Expanded Hemodialysis with Theranova 500 Improves Dialysis Adequacy and Blunts Inflammation: A 24-Week Quasi-Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
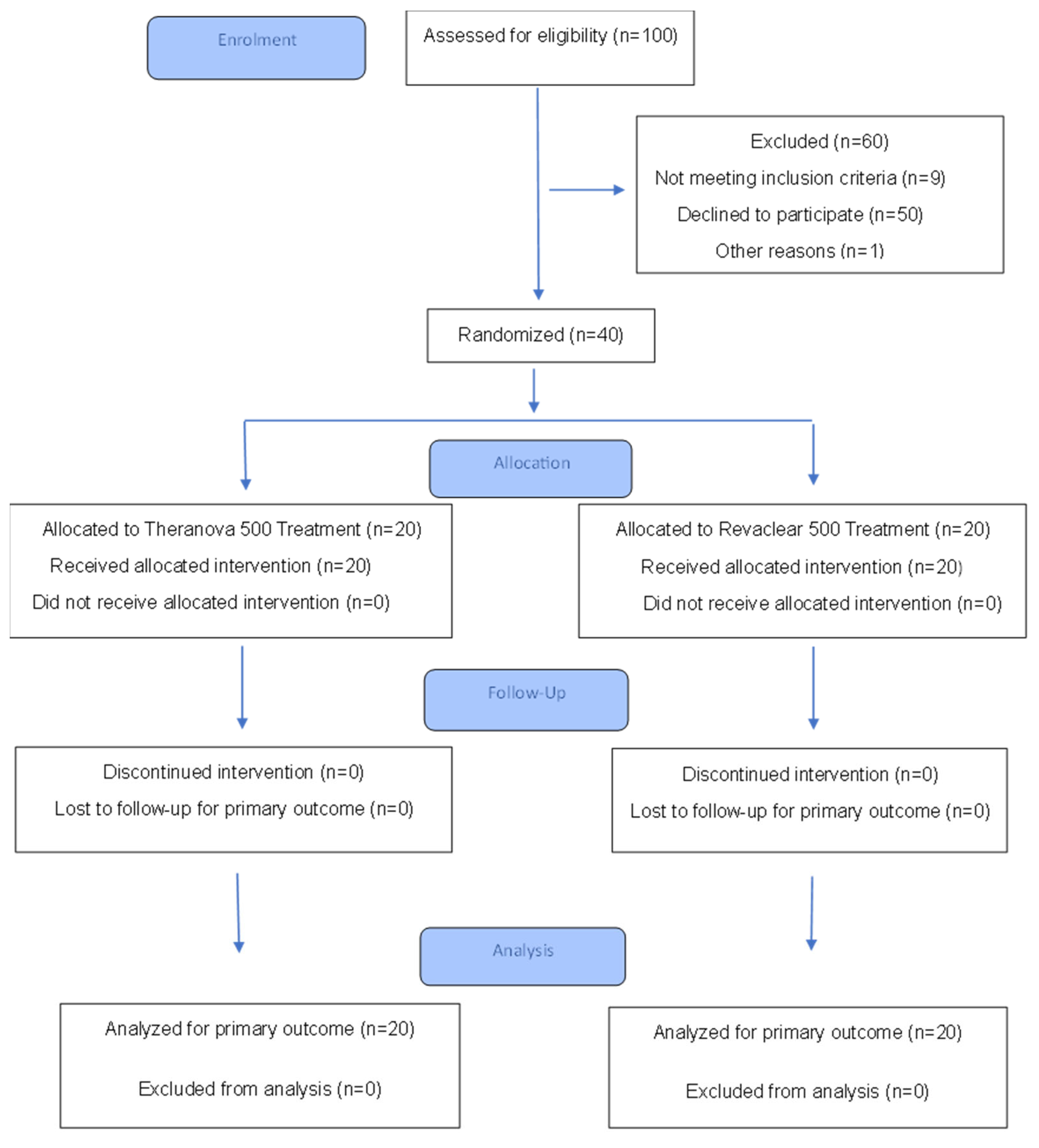
2.3. Patients Allocation and Study Groups
2.4. Study Procedures
2.5. Statistical Analysis
3. Results
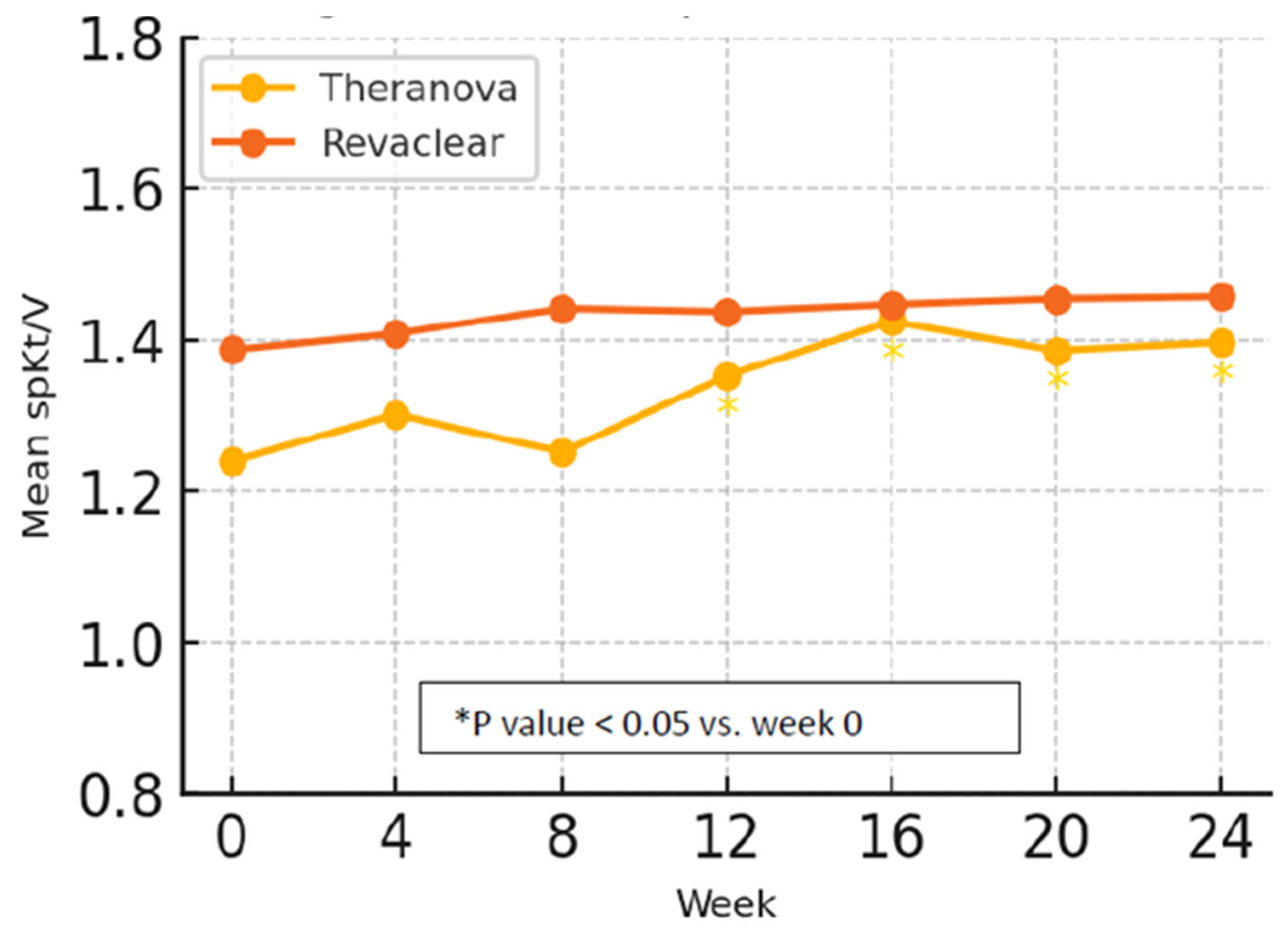
3.1. Dialysis Adequacy
3.2. Anemia, Iron Indices and Mineral Metabolism
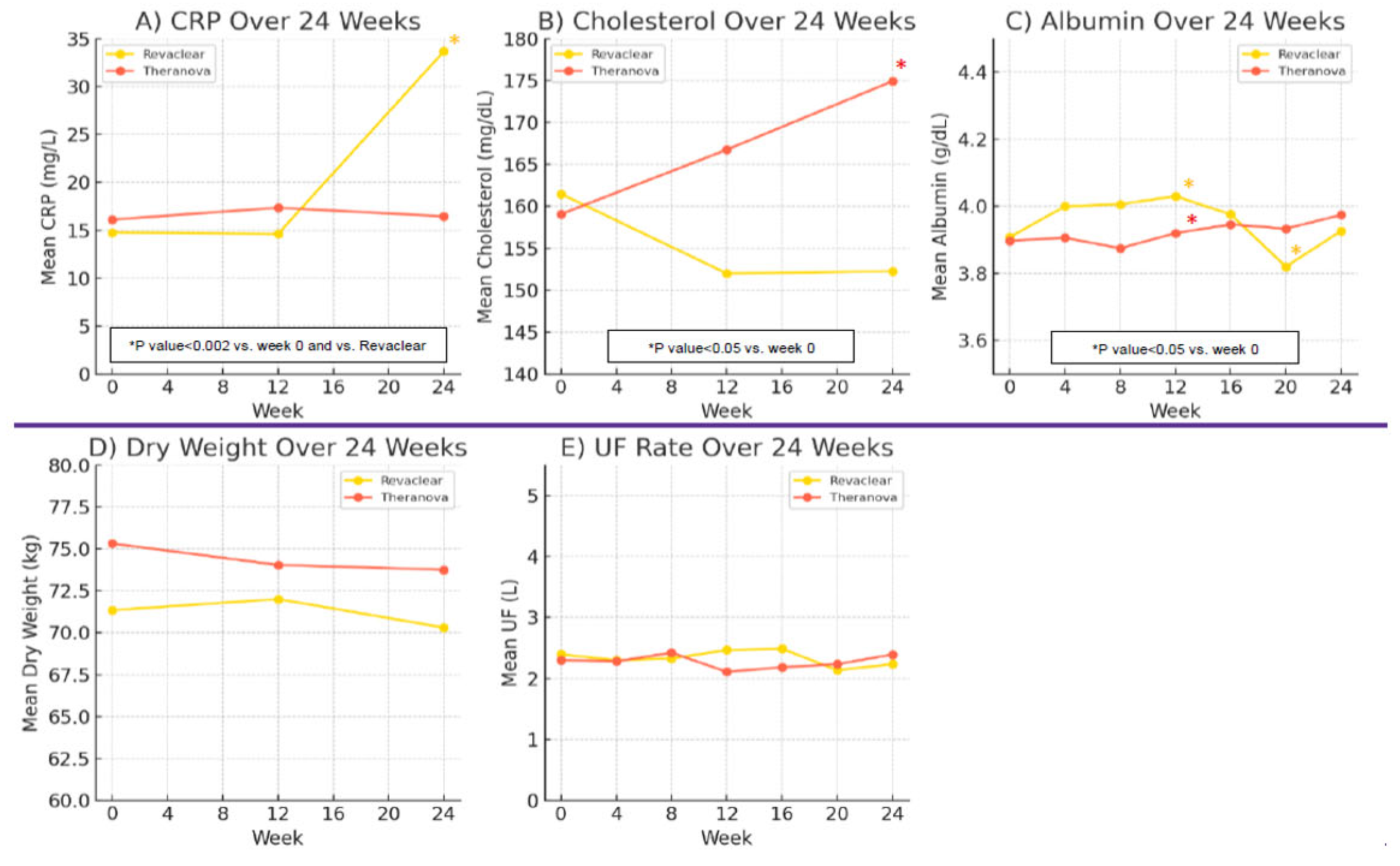
3.3. Inflammation and Nutritional Parameters
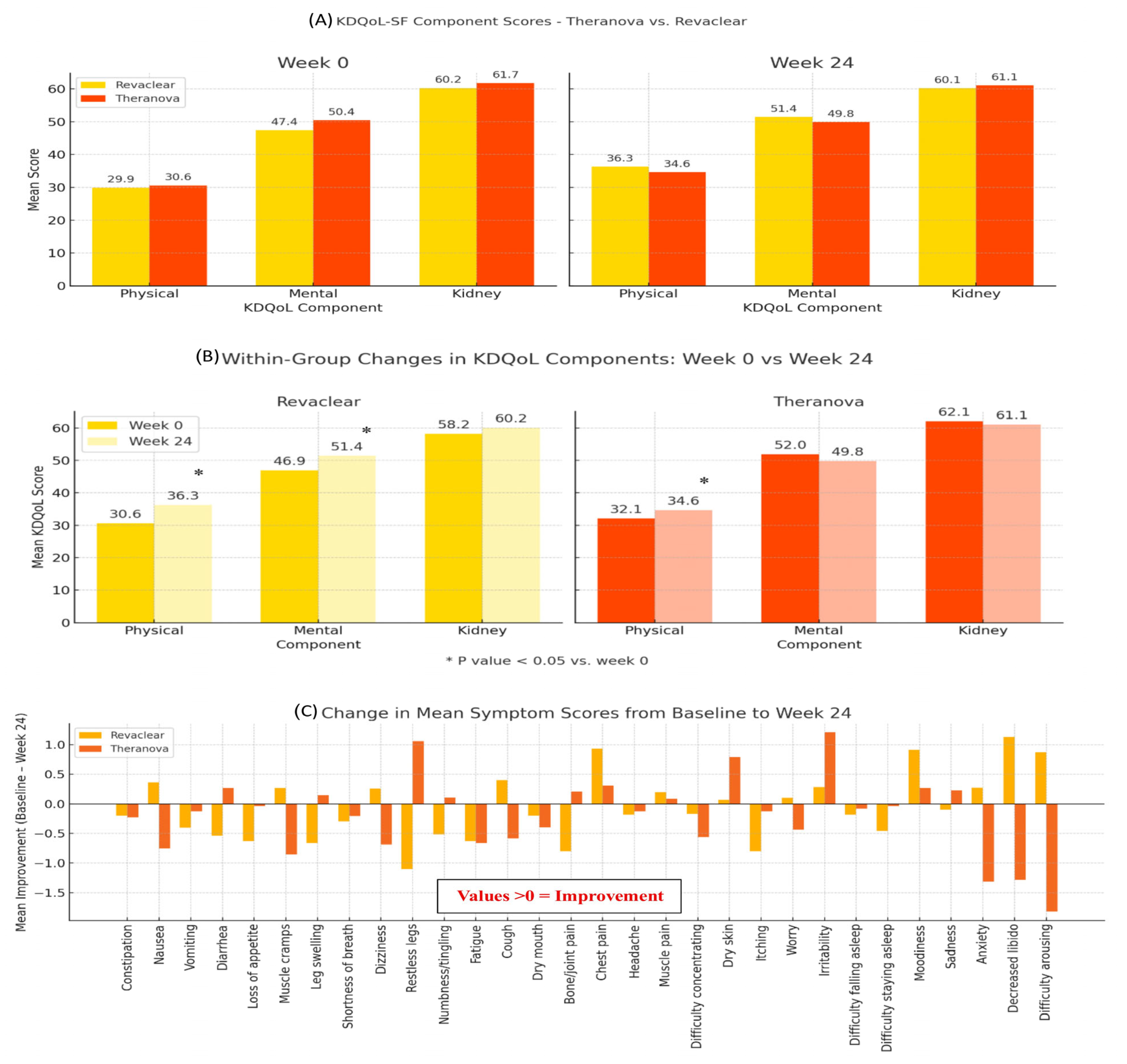
3.4. Quality of Life Assessment
3.5. Safety Monitoring, Hospitalizations and Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jnkowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argile, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.D.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Drueke, T.B. Beta2-microglobulin and Amyloidosis. Nephrol. Dial. Transpl. 2000, 15 (Suppl. S1), 17–24. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J. Mechanism of Endothelial Dysfunction in Chronic Kidney Disease. Clin. Chim. Acta 2010, 411, 1412–1420. [Google Scholar] [CrossRef]
- Cheung, A.K.; Rocco, M.V.; Yan, G.; Leypoldt, J.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.J.; Clark, W.; et al. Serum Beta-2 Microglobulin Levels Predict Mortality in Dialysis Patients: Results of the HEMO Study. J. Am. Soc. Nephrol. 2006, 17, 546–555. [Google Scholar] [CrossRef]
- Locatelli, F.; Marin-Malo, A.; Hannedouche, T.; Loureiro, A.; Papadimitriou, M.; Wizemann, V.; Jacobson, S.H.; Czekalski, S.; Ronco, C.; Vanholder, R. Membrane Permeability Outcome (MPO) Study Group. Effect of Membrane Permeability on Survival of Hemodialysis Patients. J. Am. Soc. Nephrol. 2009, 20, 645–654. [Google Scholar] [CrossRef]
- Eknoyan, G.; Beck, G.J.; Cheung, A.K.; Daugirdas, J.T.; Greene, T.; Kusek, J.W.; Allon, M.; Bailey, J.; Delmez, J.A.; Depner, T.A.; et al. Effect of Dialysis Dose and Membrane Flux in Maintenance Hemodialysis. N. Engl. J. Med. 2002, 347, 2010–2019. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, Y.; Yook, J.M.; Choi, S.Y.; Jung, H.Y.; Choi, J.Y.; Park, S.H.; Kim, C.D.; Kim, Y.L.; Cho, J.H. Randomized Controlled Trial of Medium Cut-Off versus High-Flux Dialyzers on Quality-of-Life Outcomes in Maintenance Hemodialysis Patients. Nat. Sci. Rep. 2020, 10, 7780–7790. [Google Scholar] [CrossRef]
- Bunch, A.; Sanchez, R.; Nilsson, L.G.; Bernardo, A.A.; Vesga, J.I.; Ardila, F.; Guerrerro, I.M.; Sanabria, R.M.; Rivera, A.S. The Colombian Registry of expanded Hemodialysis investigators. Medium Cut-Off Dialyzers in a Large Population of Hemodialysis Patients in Colombia: COREXH Registry. Ther. Apher. Dial. 2021, 25, 33–43. [Google Scholar] [CrossRef]
- Hays, R.D.; Kallich, J.D.; Mapes, D.L.; Coons, S.J.; Amin, N.; Carter, W.B.; Kamberg, C. Kidney Disease Quality of Life Short Form (KDQOL-SF™), Version 1.3: A Manual for Use and Scoring; RAND Health: Santa Monica, CA, USA, 1997. [Google Scholar]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison Of The Removal of Uraemic Toxins With Medium Cut-Off And High-Flux Dialysers: A Randomized Clinical Trial. Nephrol. Dial. Transplant. 2020, 35, 328–335. [Google Scholar] [CrossRef]
- Belmouaz, M.; Diolez, J.; Bauwens, M.; Duthe, F.; Ecotiere, L.; Desport, E.; Bridoux, F. Comparison of Hemodialysis with Medium Cut-Off Dialyzer and Online Hemodiafiltration on the Removal of Small and Middle-Sized Molecules. Clin. Nephrol. 2018, 89, 50–56. [Google Scholar]
- Cho, N.J.; Jeong, S.H.; Lee, K.Y.; Yu, J.Y.; Park, S.; Lee, E.Y.; Gil, H.W. Clinical Safety of Expanded Hemodialysis Compared with Hemodialysis Using High-Flux Dialyzer During a Three-Year Cohort. J. Clin. Med. 2022, 11, 2261–2272. [Google Scholar] [CrossRef]
- Weiner, D.E.; Falzon, L.; Skoufos, L.; Bernardo, A.; Beck, W.; Xiao, M.; Tran, H. Efficacy and Safety of Expanded Hemodialysis with the Theranova 400 Dialyzer. A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1310–1319. [Google Scholar] [CrossRef]
- Cozzolino, M.; Magagnoli, L.; Ciceri, P.; Conte, F.; Galassi, A. Effects if a Medium Cut-Off (Theranova) Dialyser on Haemodialysis Patients: A Prospective, Cross-Over Study. Clin. Kidney J. 2019, 14, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.M.; Jasmin, V.; Rivera, A.; Rutherford, P.; Sanchez, R.; Oliveros, H.; Lindholm, B.; Sanabria, M. Variations in Serum Albumin Levels Over Time in Patients Treated with Conventional Hemodialysis or Expanded Hemodialysis: A Cohort Study. Hemodial. Int. 2025, 29, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Hadad-Arrascue, F.; Nilsson, L.G.; Rivera, A.S.; Bernardo, A.A.; Romero, C.J.B. Expanded Hemodialysis as Effective Alternative to Online Hemodiafiltration: A Randomized Mid-Term Clinical Trial. Ther. Apher. Dial. 2022, 26, 37–44. [Google Scholar] [CrossRef]
- Sahutoglu, T.; Erinc, O.; Avsar, F.N. Theranova Versus FX80: The Impact on Anemia Management in Hemodialysis. Int. J. Artif. Organs 2024, 47, 260–268. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, M.J.; Jeon, J.; Lee, J.E.; Huh, W.; Choi, B.S.; Park, C.W.; Chin, H.J.; Kang, C.L.; Kim, D.K.; et al. Cardiovascular Risk Comparison between Expanded Hemodialysis Using Theranova and Online Hemodiafltration (CARTOON): A Multicenter Randomized Controlled Trial. Nat. Sci. Rep. 2021, 11, 10807–110815. [Google Scholar]
- Bolton, S.; Gair, R.; Nilsson, L.G.; Matthews, M.; Stewart, L.; McCullagh, N. Clinical Assessment of Dialysis Recovery Time and Symptom Burden: Impact of Switching Hemodialysis Therapy Mode. Patient Relat. Outcome Meas. 2021, 12, 315–321. [Google Scholar] [CrossRef]
- Blackowicz, M.J.; Falzon, L.; Beck, W.; Tran, H.; Weiner, D.E. Economic Evaluation of Expanded Hemodialysis with the Theranova 400 Dialyzer: A Post Hoc Evaluation of a Randomized Clinical Trial in the United States. Hemodial. Int. 2022, 26, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ariza, J.A.; Walton, S.M.; Suarez, A.M.; Sanabria, M.; Vesga, J. An Initial Evaluation of Expanded Hemodialysis on Hospitalizations, Drug Utilization, Costs, and Patient Utility in Colombia. Ther. Apher. Dial. 2021, 25, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, R.M.; Hutchison, C.A.; Vesga, J.; Ariza, J.A.; Sanchez, R.; Suarez, A.M. Expanded Hemodialysis and its Effects on Hospitalizations and Medication Usage: A Cohort Study. Nephron 2021, 145, 179–187. [Google Scholar] [CrossRef] [PubMed]
| Dialyzer Type | Dialyzer Membrane | Polymer Membrane Type | Inner Diameter (μm) | Wall Thickness (μm) | Effective Surface Area (m2) | Ultrafiltration Coefficient (mL/h/mmHg) | Priming Volume (mL) | Sterilization Method |
|---|---|---|---|---|---|---|---|---|
| Theranova 500 | Medium cutoff | PAES/PVP, BPA-free | 180 | 35 | 2 | 59 | 105 | Steam |
| Revaclear 500 | High flux | PAES/PVP, BPA-free | 190 | 35 | 2.1 | 65 | 106 | Steam |
| Baseline Characteristic | Theranova 500 (n = 20) | Revaclear 500 (n = 20) | p Value |
|---|---|---|---|
| Age, years (mean ± SD; range) | 65 ± 14 (43–87) | 69 ± 11 (54–84) | 0.44 |
| Hemodialysis vintage, months (mean ± SD; range) | 55 ± 29 (32–87) | 52 ± 29 (21–107) | 0.66 |
| ESRD cause (n,%) | 0.26 | ||
| Diabetic kidney disease | 8 (40) | 12 (60) | |
| Nephrosclerosis | 4 (20) | 2 (10) | |
| Cardiorenal syndrome | 1 (5) | 2 (10) | |
| Other/Unknown | 7 (35) | 4 (20) | |
| Dialysis access type (n,%) | 0.36 | ||
| AVF | 13 (65) | 15 (75) | |
| AVG | 7 (35) | 4 (20) | |
| Permcath | 0 (0) | 1 (5) | |
| Medical history (n,%) | 0.06 | ||
| Diabetes mellitus | 8 (40) | 12 (60) | |
| Hypertension | 15 (75) | 14 (70) | |
| Dyslipidemia | 5 (25) | 8 (40) | |
| Congestive heart failure | 10 (50) | 10 (50) | |
| Coronary artery disease | 5 (25) | 7 (35) | |
| Atrial fibrillation | 9 (45) | 12 (60) |
| Theranova (n = 20) | Revaclear (n = 20) | p Value | |
|---|---|---|---|
| spKt/V (mean ± SD) | |||
| Week 0 | 1.24 ± 0.33 | 1.39 ± 0.32 | 0.213 |
| Week 12 | 1.35 ± 0.27 | 1.44 ± 0.26 | 0.424 |
| Week 24 | 1.4 ± 0.36 | 1.46 ± 0.27 | 0.645 |
| Dry weight, Kg (mean ± SD) | |||
| Week 0 | 75.31 ± 20.93 | 71.34 ± 13.3 | 0.536 |
| Week 12 | 74.04 ± 17.85 | 72 ± 13.54 | 0.743 |
| Week 24 | 73.75 ± 17.49 | 70.31 ± 13.85 | 0.598 |
| UF, L (mean ± SD) | |||
| Week 0 | 2.3 ± 1.48 | 2.39 ± 0.63 | 0.823 |
| Week 12 | 2.11 ± 1.1 | 2.46 ± 0.71 | 0.328 |
| Week 24 | 2.39 ± 1.28 | 2.23 ± 1.01 | 0.74 |
| Theranova (n = 20) | Revaclear (n = 20) | p Value | |
|---|---|---|---|
| Hemoglobin, g/dL (mean ± SD) | |||
| Week 0 | 10.86 ± 1.2 | 10.86 ± 1.22 | 1 |
| Week 12 | 11.04 ± 1.04 | 10.84 ± 0.86 | 0.6 |
| Week 24 | 10.58 ± 1.15 | 11.53 ± 0.84 | 0.03 |
| Ferritin, ng/mL (mean ± SD) | |||
| Week 0 | 672.61 ± 540.33 | 480.47 ± 303.02 | 0.236 |
| Week 12 | 574.31 ± 401.21 | 412.04 ± 239.83 | 0.215 |
| Week 24 | 674.53 ± 415.86 | 681.62 ± 751.8 | 0.977 |
| Transferrin saturation, % (mean ± SD | |||
| Week 0 | 25.82 ± 10.64 | 30.36 ± 16.54 | 0.368 |
| Week 12 | 28.19 ± 8.36 | 22.14 ± 8.65 | 0.084 |
| Week 24 | 29.08 ± 12.52 | 25.2 ± 7.62 | 0.37 |
| Calcium, mg/dL (mean ± SD) | |||
| Week 0 | 8.49 ± 0.85 | 8.49 ± 0.62 | 0.983 |
| Week 12 | 8.34 ± 1 | 8.59 ± 0.62 | 0.455 |
| Week 24 | 8.17 ± 0.69 | 8.64 ± 0.78 | 0.128 |
| Phosphorus, mg/dL (mean ± SD) | |||
| Week 0 | 5.86 ± 1.54 | 6 ± 1.3 | 0.791 |
| Week 12 | 6.66 ± 2.03 | 5.5 ± 0.62 | 0.053 |
| Week 24 | 6.02 ± 1.58 | 5.71 ± 1.29 | 0.596 |
| PTH, pg/mL (mean ± SD) | |||
| Week 0 | 473.04 ± 484.91 | 415.45 ± 327.39 | 0.703 |
| Week 12 | 703.28 ± 894.63 | 438.76 ± 464.86 | 0.343 |
| Week 24 | 769.76 ± 1001.91 | 505.27 ± 598.37 | 0.441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levin Iaina, N.; Rotshild, E.; Mini Goldberg, S.; Beckerman, P. Expanded Hemodialysis with Theranova 500 Improves Dialysis Adequacy and Blunts Inflammation: A 24-Week Quasi-Randomized Trial. J. Clin. Med. 2025, 14, 8853. https://doi.org/10.3390/jcm14248853
Levin Iaina N, Rotshild E, Mini Goldberg S, Beckerman P. Expanded Hemodialysis with Theranova 500 Improves Dialysis Adequacy and Blunts Inflammation: A 24-Week Quasi-Randomized Trial. Journal of Clinical Medicine. 2025; 14(24):8853. https://doi.org/10.3390/jcm14248853
Chicago/Turabian StyleLevin Iaina, Nomy, Elena Rotshild, Sharon Mini Goldberg, and Pazit Beckerman. 2025. "Expanded Hemodialysis with Theranova 500 Improves Dialysis Adequacy and Blunts Inflammation: A 24-Week Quasi-Randomized Trial" Journal of Clinical Medicine 14, no. 24: 8853. https://doi.org/10.3390/jcm14248853
APA StyleLevin Iaina, N., Rotshild, E., Mini Goldberg, S., & Beckerman, P. (2025). Expanded Hemodialysis with Theranova 500 Improves Dialysis Adequacy and Blunts Inflammation: A 24-Week Quasi-Randomized Trial. Journal of Clinical Medicine, 14(24), 8853. https://doi.org/10.3390/jcm14248853






