n (%) | 39 (60.0) |
| Incontinence observed in VUD n (%) | 3 (6.4) |
| Detrusor contractility during voiding n (%) | 11 (17.2) |
| Post-void residual volume (mL) (mean ± SD) | 488.69 (±236.03) |
| Successful VUD n (%) | 43 (62.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, S.; Grassner, L.; Maier, D.; Aigner, L.; Lusuardi, L.; Peters, J.; Mach, O.; Roider, K.; Beyerer, E.; Kleindorfer, M.; et al. Early Sacral Neuromodulation: A Promising Opportunity or an Overload for Patients with a Recent Spinal Cord Injury? A Cross-Sectional Study. J. Clin. Med. 2025, 14, 1031. https://doi.org/10.3390/jcm14031031
Bauer S, Grassner L, Maier D, Aigner L, Lusuardi L, Peters J, Mach O, Roider K, Beyerer E, Kleindorfer M, et al. Early Sacral Neuromodulation: A Promising Opportunity or an Overload for Patients with a Recent Spinal Cord Injury? A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(3):1031. https://doi.org/10.3390/jcm14031031
Chicago/Turabian StyleBauer, Sophina, Lukas Grassner, Doris Maier, Ludwig Aigner, Lukas Lusuardi, Julia Peters, Orpheus Mach, Karin Roider, Evelyn Beyerer, Michael Kleindorfer, and et al. 2025. "Early Sacral Neuromodulation: A Promising Opportunity or an Overload for Patients with a Recent Spinal Cord Injury? A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 3: 1031. https://doi.org/10.3390/jcm14031031
APA StyleBauer, S., Grassner, L., Maier, D., Aigner, L., Lusuardi, L., Peters, J., Mach, O., Roider, K., Beyerer, E., Kleindorfer, M., Wolff, A., Leister, I., & Keller, E. E. (2025). Early Sacral Neuromodulation: A Promising Opportunity or an Overload for Patients with a Recent Spinal Cord Injury? A Cross-Sectional Study. Journal of Clinical Medicine, 14(3), 1031. https://doi.org/10.3390/jcm14031031







