AI vs. MD: Benchmarking ChatGPT and Gemini for Complex Wound Management
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LLMs | Large Language Models |
| TIME | Tissue, Infection/Inflammation, Moisture balance, Edge |
| TIME CDST | TIME Clinical Decision Support Tool |
| TIME-H | TIME Health model |
| NPWT | Negative Pressure Wound Therapy |
| AI | Artificial Intelligence |
| MRI | Magnetic Resonance Imaging |
| USD | United States Dollar |
References
- Swanson, T.; Ousey, K.; Haesler, E.; Bjarnsholt, T.; Carville, K.; Idensohn, P.; Kalan, L.; Keast, D.H.; Larsen, D.; Percival, S.; et al. IWII Wound Infection in Clinical Practice consensus document: 2022 update. J. Wound Care 2022, 31, S10–S21. [Google Scholar] [CrossRef] [PubMed]
- Ivory, J.D.; Vellinga, A.; O’Gara, J.; Gethin, G. A scoping review protocol to identify clinical signs, symptoms and biomarkers indicative of biofilm presence in chronic wounds [version 2]. HRB Open Res. 2021, 4, 1–5. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janowska, A.; Dini, V.; Oranges, T.; Iannone, M.; Loggini, B.; Romanelli, M. Atypical Ulcers: Diagnosis and Management. Clin. Interv. Aging 2019, 14, 2137–2143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hongying, Z.; Chunmei, H.; Lijuan, C.; Miao, T.; Jingying, X.; Hongmei, J.; Jing, H.; Yang, H.; Min, Y. The current status and influencing factors of quality of life of chronic wound patients based on Wound-QoL scale: A cross-sectional study. Medicine 2025, 104, e42961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sen, C.K. Human Wound and Its Burden: Updated 2025 Compendium of Estimates. Adv. Wound Care 2025, 14, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, D.O.; Tettelbach, W.H.; Ciprandi, G.; Downie, F.; Hampton, J.; Hodgson, H.; Lazaro-Martinez, J.L.; Probst, A.; Schultz, G.; Stürmer, E.K.; et al. Best practice for wound debridement. J. Wound Care 2024, 33, S1–S32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, S.; Wang, H.; Wang, Y. Biofilm therapy for chronic wounds. Int. Wound J. 2024, 21, e14667. [Google Scholar] [CrossRef]
- Beraja, G.E.; Gruzmark, F.; Pastar, I.; Lev-Tov, H. What’s New in Wound Healing: Treatment Advances and Microbial Insights. Am. J. Clin. Dermatol. 2025, 26, 677–694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falanga, V.; Isaacs, C.; Paquette, D.; Downing, G.; Kouttab, N.; Butmarc, J.; Badiavas, E.; Hardin-Young, J. Wounding of bioengineered skin: Cellular and molecular aspects after injury. J. Investig. Dermatol. 2002, 119, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Moore, Z.; Dowsett, C.; Smith, G.; Atkin, L.; Bain, M.; Lahmann, N.A.; Schultz, G.S.; Swanson, T.; Vowden, P.; Weir, D.; et al. TIME CDST: An updated tool to address the current challenges in wound care. J. Wound Care 2019, 28, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Guarro, G.; Cozzani, F.; Rossini, M.; Bonati, E.; Del Rio, P. The modified TIME-H scoring system, a versatile tool in wound management practice: A preliminary report. Acta Biomed. 2021, 92, e2021226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, S.; Lay, B.; Johnson, A.R., Jr. Artificial Intelligence in Skin and Wound Care: Enhancing Diagnosis and Treatment With Large Language Models. Adv. Ski. Wound Care 2025, 38, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Schellaert, W.; Martínez-Plumed, F.; Moros-Daval, Y.; Ferri, C.; Hernández-Orallo, J. Larger and more instructable language models become less reliable. Nature 2024, 634, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pressman, S.M.; Borna, S.; Gomez-Cabello, C.A.; Haider, S.A.; Haider, C.R.; Forte, A.J. Clinical and Surgical Applications of Large Language Models: A Systematic Review. J. Clin. Med. 2024, 13, 3041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, W.; Zhang, Y.; Wu, Z.; Lepp, H.; Ji, W.; Zhao, X.; Cao, H.; Liu, S.; He, S.; Huang, Z.; et al. Quantifying large language model usage in scientific papers. Nat. Hum. Behav. 2025, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, G.; Corradini, L.; Shadid, O.; Seth, I.; Rozen, W.M.; Grimaldi, L.; Cuomo, R. From Prompts to Practice: Evaluating ChatGPT, Gemini, and Grok Against Plastic Surgeons in Local Flap Decision-Making. Diagnostics 2025, 15, 2646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sallam, M. ChatGPT Utility in Healthcare Education, Research, and Practice: Systematic Review on the Promising Perspectives and Valid Concerns. Healthcare 2023, 11, 887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asgari, E.; Montaña-Brown, N.; Dubois, M.; Khalil, S.; Balloch, J.; Yeung, J.A.; Pimenta, D. A framework to assess clinical safety and hallucination rates of LLMs for medical text summarisation. npj Digit. Med. 2025, 8, 274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, Y.; Ma, W.; Sivarajkumar, S.; Zhang, H.; Sadhu, E.M.; Li, Z.; Wu, X.; Visweswaran, S.; Wang, Y. Mitigating the risk of health inequity exacerbated by large language models. npj Digit. Med. 2025, 8, 246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.S.; Baek, S.J.; Ryu, H.S.; Choo, J.M.; Cho, E.; Kwak, J.M.; Kim, J. Using large language models for clinical staging of colorectal cancer from imaging reports: A pilot study. Ann. Surg. Treat. Res. 2025, 109, 318–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zada, T.; Tam, N.; Barnard, F.; Van Sittert, M.; Bhat, V.; Rambhatla, S. Medical Misinformation in AI-Assisted Self-Diagnosis: Development of a Method (EvalPrompt) for Analyzing Large Language Models. JMIR Form. Res. 2025, 9, e66207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seth, I.; Marcaccini, G.; Lim, B.; Novo, J.; Bacchi, S.; Cuomo, R.; Ross, R.J.; Rozen, W.M. The Temporal Evolution of Large Language Model Performance: A Comparative Analysis of Past and Current Outputs in Scientific and Medical Research. Informatics 2025, 12, 86. [Google Scholar] [CrossRef]
- Reifs Jiménez, D.; Casanova-Lozano, L.; Grau-Carrión, S.; Reig-Bolaño, R. Artificial Intelligence Methods for Diagnostic and Decision-Making Assistance in Chronic Wounds: A Systematic Review. J. Med. Syst. 2025, 49, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salbas, A.; Buyuktoka, R.E. Performance of Large Language Models in Recognizing Brain MRI Sequences: A Comparative Analysis of ChatGPT-4o, Claude 4 Opus, and Gemini 2.5 Pro. Diagnostics 2025, 15, 1919. [Google Scholar] [CrossRef]
- Abdul Sami, M.; Abdul Samad, M.; Parekh, K.; Suthar, P.P. Comparative Accuracy of ChatGPT 4.0 and Google Gemini in Answering Pediatric Radiology Text-Based Questions. Cureus 2024, 16, e70897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aljindan, F.K.; Shawosh, M.H.; Altamimi, L.; Arif, S.; Mortada, H. Utilization of ChatGPT-4 in Plastic and Reconstructive Surgery: A Narrative Review. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez-Cabello, C.A.; Borna, S.; Pressman, S.M.; Haider, S.A.; Forte, A.J. Large Language Models for Intraoperative Decision Support in Plastic Surgery: A Comparison between ChatGPT-4 and Gemini. Medicina 2024, 60, 957. [Google Scholar] [CrossRef] [PubMed]
- Soenksen, L.R.; Kassis, T.; Conover, S.T.; Marti-Fuster, B.; Birkenfeld, J.S.; Tucker-Schwartz, J.; Naseem, A.; Stavert, R.R.; Kim, C.C.; Senna, M.M.; et al. Using deep learning for dermatologist-level detection of suspicious pigmented skin lesions from wide-field images. Sci. Transl. Med. 2021, 13, eabb3652. [Google Scholar] [CrossRef] [PubMed]
- Daneshjou, R.; Smith, M.P.; Sun, M.D.; Rotemberg, V.; Zou, J. Lack of Transparency and Potential Bias in Artificial Intelligence Data Sets and Algorithms: A Scoping Review. JAMA Dermatol. 2021, 157, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Case ID | Clinical Case | ChatGPT—Wound Description | ChatGPT—Suggested Management | Gemini—Wound Description | Gemini—Suggested Management | Surgeons’ Consensus on Wound Description | Surgeons’ Consensus on Recommended Management |
|---|---|---|---|---|---|---|---|
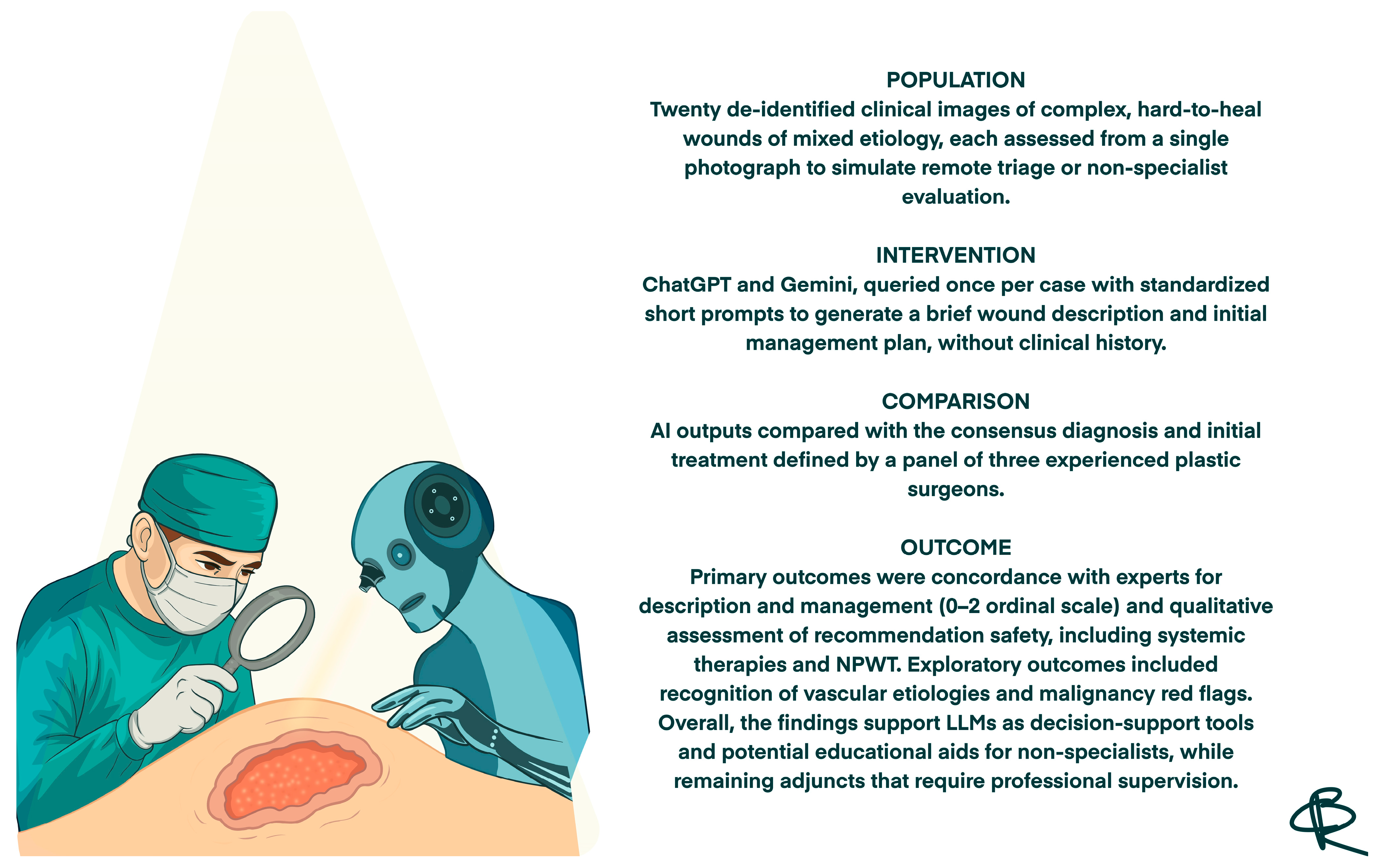
| 1 | 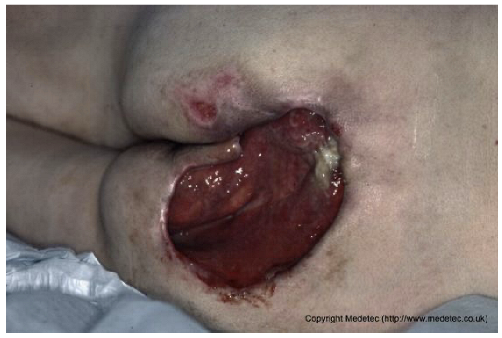 | Large sacral pressure ulcer with exposed subcutaneous tissue and muscle, showing moist red granulation tissue mixed with areas of fibrinous slough. The wound edges are irregular and macerated, with surrounding erythema and a smaller superficial lesion superiorly. | Alginate or hydrofiber dressing with secondary foam cover; initiate pressure relief, wound debridement and/or NPWT as needed. | The image displays a large, deep wound located over the gluteal/sacral region. The wound bed appears to be filled with exposed, deep red, viable tissue (likely muscle/fat) and exhibits significant tissue loss, consistent with a full-thickness injury. There is minimal surrounding erythema, and some slough/exudate is visible in the wound base. | Wound management should involve debridement if necessary, followed by a deeply absorptive dressing (e.g., foam or alginate) or negative pressure wound therapy (NPWT) to promote granulation and manage high exudate, along with infection control. | Extensive stage IV pressure ulcer with muscular exposure involving the sacral and left gluteal regions. The wound bed is exudative, showing evident granulation tissue with a limited area of fibrin deposition in the cranial portion. Wound edges are irregular, undermined, and slightly macerated. The perilesional skin appears edematous. On the right sacral area, a smaller stage III pressure ulcer is also present. | Surgical debridement of the limited area of fibrin deposition, protease-modulating matrix and foam; alternatively, NPWT. Pressure-relieving devices and alternative patient repositioning. |
| 2 |  | Deep sacral ulcer with central necrotic slough and purulent exudate, surrounded by erythematous and inflamed skin. The wound margins are irregular and macerated, with signs of local infection. | Debridement of necrotic tissue, application of hydrofiber or alginate dressing with antimicrobial properties, and consistent pressure off-loading. | The image shows a wound in the sacral/gluteal region with surrounding intense erythema and inflammation. The wound bed contains a significant amount of thick, yellow-brown slough/necrotic tissue and purulent exudate. The wound appears to be a full-thickness injury with clear signs of local infection/inflammation (cellulitis). | Aggressive management for presumed infection is required, including systemic antibiotics and urgent surgical or sharp debridement to remove the necrotic tissue. Topical management should include an antimicrobial dressing (e.g., silver) or antiseptic cleanser/gel. | Extensive pressure ulcer of probable stage III–IV etiology, located in the coccygeal region. The wound bed is exudative, with abundant moist necrotic tissue and slough. The presence of an infectious component cannot be excluded. Wound margins are irregular, exhibiting an erythematous peripheral halo. The perilesional skin shows extensive and marked erythema, likely of infectious or inflammatory origin. | Perform a wound culture swab. Surgical debridement of the necrotic tissue and slough down to a well-vascularized tissue; hydrofiber or antimicrobial dressing (i.e., silver containing dressing) and foam. Evaluate the administration of systemic antibiotics Pressure-relieving devices and alternative patient repositioning. |
| 3 | 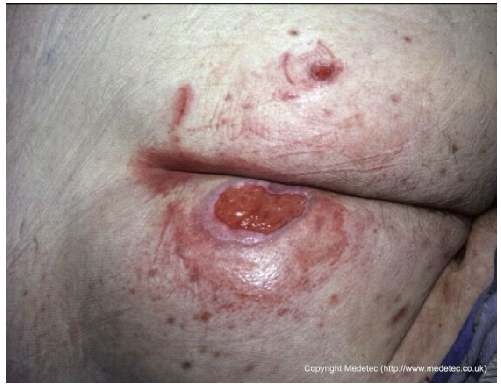 | Shallow sacral ulcer with clean red granulation tissue and minimal exudate, surrounded by erythematous, mildly macerated skin. Wound edges are well defined without visible necrosis. | Hydrocolloid or foam dressing to maintain a moist environment, with regular repositioning and skin protection from friction and moisture. | The image shows a partial-thickness wound over the gluteal/sacral region with a clean, granulating red base and no visible slough or necrosis. The periwound skin is intensely erythematous, macerated/denuded, and excoriated, suggesting significant moisture-associated skin damage (MASD) or incontinence-associated dermatitis (IAD). The primary wound is small and shallow with clear margins. | Manage periwound moisture-associated skin damage (MASD) with a skin barrier cream/ointment (or liquid film). Apply a hydrocolloid or thin foam dressing to the main wound to maintain a moist environment for healing. | Right sacral ulcer, likely stage III pressure-related, oval in shape with an approximate diameter of 5 cm. The wound bed appears clean, granulating, and with minimal clear exudate. Wound margins are well-defined and display a pinkish-violet hue, suggestive of re-epithelialization. The perilesional skin is erythematous, with evidence of intertrigo along the intergluteal fold. A similar ulcer, though smaller in size, is present in the left sacral region. | Thin hydrocolloid or low-adherence polyurethane foam dressing. Skin barrier cream on perilesional skin. Pressure-relieving devices and alternative patient repositioning. |
| 4 |  | Oval-shaped ulcer on the lower leg with a central area of yellow fibrinous slough, surrounded by erythematous and mildly edematous skin. The wound edges are well defined and slightly raised. | Hydrofiber or alginate dressing with gentle debridement and compression therapy if venous etiology is confirmed. | The image shows a small, shallow, circular wound on the lower leg with a well-defined rolled/epibole edge. The wound base contains a moderate amount of pale yellow, translucent slough/fibrin, with minimal surrounding erythema. The periwound skin is intact but appears glossy and slightly edematous. | Manage the epibole with gentle debridement/abrasion of the wound edges. Apply a hydrogel to the sloughy base under a semi-occlusive film or hydrocolloid to promote autolytic debridement and maintain moisture. | Oval-shaped ulcer of probable venous etiology, approximately 2 cm in diameter, located on the anterior aspect of the leg. The wound bed is exudative and covered with slough. Wound margins are well-defined, irregular, and erythematous. The perilesional skin appears mildly edematous. | Surgical debridement or mechanical abrasion of the slough down to a well-vascularized tissue; hydrofiber and low-adherence polyurethane foam dressing; evaluate compression therapy. |
| 5 | 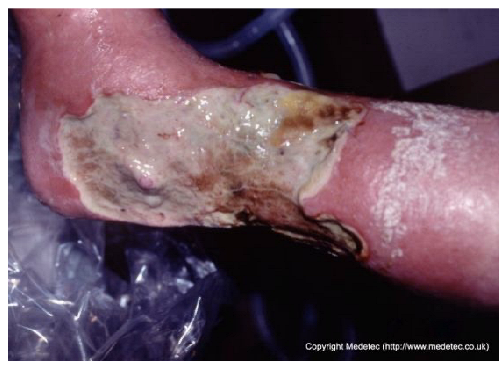 | Extensive ulcer on the lower leg with thick yellow-green necrotic slough and areas of black eschar, surrounded by erythematous and edematous skin. The wound surface appears moist with signs of infection. | Sharp or enzymatic debridement, application of antimicrobial alginate or silver dressing, and initiation of systemic antibiotics if infection is confirmed. | The image displays a large, irregular wound on the lower leg/ankle, primarily covered by thick, pale yellow slough and central black eschar/necrosis. The wound depth appears superficial/partial-thickness. The surrounding skin is hyper-pigmented, significantly erythematous, and possibly weeping, suggesting chronic venous insufficiency and acute inflammation. | Prioritize compression therapy for underlying venous insufficiency. Debridement is necessary for the slough and eschar; apply a hydrogel or cadexomer iodine under a highly absorptive dressing (e.g., foam) to manage exudate and promote autolytic debridement. | Large ulcer of probable venous etiology, located on the medial and distal aspects of the right leg. The lesion shows full-thickness tissue loss, with a necrotic and slough-covered exudative wound bed. A dry eschar is present in the proximal-posterior portion. An infectious component is considered plausible. Wound margins are irregular, macerated, and undermined, particularly in the proximal area. The perilesional skin exhibits marked edema and erythema, with desquamation in the proximal portion. | Surgical debridement of necrotic tissue, slough and dry eschar; antimicrobial dressing (i.e., silver containing dressing) or alginate and foam. Evaluate the administration of systemic antibiotics, compression therapy and hyperbaric oxygen therapy. |
| 6 | 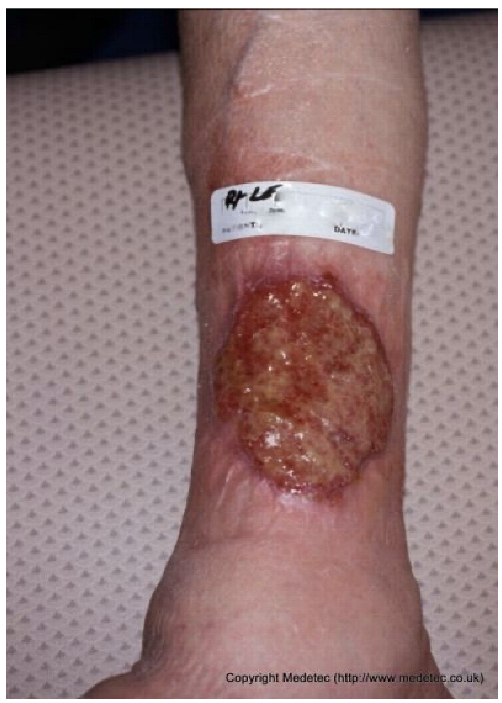 | Oval ulcer on the lower leg with a moist granulating base and minimal fibrin, surrounded by erythematous and slightly thickened skin. The wound yet edges are regular and well defined. | Hydrogel dressing to promote autolytic debridement of slough; alginate and foam dressing to maintain moisture balance, combined with graduated compression therapy if venous origin is suspected. | The image shows an ulcer on the lower leg with an irregular, full-thickness appearance. The wound bed is mostly covered by mixed tissue: active, highly vascular granulation tissue (red) interspersed with moderate, loose, pale-yellow slough/fibrin. There is minimal erythema surrounding the wound, and the periwound skin is intact. | Manage the mixed wound bed by applying a calcium alginate or hydrofiber dressing to manage exudate and promote autolytic debridement of the slough. Ensure underlying compression therapy is optimized for this likely venous ulcer. | Oval-shaped ulcer, approximately 8 cm in maximum diameter, of probable venous etiology, located on the distal portion of the right leg. The wound bed is exudative, with granulation tissue and areas covered by a modest amount of slough. Wound margins are regular, surrounded by an erythematous halo. The perilesional skin is edematous, with mild erythema. | Amorphous hydrogel or mechanical abrasion; alginate and polyurethane foam. Evaluate compression therapy. |
| 7 | 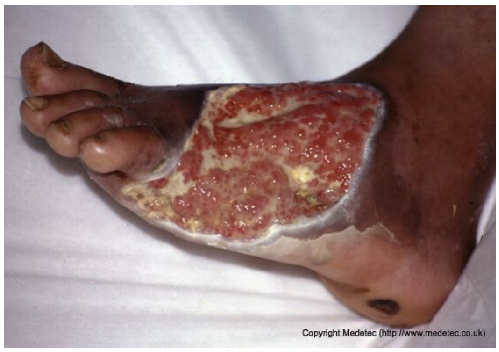 | Large dorsal foot ulcer with mixed red granulation tissue and yellow fibrinous areas, partial epithelialization at the edges, and surrounding dusky, macerated skin. Moderate exudate present. | Hydrogel, hydrofiber or alginate dressing with antimicrobial coverage, strict off-loading, and regular debridement to promote granulation and prevent infection. | The image displays a very large, irregular, full-thickness ulcer on the plantar and medial aspect of the foot, extending to the toes. The wound bed is a mix of abundant, friable, pale-red granulation tissue and scattered areas of pale-yellow slough/fibrin. There is significant periwound maceration/peeling, and hyperkeratosis of the surrounding foot skin is evident. | Immediate and strict offloading is mandatory, as this is a likely diabetic foot ulcer. Use a highly absorptive hydrofiber or alginate dressing to manage exudate, combined with an antimicrobial component (e.g., silver) if infection is suspected. | Probable diabetic ulcer involving the plantar, lateral, and dorsal aspects of the left foot, extending from the tarsus to the base of the toes. Full-thickness tissue loss is present, with bright red granulation tissue and multiple areas covered by slough. Wound margins are well-defined and hyperkeratotic. The perilesional skin appears intact. | Amorphous hydrogel and antimicrobial dressing (i.e., silver containing dressing or cadexomer iodine); hydrofiber or alginate dressing and polyurethane foam. Evaluate compression therapy, off-loading, glycemic monitoring and corresponding antidiabetic therapy. |
| 8 | 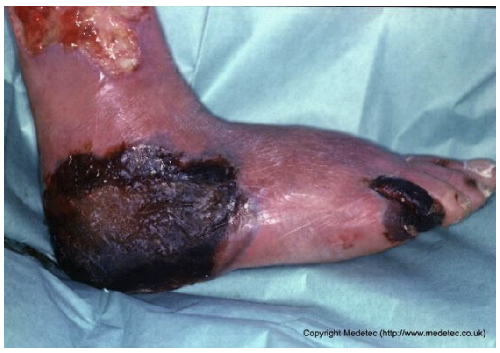 | Extensive necrotic ulcer on the lateral and plantar aspects of the foot with black eschar and surrounding erythematous, edematous skin. Distal tissue appears ischemic with early demarcation. | Urgent vascular assessment, dry necrosis management with protective dry dressing, and surgical or enzymatic debridement if perfusion is restored. | The image shows extensive necrotic tissue (black eschar) covering the lateral malleolus and posterior aspect of the foot/heel. The surrounding skin is severely erythematous and dusky/mottled with significant edema, suggesting deep tissue injury, severe ischemia, or gangrene. A separate, smaller eschar is visible near the fifth toe. | Urgent vascular assessment is essential to determine limb viability and potential for revascularization; sharp/surgical debridement of the eschar should only be performed after this assessment. Keep the dry, stable eschar intact (do not debride) and apply a dry, non-adherent dressing until definitive management is planned. | Multifocal ulcers of the right lower limb, likely of mixed arterial, venous, and/or diabetic etiology. At the heel, a large area of demarcated eschar with relatively well-defined margins is present. At the level of the lateral malleolus, a full-thickness irregular ulcer (partially visible) is noted, with an exudative, bleeding, and slough-covered wound bed; margins are violaceous and macerated. Distal eschars are present at the stump of the V toe, the entire IV toe, and the periungual areas of the III and II toes. The perilesional skin is reddish-violet, edematous, and exhibits desquamation in the dorsal region. | Urgent vascular assessment. Dry dressing on the eschars; hydrofiber or alginate dressing and polyurethane foam on the malleolar ulcer. |
| 9 | 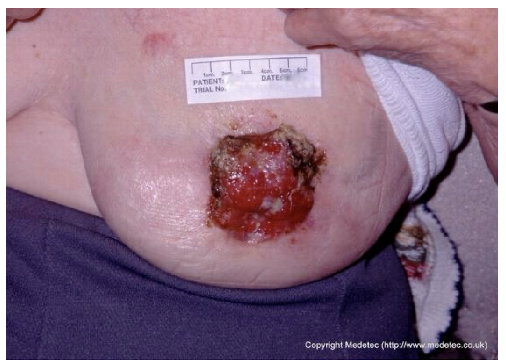 | Ulcerated lesion on the lateral breast, approximately 4–5 cm in diameter, with irregular margins, mixed red granulation and yellow-brown necrotic tissue. Surrounding skin shows erythema and mild edema. | Cleanse with saline and apply hydrocolloid or hydrogel dressing to maintain moisture; consider alginate for exudate control and protect periwound skin. | The image shows an irregular, full-thickness wound, approximately 2–3 cm in diameter, located in the infra-mammary/chest wall region. The wound bed is a mix of bright red, friable granulation tissue and peripheral slough/necrotic tissue with a dark, rolled border. The appearance is concerning for a malignant ulcer (e.g., fungating wound) or pyoderma gangrenosum. | Prioritize differential diagnosis/biopsy if malignancy is suspected. Manage symptoms with a non-adherent, lightly absorbent dressing (e.g., silicone foam or hydrogel). Use a topical hemostatic agent (e.g., alginate) if bleeding is a concern. | Probable neoplastic ulcer, round in shape, approximately 5 cm in diameter, located on the left breast at the level of the NAC. The wound bed is exudative, with exuberant granulation tissue, multiple slough-covered areas and small eschars in the superolateral and medial portions. Wound margins are irregular and elevated. The surrounding skin is erythematous and taut. | Punch biopsy of the ulcer. Hydrogel and alginate or hydrofiber dressing and foam. |
| 10 | 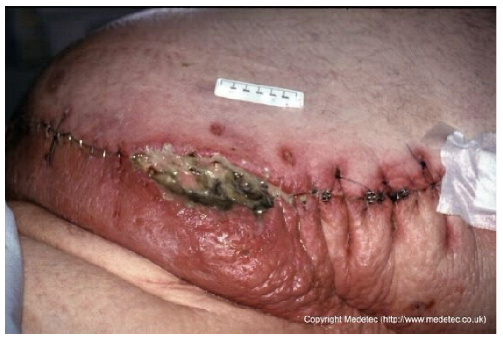 | Post-surgical wound dehiscence along a stapled incision line with central necrotic slough and purulent exudate. Surrounding skin is erythematous, edematous, and tense, suggesting local infection or inflammation. | Cleanse with saline, apply antiseptic or silver-impregnated dressing, and consider negative pressure therapy after infection control. Start systemic antibiotics and monitor for surical site infection. | The image shows a poorly healing surgical incision with steel staples, located on the abdomen. A significant portion of the incision line has dehisced, forming a large defect with a base covered by thick, tenacious, dark slough and necrosis. There is marked surrounding erythema, edema, and cellulitis, suggesting severe surgical site infection (SSI) and wound dehiscence. | Urgent surgical intervention is required for debridement of necrotic tissue and source control of the severe infection. Post-debridement, manage the open abdomen with Negative Pressure Wound Therapy (NPWT) or highly absorbent antimicrobial dressings. | Dehiscence of an infected surgical wound, approximately 12 × 3 cm, located in the distal left abdomen. The wound bed is highly exudative, with areas of necrosis, slough, and abundant purulent material; assessment of lesion depth is difficult. Wound margins are irregular, markedly erythematous, and edematous. The perilesional skin is red with intense edema, particularly in the area inferior to the dehiscence and at the sites of sutures and steel staples. | Surgical debridement of necrotic tissue; antimicrobial dressing (i.e., silver containing dressing or cadexomer iodine); hydrofiber dressing and foam. Evaluate the administration of systemic antibiotics, partial or complete sutures and steel staples removal and urgent CT scan. |
| 11 | 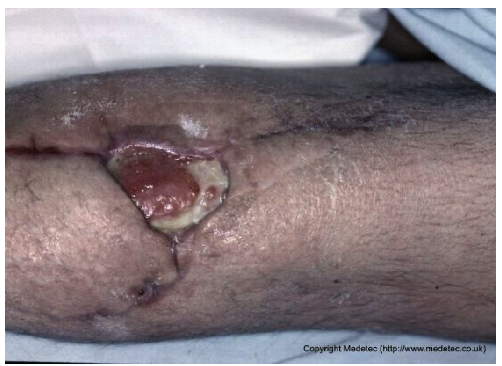 | Localized dehiscence over a surgical scar with exposed granulation tissue and yellow fibrinous slough. Margins appear rolled and indurated, with mild surrounding erythema. | Cleanse with saline and apply hydrogel and hydrofiber dressing; protect edges with barrier film. | The image shows a dehisced surgical incision on an extremity, where the wound edges have separated. The resulting defect reveals a wound bed with a mix of fragile, red granulation tissue and pale-yellow slough/fibrin. There is notable serous or purulent exudate and minimal surrounding erythema. | Focus on achieving secondary wound closure: use a hydrofiber or alginate to manage exudate and a hydrogel to autolytically debride the slough. Consider Negative Pressure Wound Therapy (NPWT) if the defect is large and not progressing. | Full-thickness oval-shaped ulcer, likely resulting from partial dehiscence of a surgical wound at the level of the left knee. The wound bed is exudative, partially granulating, with a broad proximal area covered by slough. Wound margins are well-defined but undermined. The perilesional skin shows desquamation, with visible remaining portions of the suture line, which appear hyperpigmented and in the maturation phase. Along the lateral suture, a subcentimetric area of early ulceration is present. | Amorphous hydrogel or selective surgical debridement of slough; hydrofiber and polyurethane foam dressing. Analogous treatment for smaller ulcer. |
| 12 | 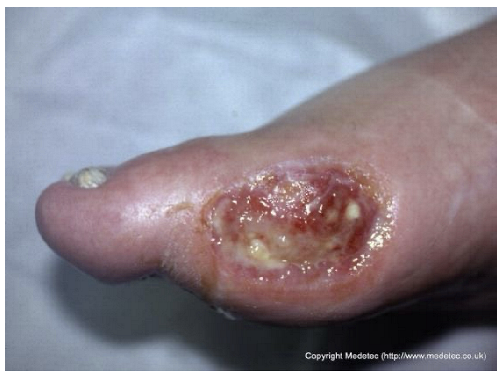 | Ulcer on the distal toe with irregular margins, red granulation tissue, and yellow fibrinous exudate. Surrounding skin shows mild maceration and erythema. | Cleanse with saline and apply hydrofiber or alginate dressing; maintain moisture balance and offload pressure from the affected toe. | The image shows an ulcer on the plantar surface of the great toe, likely a diabetic foot ulcer. The wound bed has a mixture of red, moist granulation tissue and moderate amounts of pale-yellow, soft slough. The periwound skin is slightly macerated, and the wound edges appear calloused/hyperkeratotic. | Mandatory strict offloading is required to remove pressure from the ulcer. Debride the hyperkeratotic edges and slough, then apply a moist wound dressing like a hydrogel covered by a foam or hydrocolloid. | Supposed stage III elliptical-shaped pressure ulcer located on the lateral aspect of the first metatarsal head of the right foot. The wound bed is highly exudative and granulating, with evident islands of slough noted primarily in the inferior portion. The margins are well-demarcated and hyperkeratotic superiorly. The perilesional skin is mildly erythematous. | Surgical debridement of islands of slough and hyperkeratotic margins down to a well-vascularized tissue; hydrofiber and polyurethane foam. Evaluate off-loading. |
| 13 | 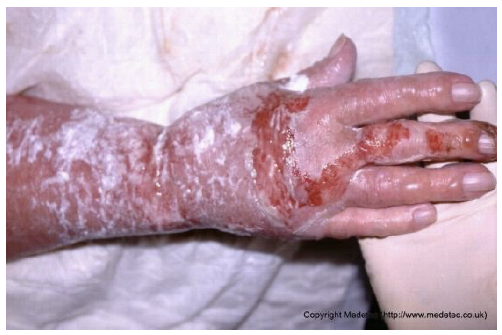 | Superficial partial-thickness burn on the dorsal hand and wrist with areas of erythema, serous exudate, and superficial skin loss. No visible necrosis or deep tissue exposure. | Cleanse gently and apply silver sulfadiazine dressing and cover with a non-adherent absorbent dressing; maintain moisture and protect from friction or contamination. | The image shows an injury to the hand and forearm, characterized by a mix of partial-thickness and deeper burns. The forearm is covered by a white, creamy substance (likely a topical cream/ointment), while the dorsum of the hand and fingers show areas of raw, weeping, bright red dermis (superficial/partial thickness) and possible deeper injury on the fingers. There is surrounding erythema and significant edema of the hand. | Management involves thorough wound cleansing and application of a topical antimicrobial agent (e.g., silver sulfadiazine, honey, or a silver dressing) followed by a non-adherent dressing. Control edema with elevation and consider physical therapy to prevent contractures. | Superficial partial-thickness burn to the dorsal aspect of the right hand, involving the metacarpal area and the third digit. The wound bed is minimally exudative, demonstrating alternating areas of granulation and re-epithelialization. The margins are irregular and indistinct. The perilesional skin is edematous and covered with a difficult-to-identify whitish material (likely an emollient or cream). | Gentle wound cleasing; hydrofiber with silver and polyurethane foam. On the perilesional skin: gently remove the cream, petrolatum gauze and sterile gauze. |
| 14 | 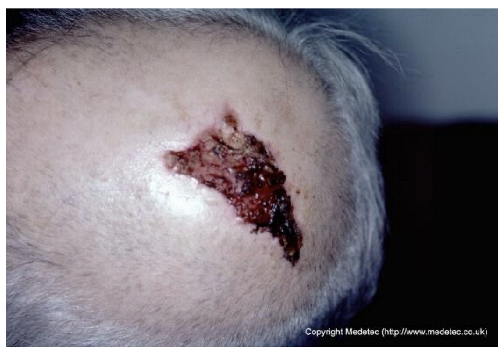 | Superficial ulcer on the scalp with mixed red granulation and brown necrotic tissue. Surrounding skin appears mildly erythematous without evident edema. | Cleanse with saline and apply hydrocolloid or hydrogel dressing to maintain a moist environment; protect surrounding skin. | The image displays an irregular, partial-to-full-thickness wound on the scalp/forehead. The wound base is predominantly dark red, bloody, and moist granulation tissue interspersed with adherent dark brown/black eschar/necrotic tissue. There is minimal surrounding erythema, and the wound edges are slightly rolled, raising suspicion for a potential malignancy (e.g., basal or squamous cell carcinoma). | Due to the appearance, obtain an urgent biopsy to rule out malignancy. If non-malignant, use a hydrogel dressing to promote autolytic debridement of the necrotic tissue and cover with a non-adherent foam dressing. | Triangular-shaped ulcer on the left parietal region, with the apex pointing inferolaterally, most likely partial-thickness. The wound base is bleeding and crusted-covered in the inferior portion. The margins are erythematous, serrated, yet well-defined. Neoplastic etiology cannot be excluded. Mild erythema of the surrounding skin. | Punch biopsy of the ulcer; amorphous hydrogel; thin hydrocolloid dressing. |
| 15 | 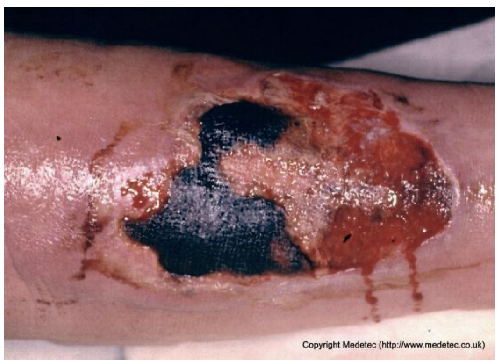 | Extensive ulcer on the lower limb with central black eschar and surrounding red granulation tissue. Margins irregular, with moderate exudate and peripheral erythema. | Perform gentle debridement or apply hydrogel or enzymatic dressing to soften necrosis; use hydrofiber and absorbent secondary dressing for exudate control. Monitor for systemic signs. | The image displays a large, irregular ulcer on the lower extremity with a mixed wound bed. Approximately half the bed is covered by hard, black, adherent eschar/necrosis, and the remainder shows bright red, moist granulation tissue. The periwound skin is erythematous and appears macerated/weeping, with serosanguinous exudate visible. | Management requires debridement of the eschar. Apply a hydrogel or enzyme debrider to the necrotic areas and cover with a moisture-retaining, absorptive foam or hydrocolloid dressing to facilitate autolytic debridement and manage exudate. | Extensive, full-thickness ulcer of oval shape on the lower leg, with a proximal eschar and a distal exudative and granulating area. The margins are irregular, yet sharp and well-demarcated. The perilesional skin is edematous and slightly erythematous. | Surgical debridement of the eschar; hydrofiber with silver and polyurethane foam. |
| 16 | 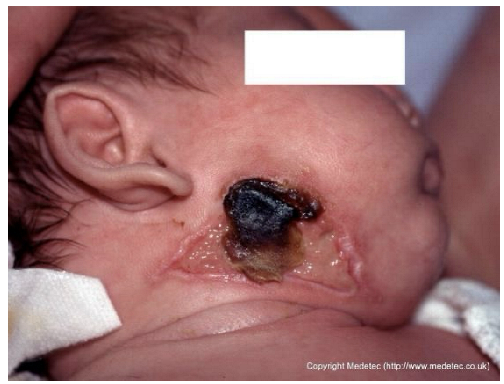 | Necrotic lesion with black eschar on the right mandibular and subauricular region, surrounded by erythematous margins and partial epidermal loss. Mild serous exudate present with early demarcation between necrotic and viable tissue. | Cleanse gently with saline, apply hydrogel or enzymatic dressing to promote autolytic debridement, cover with non-adhesive moist dressing and protect surrounding skin with barrier film. Consult a pediatrician to investigate the etiology. | The image shows an ulcerated lesion on the face/neck of an infant, primarily characterized by a central area of black, hard eschar/necrosis. Surrounding the eschar are areas of yellow slough and pale-pink, fragile skin breakdown. The periwound skin is erythematous and intact, and the appearance is concerning for a deep ischemic or infectious process (e.g., Ecthyma gangrenosum). | This wound requires urgent pediatric consultation for underlying etiology and management, likely involving systemic antibiotics and potential surgical debridement if an infectious or ischemic process is confirmed. Topically, maintain a moist environment with a hydrogel under a light, non-occlusive dressing. | Deep ulcer of a trapezoidal shape located on the right mandibular area, consistent with a pressure ulcer. The wound base exhibits abundant slough and eschar in the middle and superior portions. The margins are undulating and well-demarcated. The perilesional skin appears erythematous and edematous. | Amorphic hydrogel on eschar and slough; polyurethane foam. Evaluate pressure-relieving devices and alternative patient repositioning. |
| 17 | 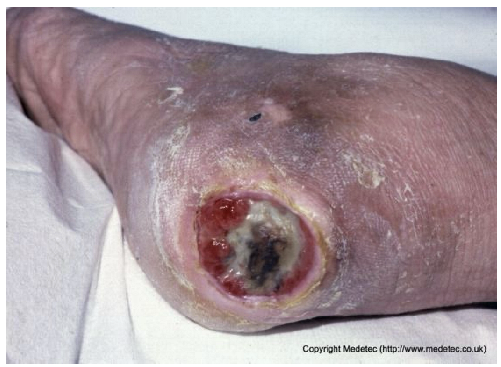 | Circular ulcer on the heel with necrotic tissue at the base, yellow slough, and peripheral granulation. Surrounding skin shows erythema, dryness, and scaling, consistent with a chronic pressure ulcer. | Debridement of necrotic tissue followed by hydrocolloid or hydrogel dressing; maintain moist wound environment and offload pressure. | The image shows a deep, circular ulcer on the heel with a well-defined, hyperkeratotic/calloused border, characteristic of a neuropathic/pressure ulcer. The wound base contains a mix of red granulation tissue, yellow slough, and a small amount of central black eschar. The surrounding skin is dry and hyperkeratotic. | Implement immediate and strict offloading for the heel. Debride the calloused edges and necrotic/sloughy center. Apply a hydrogel to the base under an absorptive foam dressing for autolytic debridement and moisture balance. | Plausible stage III or stage IV pressure ulcer, rounded shaped, on the posterior aspect of the right heel. The wound bed is exudative, with peripheral and distally exuberant granulation tissue and a central area of slough and necrosis. The margins are well-defined, raised, and exhibit a callused border. The perilesional skin is atrophic with generalized desquamation. | Amorphic hydrogel on the necrosis and surgical debridement of the callused border; alginate and polyurethane foam. Use pressure-relieving devices and compression therapy. |
| 18 | 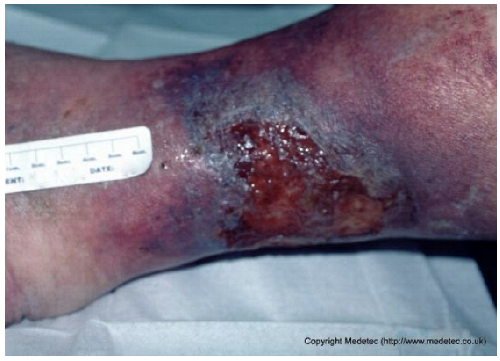 | Irregular superficial ulcer on the lower leg with a moist red base and partial epithelial loss. Surrounding skin shows hyperpigmentation, edema, and signs of chronic venous stasis. | Apply hydrocolloid and foam dressing and initiate multilayer compression therapy; elevate limb to reduce venous stasis. | The image shows a shallow, irregular ulcer located on the medial aspect of the ankle/lower leg, surrounded by pronounced hyperpigmentation and induration, characteristic of lipodermatosclerosis. The wound bed is mainly covered by clean, red, moist granulation tissue with low exudate and minimal slough. | Strictly maintain compression therapy for the underlying venous insufficiency. Apply a non-adherent contact layer or a thin hydrocolloid dressing to protect the granulation tissue and promote healing with minimal disruption. | Superficial ulcer of an irregularly triangular shape with a maximum length of approximately 5 cm, located on the postero-medial aspect of the right distal lower leg. Plausible venous etiology. The wound bed is exudative, with markedly edematous granulation tissue interspersed with islands of slough. The margins are irregular, violaceous and macerated. The perilesional skin is moderately edematous and darkly pigmented, highly suggestive of venous insufficiency. | Gentle surgical debridement of islands of slough; hydrofiber and polyurethane foam. Compression therapy with zinc oxide bandage. |
| 19 | 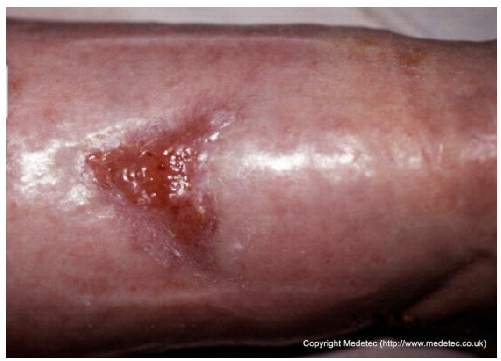 | Small superficial ulcer with partial epithelial loss and granulation tissue at the base. Surrounding skin appears shiny, taut, and mildly erythematous, suggestive of venous insufficiency. | Use hydrocolloid or foam dressing to maintain moisture and apply graduated compression therapy to address venous insufficiency. | The image displays a small, shallow, triangular ulcer on the lower leg. The wound base is primarily covered by bright red, healthy-appearing granulation tissue with a small amount of peripheral yellow slough/fibrin. The surrounding skin is erythematous, edematous, and appears glossy/taut, suggesting underlying venous disease or inflammation. | If a venous ulcer is suspected, maintain compression therapy. Apply a hydrocolloid or thin foam dressing to protect the granulation tissue and manage low exudate, or a hydrogel if slough needs autolytic debridement. | Small, triangular-shaped superficial ulcer, likely located on the anterior aspect of the lower leg. The wound bed is granulating and mildly edematous, with small areas of fibrin in the most proximal portion. The margins are irregular and sloping, showing signs of initial re-epithelialization. The surrounding skin is diffusely edematous. | Gentle surgical debridement of the small areas of fibrin; thin hydrocolloid dressing; compression therapy. |
| 20 |  | Irregular hypertrophic scar on the forearm with areas of epithelial breakdown and partial ulceration. The wound bed appears red and moist, surrounded by fibrotic, shiny scar tissue with mild local erythema. No signs of active purulent exudate are visible. | Use a non-adherent silicone or hydrogel dressing to maintain moisture balance and protect fragile tissue; consider compression and scar management therapy. | The image displays a mature, pale, slightly raised, hyper-pigmented linear scar on the forearm, suggesting a healed previous injury or incision. There are two areas of superficial wound dehiscence/breakdown along the scar line, exposing small amounts of bright red, moist granulation tissue with minimal exudate. The surrounding skin shows post-inflammatory hyperpigmentation. | Management involves minimizing tension/movement across the area. Apply a thin hydrocolloid or transparent film to protect the small areas of granulation tissue and facilitate re-epithelialization. | Superficial ulcer located along a hypertrophic and hyperpigmented scar, plausibly from a burn injury, situated on the dorsal aspect of the left forearm. The wound bed is granulating with modest peripheral fibrin. The margins are violaceous and fibrotic. Medially and more distally, there is a subcentimeter ulcer with analogous characteristics. | On main and smaller ulcer: surgical debridement of the fibrotic margins; thin hydrocolloid dressing; compression therapy. Evaluate scar management therapy. |
| Case ID | Wound Description ChatGPT | Management ChatGPT | Wound Description Gemini | Management Gemini |
|---|---|---|---|---|
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 9 | ||||
| 10 | ||||
| 11 | ||||
| 12 | ||||
| 13 | ||||
| 14 | ||||
| 15 | ||||
| 16 | ||||
| 17 | ||||
| 18 | ||||
| 19 | ||||
| 20 |
| Assessment | Model | Mean Score (SD) | Score Distribution (0/1/2) | Perfect Agreement n (%) | 95% CI | p-Value (vs. 50%) | p-Value (vs. Gemini) |
|---|---|---|---|---|---|---|---|
| Wound Description | ChatGPT | 1.50 (0.51) | 0/10/10 | 10 (50.0%) | 29.9–70.1% | 1.000 | |
| Gemini | 1.55 (0.69) | 2/5/13 | 13 (65.0%) | 43.3–81.9% | 0.263 | 0.508 | |
| Inter-model Agreement | Cohen’s κ = 0.000 | ||||||
| Management | ChatGPT | 1.60 (0.50) | 0/8/12 | 12 (60.0%) | 38.7–78.1% | 0.503 | |
| Gemini | 1.70 (0.57) | 1/4/15 | 15 (75.0%) | 53.1–88.8% | 0.041 | 0.453 | |
| Inter-model Agreement | Cohen’s κ = 0.255 | ||||||
| Overall Performance | ChatGPT | 3.10 (0.64) | — | 5 (25.0%) | — | — | |
| Gemini | 3.25 (0.97) | — | 9 (45.0%) | — | — | 0.388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corradini, L.; Marcaccini, G.; Seth, I.; Rozen, W.M.; Biagiotti, C.; Cuomo, R.; Giardino, F.R. AI vs. MD: Benchmarking ChatGPT and Gemini for Complex Wound Management. J. Clin. Med. 2025, 14, 8825. https://doi.org/10.3390/jcm14248825
Corradini L, Marcaccini G, Seth I, Rozen WM, Biagiotti C, Cuomo R, Giardino FR. AI vs. MD: Benchmarking ChatGPT and Gemini for Complex Wound Management. Journal of Clinical Medicine. 2025; 14(24):8825. https://doi.org/10.3390/jcm14248825
Chicago/Turabian StyleCorradini, Luca, Gianluca Marcaccini, Ishith Seth, Warren M. Rozen, Camilla Biagiotti, Roberto Cuomo, and Francesco Ruben Giardino. 2025. "AI vs. MD: Benchmarking ChatGPT and Gemini for Complex Wound Management" Journal of Clinical Medicine 14, no. 24: 8825. https://doi.org/10.3390/jcm14248825
APA StyleCorradini, L., Marcaccini, G., Seth, I., Rozen, W. M., Biagiotti, C., Cuomo, R., & Giardino, F. R. (2025). AI vs. MD: Benchmarking ChatGPT and Gemini for Complex Wound Management. Journal of Clinical Medicine, 14(24), 8825. https://doi.org/10.3390/jcm14248825








