1. Introduction
Triple negative breast cancer (TNBC) continues to pose significant challenges for treatment, in the perioperative as well as the metastatic setting [
1]. Despite progress in chemotherapy protocols such as the neo-adjuvant treatments, the implementation of dose-dense anthracyclines, and the incorporation of carboplatin, outcomes have still fallen short compared to other breast cancer types, particularly hormone receptor positive and HER2 positive disease [
2,
3,
4]. This issue, combined with mounting evidence suggesting that TNBCs are more immunogenic, has resulted in several phase II and III clinical trials involving early TNBC patients, in which immunotherapeutics were associated with cytotoxic chemotherapy [
5,
6].
In recent years, pembrolizumab combined with chemotherapy has become a standard treatment for advanced PD-L1 positive TNBC and has also been included as part of neoadjuvant treatment for high-risk early TNBC, following the results of KEYNOTE-522 phase III clinical trial [
7]. However, even with the practice-changing data brought about by KEYNOTE-522, crucial questions persist concerning the ideal candidate selection and the prediction of outstanding responders to chemoimmunotherapy [
8,
9]. The pathologic complete response (pCR) rate in TNBC patients receiving only chemotherapy is around 30–50%, something suggesting that a significant portion of patients could have achieved a pCR without the additional side effects of immunotherapy [
10]. Thus, the identification of predictive biomarkers remains essential for directing patient selection, enhancing clinical results, and reducing the risk of chemoimmunotherapy adverse effects [
11]. In fact, it is still unclear if specific patients undergoing neoadjuvant chemoimmunotherapy for TNBC may receive greater advantages from this treatment [
12,
13]. Since
BRCA-mutated tumors display homologous recombination deficiency (HRD) and high genomic instability, they have been suggested to be more immunogenic and sensitive to immune checkpoint inhibitors. Based on these premises, we sought to evaluate the association between
BRCA1/2 mutations and pCR in a real-world cohort of TNBC patients treated with pembrolizumab-based neoadjuvant chemotherapy (NACT). The present analysis aimed to investigate the association between BRCA1/2 mutational status and pathologic complete response (pCR) in a real-world multicenter cohort of patients with triple-negative breast cancer (TNBC) treated with pembrolizumab-based neoadjuvant chemotherapy.
2. Materials and Methods
2.1. Study Population
This retrospective multicenter study included 184 consecutive patients with stage II–III TNBC treated between January 2021 and April 2024 across eleven Italian oncology centres. Eligible patients were aged ≥ 18 years and had histologically confirmed TNBC, defined as estrogen receptor (ER) and progesterone receptor (PR) expression < 1% and HER2 0–1+ or 2+ with negative in situ hybridization (ISH). Patients with de novo metastatic disease, pure metaplastic histology, prior systemic therapy for breast cancer, or missing pathological data were excluded.
All patients received pembrolizumab-based therapy following the KEYNOTE-522 regimen, which represents the current standard of care for high-risk early-stage TNBC after demonstrating a significant improvement in pCR (64.8% vs. 51.2%) and event-free survival in the phase III trial. The regimen consisted of pembrolizumab (200 mg every 3 weeks or 400 mg every 6 weeks) in combination with paclitaxel (80 mg/m2 weekly) and carboplatin (AUC 5 every 3 weeks or AUC 1.5 weekly) for 12 weeks, followed by doxorubicin (60 mg/m2) or epirubicin (90 mg/m2) with cyclophosphamide (600 mg/m2) every 3 weeks for four cycles. Surgery was performed 3–6 weeks after completion of neoadjuvant therapy, and adjuvant pembrolizumab was continued to complete one year of therapy whenever feasible, according to institutional practice and national guidelines.
Germline BRCA1 and BRCA2 mutational analyses were performed on peripheral blood DNA using next-generation sequencing (NGS) hereditary cancer panels validated for clinical use. Variant classification followed the ACMG/AMP 2015 criteria for the interpretation of sequence variants [
14]. Only pathogenic or likely pathogenic variants were considered BRCA-mutated, whereas variants of uncertain significance (VUS) or benign variants were categorized as wild-type, consistent with current practice in clinical and translational BRCA-focused analyses. Testing was conducted in certified molecular pathology laboratories at each participating institution, ensuring standardized procedures for variant interpretation and reporting.
2.2. Study Endpoints
The primary endpoint was the rate of pCR according to BRCA1/2 mutational status. Pathologic complete response (pCR) was defined as ypT0/is ypN0, indicating the absence of residual invasive carcinoma in both breast and axillary lymph nodes, irrespective of residual in situ disease. Pathological evaluation was performed by institutional breast pathologists following international recommendations for post-neoadjuvant specimen assessment. Clinical and pathological data were retrospectively extracted from institutional electronic medical records and entered into a centralized anonymized database. Collected variables included demographic characteristics (age, menopausal status, body mass index), tumor features (clinical stage per AJCC, histologic grade, Ki-67 index, and tumor-infiltrating lymphocytes), and BRCA1/2 mutational status.
2.3. Statistical Analysis
Continuous variables were expressed as medians and interquartile ranges (IQR), and categorical variables as frequencies and percentages. Comparisons between groups (BRCA1-mutated, BRCA2-mutated, and wild-type) were performed using the Fisher’s exact test or χ2 test, as appropriate. Proportions were reported with 95% confidence intervals (CI) calculated by the Wilson method, which provides accurate interval estimation for binomial proportions in moderately sized samples. A two-sided p-value < 0.05 was considered statistically significant.
To improve interpretability and align with prior real-world series, BRCA1 and BRCA2 mutations were also analyzed together as a combined “BRCA-mutated” cohort, acknowledging the limited frequency of BRCA2 alterations in TNBC.
To explore clinical and pathological predictors of pCR, a univariate and multivariate logistic regression analysis was performed including the following covariates: age (continuous), clinical stage (II vs. III), ECOG performance status (0 vs. ≥1), body-mass index (BMI, continuous), tumor-infiltrating lymphocytes (TILs ≥ 30% vs. <30%) [
14], and BRCA mutational status (mutated vs. wild-type). Variables with a
p < 0.10 in univariate analysis were subsequently entered into the multivariate model. Odds ratios (OR) and 95% confidence intervals (CI) were estimated. A two-sided
p < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics v29.0 (IBM Corp., Armonk, NY, USA).
The study was approved by the Comitato Etico Territoriale Regione Puglia—Azienda Ospedaliero-Universitaria “Consorziale Policlinico” (study code 7889) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained when required by national and institutional regulations.
3. Results
A total of 184 patients with stage II–III TNBC treated with pembrolizumab-based neoadjuvant chemotherapy were included in this analysis. All patients were enrolled consecutively between January 2021 and April 2024 across eleven Italian oncology centers. The baseline clinical and pathological characteristics of the entire cohort are summarized in
Table 1.
The median age at diagnosis was 48 years (range 42–55), and most of patients (n = 112, 60.9%) were pre-menopausal. The median body-mass index (BMI) was 24.6 kg/m2 (range 22.1–27.5). With respect to functional status, most patients had ECOG 0 (n = 152, 82.6%), while 32 patients (17.4%) presented with an ECOG performance status ≥ 1.
Regarding disease stage, 119 patients (64.7%) were classified as stage II, and 65 (35.3%) as stage III. Most tumors were high-grade (grade 3) lesions (n = 156, 84.8%). The median Ki-67 proliferation index was 70%, reflecting the typically high proliferative activity of TNBC.
The tumor microenvironment was characterized by variable immune infiltration. The median percentage of tumor-infiltrating lymphocytes (TILs) was 28% (range 15–45), and 82 patients (44.6%) had high TILs (≥30%), whereas 102 (55.4%) showed low infiltration (<30%). A total of 137 patients (74.5%) had experienced at least one pregnancy before diagnosis.
Comorbid conditions were documented in 86 patients (46.7%). Among these, thyroid disorders were the most frequent (21 patients, 11.4%), followed by autoimmune or inflammatory diseases (12 patients, 6.5%). Other comorbidities included hypertension (5, 2.7%), gastrointestinal disorders (4, 2.2%), hepatic disease (2, 1.1%), and single cases of cardiovascular, respiratory, renal, or psychiatric disorders (each 0.5%).
Germline BRCA testing was performed in all patients. Overall, 37 patients (20.1%) carried a pathogenic BRCA1/2 variant, including 25 BRCA1 (13.6%) and 12 BRCA2 (6.5%), while 147 patients (79.9%) were wild-type. The distribution of age, stage, and TIL levels was similar across BRCA-mutated and wild-type groups, with no significant baseline imbalances.
All patients received neoadjuvant treatment according to the KEYNOTE-522 regimen, consisting of pembrolizumab combined with platinum- and taxane-based chemotherapy followed by anthracycline and cyclophosphamide. Surgery was performed 3–6 weeks after the completion of chemotherapy, followed by adjuvant pembrolizumab administration in eligible patients according to institutional practice.
The overall pathologic complete response (pCR) rate in the study cohort was 63.6% (117 of 184 patients). Pathologic response data were available for all patients.
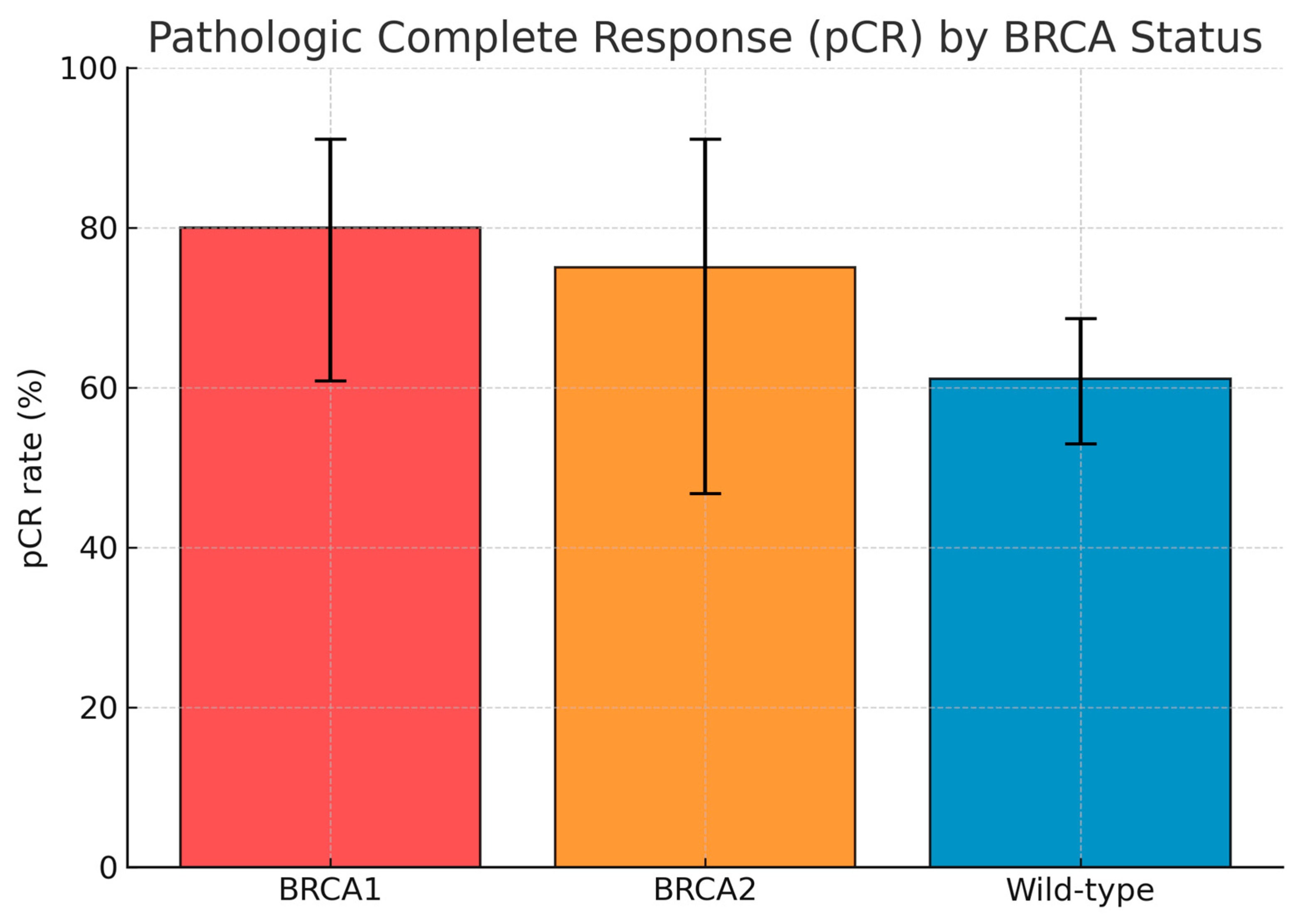
By subgroup, pCR was achieved in 20 of 25 BRCA1-mutated cases (80.0%; 95% CI 60.9–91.1), 9 of 12 BRCA2-mutated cases (75.0%; 95% CI 46.8–91.1), and 88 of 144 BRCA wild-type cases (61.1%; 95% CI 53.0–68.7). When BRCA1 and BRCA2 carriers were considered together, the combined BRCA1/2-mutated group achieved a pCR rate of 78.4% (95% CI 62.8–88.6), compared with 61.1% (95% CI 53.0–68.7) in the wild-type population. The difference approached statistical significance (p = 0.056, Fisher’s exact test).
Among the 67 patients (36.4%) who did not achieve pCR, 47 (25.5%) had residual invasive disease confined to the breast, while 20 (10.9%) presented with residual involvement of both breast and axillary lymph nodes. The pattern of residual disease did not differ substantially between BRCA-mutated and wild-type patients. The distribution of pCR outcomes according to BRCA1, BRCA2, and wild-type subgroups is summarized in
Table 2 and depicted graphically in
Figure 1, which shows the relative pCR proportions with 95% confidence intervals for each subgroup; pCR according to BRCA status were reported in
Table 3.
In the univariate Cox model, BRCA mutations (OR 2.26; 95% CI, 1.00–5.12;
p = 0.049) and high TILs (OR 1.98; 95% CI, 1.05–3.75;
p = 0.034) were significantly associated with pCR; BRCA status was also associated with pCR in the multivariate model (
Table 4) (OR 2.17; 95% CI, 1.01–4.97;
p = 0.048).
4. Discussion
The introduction of pembrolizumab into the neoadjuvant setting has marked a turning point in the management of early-stage TNBC. Through this multicenter, retrospective analysis, we aimed to explore whether germline BRCA1/2 mutations could influence pCR to pembrolizumab-based NACT in everyday clinical practice. In our experience, BRCA-mutated tumors achieved numerically higher pCR rates compared with wild-type cases (78.4% vs. 61.1%), supporting a potential biological link between HRD and enhanced sensitivity to chemo-immunotherapy.
The overall pCR rate observed in our cohort (63.6%) closely mirrors the results of the KEYNOTE-522 trial (64.8%) and aligns with other real-world studies reporting rates between 45% and 65% [
15,
16]. Within this context, the apparent advantage among BRCA-mutated patients offers a clinically relevant observation. In our logistic regression model, BRCA mutations were associated with higher odds of achieving pCR (OR = 2.17; 95% CI 1.01–4.97;
p = 0.056), reflecting a trend toward statistical significance. While this result does not meet conventional significance thresholds, we believe it remains biologically plausible given the well-known immunogenic profile of BRCA-deficient TNBC. These tumors typically display greater genomic instability, higher TIL levels, and increased neoantigen load factors that may contribute to heightened sensitivity to immune checkpoint blockade. In keeping with this hypothesis, high TILs (≥30%) were also associated with higher pCR rates in our cohort, in line with previous data underscoring immune activation as a predictive marker for ICI efficacy [
17,
18,
19,
20,
21].
Several aspects of our analysis deserve careful interpretation. The relatively small number of BRCA2-mutated patients (n = 12) limits the power to discern distinct effects between BRCA1 and BRCA2. For this reason, we opted to analyze BRCA1/2 carriers as a combined group, though we acknowledge that this approach may have concealed potential subtype-specific differences. Moreover, as this was a retrospective study, granular data on dose intensity, treatment discontinuation, and immune-related adverse events were not systematically captured. In our clinical experience, some degree of treatment modulation is frequent in real-world settings, especially in younger patients balancing aggressive regimens with tolerability. These aspects, however, could not be formally evaluated here. Finally, survival outcomes such as EFS or OS were not assessed, as follow-up remains immature, and toxicity data were not reported. Future longitudinal analyses will help clarify whether the numerical advantage in pCR observed among BRCA-mutated patients translates into meaningful survival benefits.
In addition, we acknowledge some potentially relevant factors have not been included in some analysis, such as tumor size, nodal status, chemotherapy dose intensity, treatment delays, and PD-L1 expression. We are aware that the lack of some factors may have introduced some bias. Moreover, since toxicity, dose adjustment, and immune-related adverse events may have a strong influence on neoadjuvant therapy response, all these missing data may have biased pCR outcomes.
Despite these limitations, this study has important strengths. It represents one of the few real-world, multicenter efforts to address the relationship between BRCA status and immunotherapy response in TNBC. The inclusion of eleven Italian oncology centers reflects the collaborative nature of contemporary oncologic practice, where shared data can illuminate subtle biological and clinical patterns not easily discerned in single-institution experiences. The BRCA mutation frequency observed in our cohort (20.1%) closely parallels that of major TNBC series, reinforcing the representativeness and external validity of our population.
From a clinical standpoint, our findings highlight the importance of integrating germline BRCA testing into the neoadjuvant treatment pathway for TNBC. Identifying BRCA-mutated patients at diagnosis not only refines prognostic assessment but also facilitates a more informed discussion with patients regarding the potential for enhanced chemo-immunotherapy responsiveness. In our daily clinical practice, we often observe that BRCA-mutated TNBC exhibits a more rapid and profound response to systemic therapy. Nevertheless, the interplay between BRCA status, immune infiltration, and PD-L1 expression warrants deeper molecular investigation in prospective studies.
In conclusion, BRCA1/2-mutated TNBC patients treated with pembrolizumab-based NACT demonstrated a trend toward higher pCR rates compared with wild-type counterparts. These observations suggest that HRD may enhance both chemosensitivity and immune responsiveness.
5. Conclusions
In this multicenter real-world analysis, BRCA1/2-mutated TNBC showed numerically higher pCR rates with pembrolizumab-based NACT compared with wild-type tumors, suggesting enhanced chemosensitivity and immune responsiveness in BRCA-deficient disease. These findings underline the clinical value of BRCA testing to refine risk stratification and treatment decision-making in the neoadjuvant setting and further highlight the importance and potential clinical implications of BRCA testing in early TNBC, the role of BRCA status in neoadjuvant treatment decisions, and the need for prospective clinical trials that could validate these results.
Author Contributions
Conceptualization, P.F. and D.B. methodology, S.L.S. and A.G.; software, M.L.; validation, P.F. and F.F.; formal analysis, A.L. and A.T.; investigation, M.M.; resources, F.G.; data curation, P.F. and L.L.; writing—original draft preparation, P.F., and A.R.; writing—review and editing, A.M. and A.R.; visualization, G.G.-C. and L.M.; supervision, A.R. and G.C.; project administration, R.A.; funding acquisition, P.F. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This retrospective, multicenter study was conducted in accordance with the Declaration of Helsinki and approved by the Territorial Ethics Committee of Regione Puglia—Azienda Ospedaliero-Universitaria “Consorziale Policlinico” (study code 7889, date of approval: 30 July 2025).
Informed Consent Statement
Where feasible at participating centers, written informed consent was obtained from patients prior to data use. In cases where this was not possible (e.g., deceased patients or those who could not be contacted), consent was waived by the Institutional Review Board. All data used were fully anonymized and no individual, identifiable patient information is reported.
Data Availability Statement
Due to the retrospective design and anonymization, the data are not shared publicly to protect patient privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harbeck, N. Neoadjuvant and adjuvant treatment of patients with HER2-positive early breast cancer. Breast 2022, 62 (Suppl. S1), S12–S16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Rourke, H.; Hart, C.; De Boer, R.H. Current usage of pembrolizumab in triple negative breast cancer (TNBC). Expert Rev. Anticancer. Ther. 2024, 24, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Leon-Ferre, R.A.; Goetz, M.P. Advances in systemic therapies for triple negative breast cancer. BMJ 2023, 381, e071674. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kümmel, S.; Bergh, J.; Park, Y.H.; Hui, R.; et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: Exploratory analysis from KEYNOTE-522. Ann. Oncol. 2024, 35, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kümmel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Connors, C.; Valente, S.A.; ElSherif, A.; Escobar, P.; Chichura, A.; Kopicky, L.; Roesch, E.; Ritner, J.; McIntire, P.; Wu, Y.; et al. Real-World Outcomes with the KEYNOTE-522 Regimen in Early-Stage Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2025, 32, 912–921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Downs-Canner, S.; Mittendorf, E.A. Preoperative Immunotherapy Combined with Chemotherapy for Triple-Negative Breast Cancer: Perspective on the KEYNOTE-522 Study. Ann. Surg. Oncol. 2023, 30, 3166–3169, Erratum in Ann. Surg. Oncol. 2023, 30, 3286. https://doi.org/10.1245/s10434-023-13448-w. PMID: 36897418.. [Google Scholar] [CrossRef]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Pinto, L.; Almeida-Santos, T. Efficacy of different neoadjuvant treatment regimens in BRCA-mutated triple negative breast cancer: A systematic review and meta-analysis. Hered. Cancer Clin. Pract. 2022, 20, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonadio, R.C.; Tarantino, P.; Testa, L.; Punie, K.; Pernas, S.; Barrios, C.; Curigliano, G.; Tolaney, S.M.; Barroso-Sousa, R. Management of patients with early-stage triple-negative breast cancer following pembrolizumab-based neoadjuvant therapy: What are the evidences? Cancer Treat. Rev. 2022, 110, 102459. [Google Scholar] [CrossRef] [PubMed]
- Leon-Ferre, R.A.; Jonas, S.F.; Salgado, R.; Loi, S.; de Jong, V.; Carter, J.M.; Nielsen, T.O.; Leung, S.; Riaz, N.; Chia, S.; et al. Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. JAMA 2024, 331, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mezzanotte-Sharpe, J.; Hsu, C.Y.; Choi, D.; Sheffield, H.; Zelinskas, S.; Proskuriakova, E.; Montalvo, M.; Lee, D.S.; Whisenant, J.G.; Gaffney, K.; et al. Adverse events in patients treated with neoadjuvant chemo/immunotherapy for triple negative breast cancer: Results from seven academic medical centers. Breast Cancer Res. Treat. 2025, 213, 71–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrade, M.O.; Gutierres, I.G.; Tavares, M.C.; de Sousa, I.M.; Balint, F.C.; Marin Comini, A.C.; Gouveia, M.C.; Bines, J.; Madasi, F.; Ferreira, R.D.P.; et al. Immune-related adverse events among patients with early-stage triple-negative breast cancer treated with pembrolizumab plus chemotherapy: Real-world data from the Neo-Real/GBECAM 0123 study. Breast 2025, 83, 104473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, P.; Stecklein, S.R.; Yoder, R.; Staley, J.M.; Schwensen, K.; O’Dea, A.; Nye, L.; Satelli, D.; Crane, G.; Madan, R.; et al. Clinical and Biomarker Findings of Neoadjuvant Pembrolizumab and Carboplatin Plus Docetaxel in Triple-Negative Breast Cancer: NeoPACT Phase 2 Clinical Trial. JAMA Oncol. 2024, 10, 227–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Grazia, G.; Dri, A.; Grieco, A.; Martinelli, C.; Palleschi, M.; Martorana, F.; Barchiesi, G.; Arpino, G.; De Angelis, C.; De Laurentiis, M.; et al. Unlocking the Potential of Immune Checkpoint Inhibitors in HR+/HER2- Breast Cancer: A Systematic Review. Cancers 2025, 17, 2940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, A.Y.; Shiao, S.; Kobald, S.A.; Chen, J.; Duda, D.G.; Ly, A.; Bossuyt, V.; Cho, H.L.; Arnold, B.; Knott, S.; et al. PEARL: A Phase Ib/II Biomarker Study of Adding Radiation Therapy to Pembrolizumab Before Neoadjuvant Chemotherapy in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. J. Clin. Oncol. 2024, 42, 4282–4293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tinterri, C.; Di Maria Grimaldi, S.; Sagona, A.; Barbieri, E.; Darwish, S.; Bottini, A.; Canavese, G.; Gentile, D. Comparison of Long-Term Oncological Results in Young Women with Breast Cancer between BRCA-Mutation Carriers Versus Non-Carriers: How Tumor and Genetic Risk Factors Influence the Clinical Prognosis. Cancers 2023, 15, 4177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).