Ventilator-Associated Pneumonia in Intensive Care Units: A Comparison of Pre-Pandemic and COVID-19 Periods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
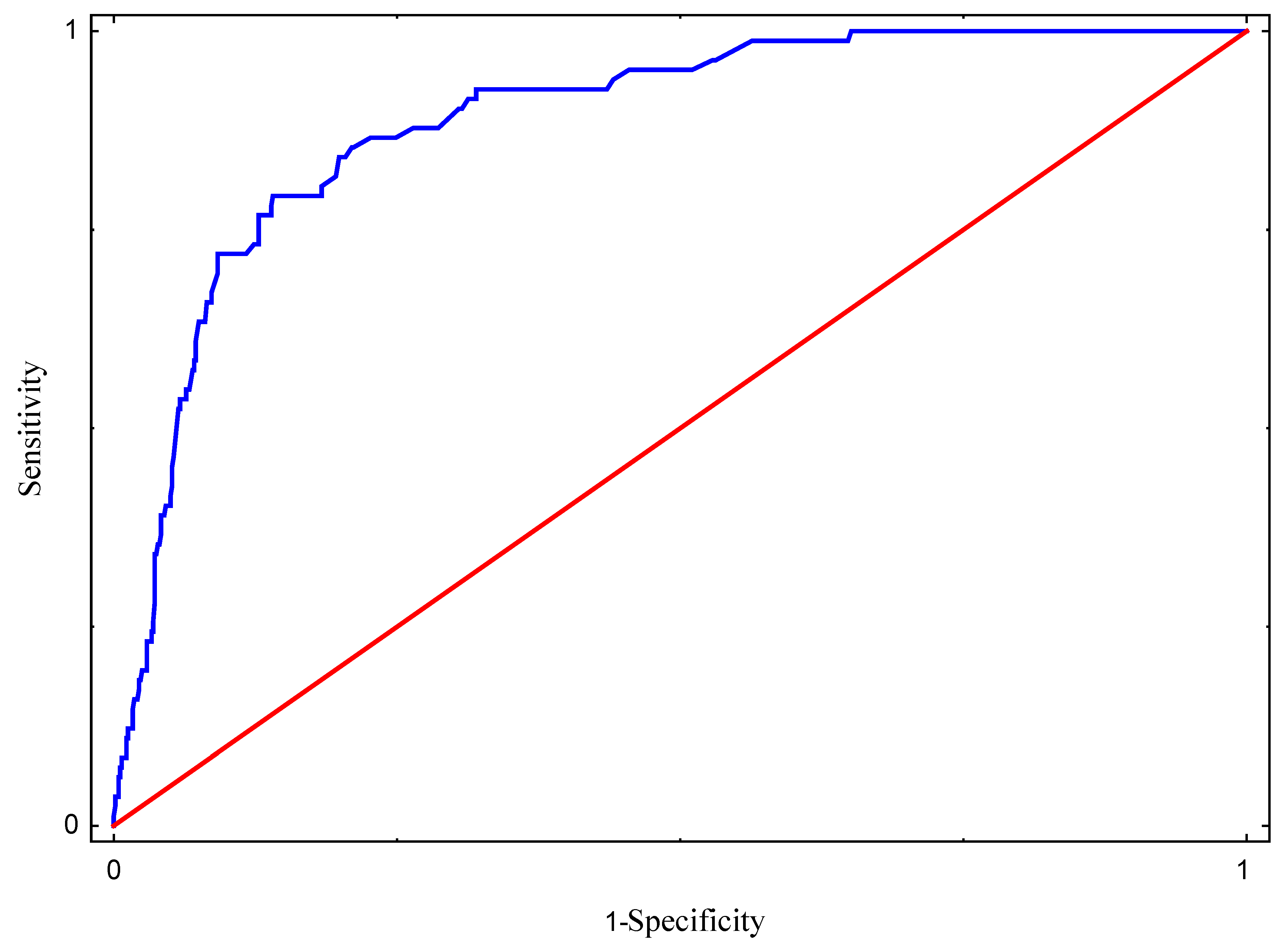
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.; Sartelli, M.; McKimm, J.; Abu, B.M. Health-care associated infections—An overview. Infect. Drug Resist. 2018, 15, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals, 2022–2023. ECDC 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/PPS-HAI-AMR-acute-care-europe-2022-2023 (accessed on 2 December 2024).
- Dudeck, M.A.; Edwards, J.R.; Allen-Bridson, K.; Gross, C.; Malpiedi, P.J.; Peterson, K.D.; Pollock, D.A.; Weiner, L.M.; Sievert, D.M. National Healthcare Safety Network report, data summary for 2013, Device-associated Module. Am. J. Infect. Control 2015, 43, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Ventilator-associated events: Surveillance, definitions, and significance. Clin. Infect. Dis. 2013, 56, 441–447. [Google Scholar]
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Kalanuria, A.A.; Zai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef]
- Kollef, M.H. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit. Care Med. 2004, 32, 1396–1405. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of Coronavirus disease 2019 (COVID-19) on Healthcare-Associated Infections. Clin. Infect. Dis. 2022, 74, 1748–1754. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- CDC NHSN. Pneumonia (Ventilator-Associated [VAP] and non-Ventilator-Associated Pneumonia [PNEU]) Event. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf (accessed on 7 December 2024).
- Public Health Authority of the Slovak Republic. Annual Report of the Regional Public Health Offices of the Slovak Republic for 2023. Available online: https://www.uvzsr.sk/web/uvz/vyrocne-spravy (accessed on 2 December 2024).
- Miron, M.; Blaj, M.; Ristescu, A.I.; Iosep, G.; Avădanei, A.-N.; Iosep, D.-G.; Crișan-Dabija, R.; Ciocan, A.; Perțea, M.; Manciuc, C.D.; et al. Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: A Literature Review. Microorganisms 2024, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, M.; Horan, T.C.; Peterson, K.D.; Allen-Bridson, K.; Morrell, G.; Anttila, A.; Pollock, D.A.; Edwards, J.R. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am. J. Infect. Control 2013, 41, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Jin, Z.; Memish, Z.A.; Rodrigues, C.; Myatra, S.N.; Kharbanda, M.; Valderrama-Beltran, S.L.; Mehta, Y.; Daboor, M.A.; Todi, S.K.; et al. Multinational prospective cohort study of rates and risk factors for ventilator-associated pneumonia over 24 years in 42 countries of Asia, Africa, Eastern Europe, Latin America, and the Middle East: Findings of the International Nosocomial Infection Control Consortium (INICC). Antimicrob. Steward. Healthc. Epidemiol. 2023, 51, 575–682. [Google Scholar]
- Klimovsky, D.; Nemec, J.; Bouckaert, G. The COVID-19 Pandemic in the Czech Republic and Slovakia. Sci. Pap. Univ. Pardubic. Ser. D Fac. Econ. Adm. 2021, 29, 1320. [Google Scholar] [CrossRef]
- Blonz, G.; Kouatchet, A.; Chudeau, N.; Pontis, E.; Lorber, J.; Lembeur, A.; Planche, L.; Lascarrou, J.-B.; Colin, G. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit. Care 2021, 25, 72. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 25. [Google Scholar] [CrossRef]
- Fumagalli, J.; Panigada, M.; Klompas, M.; Berra, L. Ventilator-associated pneumonia among SARS-CoV-2 acute respiratory distress syndrome patients. Curr. Opin. Crit. Care 2022, 28, 74–82. [Google Scholar] [CrossRef]
- Witt, L.S.; Howard-Anderson, J.R.; Jacob, J.T.; Gottlieb, L.B. The impact of COVID-19 on multidrug-resistant organisms causing healthcare-associated infections: A narrative review. JAC-Antimicrob. Resist. 2023, 5, 130. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Schreiber, M.; Cardo, D.; Srinoivasan, A. Health care safety during the pandemic and beyond—Building a system that ensures resilience. N. Engl. J. Med. 2022, 386, 609–611. [Google Scholar] [CrossRef]
- Ferrando, C.; Suarez-Sipmann, F.; Mellado-Artigas, R.; Hernández, M.; Gea, A.; Arruti, E.; Aldecoa, C.; Martínez-Pallí, G.; Martínez-González, M.A.; Slutsky, A.S.; et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020, 46, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Epidemiology, Pathogenesis, Microbiology, and Diagnosis of Hospital-Acquired and Ventilator-Associated Pneumonia in Adults. Wolters Kluwer 2024. Available online: https://sso.uptodate.com/contents/epidemiology-pathogenesis-microbiology-and-diagnosis-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults/print (accessed on 10 December 2024).
- Taniguchi, H.; Ohya, A.; Yamagata, H.; Iwashita, M.; Abe, T.; Takeuchi, I. Prolonged mechanical ventilation in patients with severe COVID-19 is associated with serial modified-lung ultrasound scores: A single-centre cohort study. PLoS ONE 2022, 17, e0271391. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Windisch, W.; McAuley, D.F.; Welte, T.; Busse, R. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir. Med. 2021, 9, e47–e48. [Google Scholar] [CrossRef]
- Zeng, Z.; Xiang, M.; Guan, H.; Liu, Y.; Zhang, H.; Xia, L.; Zhan, J.; Hu, Q. Early fibroproliferative signs on high-resolution CT are associated with mortality in COVID-19 pneumonia patients with ARDS: A retrospective study. Ther. Adv. Chronic Dis. 2021, 9, 12. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10,021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Rinaudo, M.; Terraneo, S.; de Rosa, F.; Ramirez, P.; Diaz, E.; Fernandez-Barat, L.; Li Bassi, G.L.; Ferrer, M. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J. Infect. 2015, 70, 213–222. [Google Scholar]
- Bassetti, M.; Kollef, M.H.; Timsit, J.F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 2071–2074. [Google Scholar] [CrossRef]
- Grasselli, G.; Cattaneo, E.; Florio, G. Secondary infections in critically ill patients with COVID-19. Crit. Care 2021, 25, 317. [Google Scholar] [CrossRef]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 4. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Niederman, M.S.; Torres, A. Ventilator-associated pneumonia. Intensive Care Med. 2022, 48, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.; Koulenti, D.; Dimopoulos, G.; Martin, C.; Komnos, A.; Krueger, W.A.; Spina, G.; Armaganidis, A.; Rello, J.; EU-VAP Study Investigators. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit. Care Med. 2014, 42, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Dananche, C.; Vanhems, P.; Machut, A.; Aupee, M.; Bervas, C.; L’Heriteau, F.; Lepape, A.; Lucet, J.C.; Stoeckel, V.; Timsit, J.F.; et al. Trends of Incidence and Risk Factors of Ventilator-Associated Pneumonia in Elderly Patients Admitted to French ICUs Between 2007 and 2014. Crit. Care Med. 2018, 46, 869–877. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Garnier, M.; Constantin, J.-M.; Heming, N.; Camous, L.; Ferré, A.; Razazi, K.; Lapidus, N.; the COVID-ICU Investigators. Epidemiology, risk factors and prognosis of ventilator-associated pneumonia during severe COVID-19: Multicenter observational study across 149 European Intensive Care Units. Anaesth. Crit. Care Pain Med. 2023, 42, 1. [Google Scholar] [CrossRef]
- Klompas, M.; Branson, R.; Cawcutt, K.; Crist, M.; Eichenwald, E.C.; Greene, L.R.; Lee, G.; Maragakis, L.L.; Powell, K.; Priebe, G.P.; et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2022, 43, 687–713. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical supply shortages: The need for resilient healthcare systems during pandemics. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Country Factsheet Slovakia. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/country-factsheet-slovakia (accessed on 7 December 2024).
| Variable | Variants | Total (n = 803) | Before Pandemic (n = 339) | Pandemic (n = 464) | p-Value * | VAP No (n = 721) | VAPs Yes (n = 82) | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Age Me (IQR) | Years | 64 (22) | 63 (24) | 64 (19) | 0.229 A | 64 (21) | 59 (17) | 0.032 * A |
| Gender n (%) | Male | 515 (64.1) | 226 (66.7) | 289 (62.3) | 0.201 B | 469 (64.9) | 47 (57.3) | 0.174 B |
| Female | 288 (35.9) | 113 (33.3) | 175 (37.7) | 253 (35.1) | 35 (42.7) | |||
| Hospitalization Type n (%) | Surgical | 371 (46.2) | 252 (77.3) | 119 (25.7) | < 0.001 * B | 350 (48.5) | 21 (25.6) | <0.001 * B |
| Medical | 432 (53.8) | 87 (25.7) | 345 (74.4) | 371 (51.5) | 61 (74.4) | |||
| ICU Outcome n (%) | No | 528 (65.8) | 252 (74.3) | 276 (59.5) | <0.001* B | 490 (68.0) | 38 (46.3) | <0.001 * B |
| Yes | 275 (34.3) | 87 (25.7) | 188 (40.5) | 231 (32.0) | 44 (53.7) | |||
| COVID-19 n (%) | No | 596 (74.2) | 339 (100) | 257 (55.4) | <0.001* B | 552 (76.6) | 44 (53.7) | <0.001 * B |
| Yes | 207 (25.8) | 0 (0) | 207 (44,6) | 169 (23.4) | 38 (46.3) | |||
| Pandemic COVID-19 n (%) | No | 339 (42.2) | N/A | N/A | N/A | 319 (44.2) | 20 (24.4) | <0.001 * B |
| Yes | 464 (57.8) | N/A | N/A | 402 (55.8) | 62 (75.6) |
| Variable | Total (n = 803) | Before Pandemic (n = 339) | Pandemic (n = 464) | p-Value | Pandemic | p-Value | |
|---|---|---|---|---|---|---|---|
| Yes COVID (n = 207) | Non-COVID (n = 257) | ||||||
| No. of VAP n (%) | 82 (10.2) | 20 (5.9) | 62 (13.4) | <0.001 * B | 38 (18.4) | 24 (9.3) | <0.001 * B |
| No. of Bed Days (M, SD) | 8385 (10.4 ± 11.2) | 3098 (9.1 ± 11.1) | 5299 (11.4 ± 11.2) | <0.001 * A | 2857 (13.8 ± 11.1) | 2442 (9.5 ± 10.9) | <0.001 * A |
| Mechanical Ventilator Days (M, SD) | 5836 (7.3 ± 9.8) | 2365 (7.0 ± 10.3) | 3471 (7.5 ± 9.3) | 0.701 * A | 1947 (9.4 ± 10.8) | 1524 (5.9 ±7.6) | <0.012 * A |
| Mechanical Ventilator Utilization Ratio (95 CI) | 0.70 (0.68–0.71) | 0.77 (0.73–0.79) | 0.66 (0.63–0.68) | <0.001 * A | 0.68 (0.65–0.71) | 0.62 (0.59–0.66) | 0.001 * A |
| VAP/1000 MV Days (95 CI) | 14.05 (11.25–17.35) | 8.46 (5.31–2.83) | 17.86 (13.81–2.75) | <0.001 * A | 19.52 (14.01–26.51) | 15.75 (10.32–23.07) | <0.001 * A |
| Microorganism VAPs | Total VAPs N = 82 (%) | Before Pandemic N = 20 (%) | Pandemic N = 62 (%) |
|---|---|---|---|
| Acinetobacter spp. | 18 (22.0) | 6 (30) | 12 (19.4) |
| Candida albicans | 1 (1.2) | - | 1 (1.6) |
| Corybebacterium | 1 (1.2) | - | 1 (1.6) |
| Enterobacter aerogenes | 1 (1.2) | - | 1 (1.6) |
| Enterobacter spp. | 2 (2.4) | - | 2 (3.2) |
| Enterococci sp. | 2 (2.4) | - | 2 (3.2) |
| Escherichia coli | 1 (1.2) | 1 (5) | - |
| Klebsiella pneumoniae | 21 (26.0) | 6 (30) | 15 (24.2) |
| Morganella morganii | 1 (1.2) | - | 1 (1.6) |
| Proteus mirabilis | 3 (3.7) | 1 (5) | 2 (3.2) |
| Pseudomonas aeruginosa | 23 (28.1) | 6 (30) | 17 (27.4) |
| Serratia marcescens | 5 (6.0) | - | 5 (8.1) |
| Serratia odorifera | 2 (2.4) | - | 2 (3.2) |
| Staphylococcus aureus | 1 (1.2) | - | 1 (1.6) |
| Variable—Reference Variant | Estimate of the Logistic Regression Parameter | OR (95% Cl) | p-Value |
|---|---|---|---|
| Constant Term | −4.333 | 0.013 (0.007–0.026) | <0.001 |
| MV Days | 0.067 | 1.069 (1.027–1.112) | 0.001 |
| Pandemic COVID-19 (Yes) | 1.082 | 2.950 (1.547–5.626) | 0.001 |
| Length of Stay | 0.046 | 1.047 (1.012–1.084) | 0.008 |
| VAP | COVID-19 | Pandemic COVID-19 | ICU Outcome | Observed Values No. of Patients | Expected Values No. of Patients | χ2 | p-Value |
|---|---|---|---|---|---|---|---|
| Non | Non | Non | Non | 242 | 149 | 7.667 | 0.000 |
| Non | Non | Non | Yes | 77 | 77 | 0.042 | 0.483 |
| Non | Non | Yes | Non | 177 | 203 | 1.846 | 0.032 |
| Non | Non | Yes | Yes | 56 | 106 | 4.849 | 0.000 |
| Non | Yes | Yes | Non | 71 | 71 | 0.046 | 0.482 |
| Non | Yes | Yes | Yes | 98 | 37 | 10.095 | 0.000 |
| Yes | Non | Non | Non | 10 | 17 | 1.677 | 0.047 |
| Yes | Non | Non | Yes | 10 | 9 | 0.405 | 0.343 |
| Yes | Non | Yes | Non | 17 | 23 | 1.274 | 0.101 |
| Yes | Non | Yes | Yes | 7 | 12 | 1.453 | 0.073 |
| Yes | Yes | Yes | Non | 11 | 8 | 1.048 | 0.147 |
| Yes | Yes | Yes | Yes | 27 | 4 | 11.156 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlinkova, S.; Moraucikova, E.; Strzelecka, A.; Mrazova, M.; Littva, V. Ventilator-Associated Pneumonia in Intensive Care Units: A Comparison of Pre-Pandemic and COVID-19 Periods. J. Clin. Med. 2025, 14, 1000. https://doi.org/10.3390/jcm14031000
Hlinkova S, Moraucikova E, Strzelecka A, Mrazova M, Littva V. Ventilator-Associated Pneumonia in Intensive Care Units: A Comparison of Pre-Pandemic and COVID-19 Periods. Journal of Clinical Medicine. 2025; 14(3):1000. https://doi.org/10.3390/jcm14031000
Chicago/Turabian StyleHlinkova, Sona, Eva Moraucikova, Agnieszka Strzelecka, Mariana Mrazova, and Vladimir Littva. 2025. "Ventilator-Associated Pneumonia in Intensive Care Units: A Comparison of Pre-Pandemic and COVID-19 Periods" Journal of Clinical Medicine 14, no. 3: 1000. https://doi.org/10.3390/jcm14031000
APA StyleHlinkova, S., Moraucikova, E., Strzelecka, A., Mrazova, M., & Littva, V. (2025). Ventilator-Associated Pneumonia in Intensive Care Units: A Comparison of Pre-Pandemic and COVID-19 Periods. Journal of Clinical Medicine, 14(3), 1000. https://doi.org/10.3390/jcm14031000







