Skin Autofluorescence and Perinatal Outcomes in Pregnant Women with a Positive Glucose Challenge Test: A Prospective Study with Exploratory Analyses of Oxidative Stress and CGM Metrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Measurement of SAF
2.4. Measurement of Oxidative Stress
2.5. CGM
2.6. Study Endpoints
2.7. Adverse Events
2.8. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary Endpoint: SAF
3.3. Secondary Endpoint: d-ROMs/CGM Metrics/75g-OGTT
3.4. Other Secondary Endpoints; Relationship Between SAF and d-ROMs
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full term |
| 75g-OGTT | 75-g oral glucose tolerance test |
| AGEs | Advanced glycation end products |
| ANCOVA | Analysis of covariance |
| AU | Arbitrary units |
| AUC | Area under the curve |
| BMI | Body mass index |
| CGM | Continuous glucose monitoring |
| CI | Confidence interval |
| DM | Diabetes mellitus |
| d-ROMs | diacron-reactive oxygen metabolites |
| GA | Glycated albumin |
| GCT | Glucose challenge test |
| GDM | Gestational diabetes mellitus |
| H2O2 | Hydrogen peroxide |
| HbA1c | Glycated hemoglobin |
| MAGE | Mean amplitude of glycemic excursions |
| MGL | Mean glucose level |
| NICU | Neonatal intensive care unit |
| OR | Odds ratio |
| PROM | Premature rupture of membranes |
| ROC | Receiver operating characteristic |
| SAF | Skin autofluorescence |
| SD | Standard deviation |
| TAR | Time above range |
| TBR | Time below range |
| TIR | Time in range |
| WHO | World Health Organization |
References
- American Diabetes Association. Clinical practice recommendations 2001: Gestational diabetes mellitus. Diabetes Care 2001, 24, S77–S79. [Google Scholar]
- Morikawa, M.; Sugiyama, T.; Hiramatsu, Y.; Sagawa, N. Screening methods for gestational diabetes mellitus in Japan in 2018: A retrospective cohort study using a national surveillance questionnaire. Endocr. J. 2022, 69, 1313–1322. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/tokusyu/syussyo07/dl/gaikyou.pdf (accessed on 15 May 2025).
- Lao, T.T.; Ho, L.F.; Chan, B.C.P.; Leung, W.C. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care 2006, 29, 948–949. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 161, 108034. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Coustan, D.R.; Trimble, E.R. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [PubMed]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Morse, A.N. Residual risk associations exist between hyperglycemia and adverse pregnancy outcomes despite seemingly appropriate glycemic control. Diabetes Metab. 2022, 48, 101320. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef]
- Sweeting, A.N.; Ross, G.P.; Hyett, J.; Molyneaux, L.; Constantino, M.; Harding, A.J.; Wong, J. Gestational diabetes mellitus in early pregnancy: Evidence for poor pregnancy outcomes despite treatment. Diabetes Care 2016, 39, 75–81. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Zheng, Q.-X.; Wang, H.-W.; Jiang, X.-M.; Lin, Y.; Liu, G.-H.; Pan, M.; Ge, L.; Chen, X.-Q.; Wu, J.-L.; Zhang, X.-Y.; et al. Prepregnancy body mass index and gestational weight gain are associated with maternal and infant adverse outcomes in Chinese women with gestational diabetes. Sci. Rep. 2022, 12, 2749. [Google Scholar] [CrossRef]
- Jacobsson, B.; Ladfors, L.; Milsom, I. Advanced maternal age and adverse perinatal outcome. Obstet. Gynecol. 2004, 103, 1005–1011. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Fukami, K.; Matsui, T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: A novel marker of vascular complications in high-risk patients for cardiovascular disease. Int. J. Cardiol. 2015, 185, 263–268. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Isami, F.; West, B.J.; Nakajima, S.; Yamagishi, S.I. Association of advanced glycation end products, evaluated by skin autofluorescence, with lifestyle habits in a general Japanese Population. J. Int. Med. Res. 2018, 46, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Savel, J.; Grouthier, V.; Rajaobelina, K.; Corvo, L.; Lorrain, S.; Gonzalez, C.; Gin, H.; Barberger-Gateau, P.; Rigalleau, V. Is skin autofluorescence a marker of metabolic memory in pregnant women with diabetes? Diabet. Med. 2015, 32, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, E.; Miura, J.; Iwamoto, Y.; Uchigata, Y. Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes Care 2013, 36, 2339–2345. [Google Scholar] [CrossRef][Green Version]
- Albano, E. Oxidative stress and reactive oxygen metabolites (d-ROMs) in diabetes. Clin. Chem. Lab. Med. 2005, 43, 663–666. [Google Scholar][Green Version]
- Law, G.R.; Scott, E.M.; Farmer, G.; Mackin, S.T.; Hall, R.; McCance, D.; Maresh, M.; GEMS Study Group. Association between maternal glycemic profiles in pregnancy and neonatal outcomes: A continuous glucose monitoring study. Diabetes Care 2015, 38, 1237–1244. [Google Scholar][Green Version]
- Li, A.; Brackenridge, A. The role of continuous glucose monitoring in pregnancy. Obstet. Med. 2022, 15, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.; Ögge, L.E.; Sengpiel, V.; Kjölhede, K.; Dotevall, A.; Elfvin, A.; Knop, F.K.; Wiberg, N.; Katsarou, A.; Shaat, N. Continuous glucose monitoring in pregnant women with type 1 diabetes: An observational cohort study of 186 pregnancies. BMJ Open Diabetes Res. Care 2020, 8, e001061. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, Y.; Lu, S.; Shuai, M.; Miao, Z.; Gou, W.; Shen, L.; Liang, Y.; Xu, F.; Tian, Y.; et al. Continuous glucose monitoring-derived glycemic metrics and adverse pregnancy outcomes among women with gestational diabetes: A prospective cohort study. Lancet Reg. Health West. Pac. 2023, 39, 100823. [Google Scholar] [CrossRef]
- Ohara, M.; Fukui, T.; Ouchi, M.; Watanabe, K.; Suzuki, T.; Yamamoto, S.; Yamamoto, T.; Hayashi, T.; Oba, K.; Hirano, T. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 122, 62–70. [Google Scholar] [CrossRef]
- Committee on the Diagnosis and Classification of Diabetes Mellitus, Japan Society of Obstetrics and Gynecology. Definition and classification of gestational diabetes mellitus (GDM): Revision in 2010. Acta Obstet. Gynaecol. Jpn. 2010, 62, 373–375. [Google Scholar]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar]
- Monnier, L.; Colette, C.; Wojtusciszyn, A.; Dejager, S.; Renard, E.; Molinari, N.; Owens, D.R. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017, 40, 832–838. [Google Scholar] [CrossRef]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef]
- Molnar, G.D.; Taylor, W.F.; Ho, M.M. Day-to-day variation of continuously monitored glycaemia: A further measure of diabetic instability. Diabetologia 1972, 8, 342–348. [Google Scholar] [CrossRef]
- Malaza, N.; Masete, M.; Adam, S.; Dias, S.; Nyawo, T.; Pheiffer, C. A systematic review to compare adverse pregnancy outcomes in women with pregestational diabetes and gestational diabetes. Int. J. Environ. Res. Public Health 2022, 19, 10846. [Google Scholar] [CrossRef]
- El Mallah, K.O.; Narchi, H.; Kulaylat, N.A.; Shaban, M.S. Gestational and pre-gestational diabetes: Comparison of maternal and fetal characteristics and outcome. Int. J. Gynaecol. Obstet. 1997, 58, 203–209. [Google Scholar] [CrossRef]
- World Health Organization. Preterm Birth Fact Sheet; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Cornblath, M.; Hawdon, J.M.; Williams, A.F.; Aynsley-Green, A.; Ward-Platt, M.P.; Schwartz, R.; Kalhan, S.C. Controversies regarding definition of neonatal hypoglycemia: Suggested operational thresholds. Pediatrics 2000, 105, 1141–1145. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- de Ranitz-Greven, W.L.; Kaasenbrood, L.; Poucki, W.K.; Hamerling, J.; Bos, D.C.; Visser, G.H.A.; Biesma, D.H.; Beulens, J.W.J.; de Valk, H.W. Advanced glycation end products, measured as skin autofluorescence, during normal pregnancy and pregnancy complicated by diabetes mellitus. Diabetes Technol. Ther. 2012, 14, 1134–1139. [Google Scholar] [CrossRef]
- de Ranitz-Greven, W.L.; Bos, D.C.; Poucki, W.K.; Visser, G.H.A.; Beulens, J.W.J.; Biesma, D.H.; de Valk, H.W. Advanced glycation end products, measured as skin autofluorescence, at diagnosis in gestational diabetes mellitus compared with normal pregnancy. Diabetes Technol. Ther. 2012, 14, 43–49. [Google Scholar] [CrossRef]
- Blaauw, J.; Smit, A.J.; van Pampus, M.G.; van Doormaal, J.J.; Aarnoudse, J.G.; Rakhorst, G.; Graaff, R. Skin autofluorescence, a marker of advanced glycation end products and oxidative stress, is increased in recently preeclamptic women. Am. J. Obstet. Gynecol. 2006, 195, 717–722. [Google Scholar] [CrossRef]
- Sibai, B.M. Preeclampsia as a cause of preterm and late preterm (near-term) Births. Semin. Perinatol. 2006, 30, 16–19. [Google Scholar] [CrossRef]
- Roberts, J.; Cooper, D. Pathogenesis and genetics of pre-eclampsia. Lancet 2001, 357, 53–56. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Sato, T.; Aoki, D. Changes in oxidative stress response during pregnancy. Showa Univ. J. Med. Sci. 2017, 77, 325–331. [Google Scholar]
- Fukase, M.; Watanabe, N.; Yamanouchi, K.; Tsutsumi, S.; Nagase, S. The change of oxidative stress in maternal blood during pregnancy. Reprod. Sci. 2022, 29, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Akhtar, S.; Garg, R.; Manger, P.T.; Khan, M.M. A comparative study of oxidative status in pregnant and non-pregnant women. Indian J. Basic Appl. Med. Res. 2016, 5, 225–230. [Google Scholar]
- Hashimoto, K.; Koga, M. Influence of iron deficiency on Hba1c levels in pregnant women: Comparison with non-pregnant women. J. Clin. Med. 2018, 7, 34. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Friedman, J.E.; Van Pelt, R.E.; Barbour, L.A. Patterns of glycemia in normal pregnancy: Should the current therapeutic targets be challenged? Diabetes Care 2011, 34, 1660–1668. [Google Scholar] [CrossRef]

| All Participants (n = 115) | Maternal Adverse Events (−) (n = 60) | Maternal Adverse Events (+) (n = 55) | p-Value | Neonatal Adverse Events (−) (n = 73) | Neonatal Adverse Events (+) (n = 42) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 35 (31–40) | 35 (31–39) | 36 (33–40) | 0.102 | 35 (31–39) | 36 (32–40) | 0.206 |
| Body weight (kg) | 53 (49–60) | 52.4 (49.0–56.3) | 54.0 (48.0–62.0) | 0.290 | 53.0 (48.5–60.0) | 53.5 (49.0–60.0) | 0.458 |
| Body mass index (kg/m2) | 21.3 (19.6–23.2) | 21.0 (19.4–22.9) | 21.7 (19.7–23.8) | 0.157 | 21.3 (19.5–23.4) | 21.3 (19.9–23.1) | 0.135 |
| Primiparous, n (%) | 67 (58.2) | 28 (45.0%) | 40 (72.7%) | 0.004 * | 41 (56.2) | 26 (61.9) | 0.563 |
| IVF a, n (%) | 38 (33.0) | 13 (21.6) | 25 (45.5) | 0.006 * | 23 (35.3) | 15 (30.0) | 0.556 |
| Family history of DM b, n (%) | 51 (44.3) | 27 (46.6) | 23 (41.8) | 0.601 | 33 (45.2) | 18 (42.8) | 0.452 |
| GDM c, n (%) | 45 (39.1) | 23 (38.8) | 22 (40.0) | 0.854 | 27 (37) | 18 (42) | 0.557 |
| Previous GDM, n (%) | 7 (6.1) | 6 (10) | 1 (1.8) | 0.116 | 6 (8.2) | 1 (2.4) | 0.419 |
| 50g-GCT d (mg/dL) | 156 (145–165) | 155 (144–167) | 157 (146–164) | 0.357 | 156 (144–167) | 155 (145–163) | 0.687 |
| Anesthesia delivery, n (%) | 31 (27.0) | 16 (26.6%) | 15 (27.2%) | 0.941 | 22 (30.1) | 9 (21.3) | 0.385 |
| Insulin therapy, n (%) | 17 (14.7) | 6 (10%) | 11 (20%) | 0.188 | 9 (12.3) | 8 (19.1) | 0.415 |
| Weight gain (kg) | 9.6 (7.5–12.1) | 10.3 (8.0–12.9) | 9.0 (6.3–11.3) | 0.019 * | 9.6 (7.9–12.0) | 9.6 (6.2–12.2) | 0.459 |
| Total cholesterol (mg/dL) | 260 ± 43 | 255 ± 38 | 264 ± 47 | 0.314 | 260 ± 46 | 260 ± 37 | 0.952 |
| HDL-C e (mg/dL) | 76 ± 15 | 78 ± 15 | 75 ± 15 | 0.301 | 75 ± 15 | 79 ± 15 | 0.251 |
| LDL-C f (mg/dL) | 154 ± 43 | 150 ± 39 | 158 ± 46 | 0.355 | 156 ± 47 | 152 ± 46 | 0.690 |
| Triglycerides (mg/dL) | 203 (165–256) | 193 (160–239) | 207 (170–282) | 0.106 | 202 (163–236) | 206 (167–273) | 0.467 |
| Hemoglobin (mg/dL) | 11.2 ± 0.9 | 11.1 ± 0.8 | 11.4 ± 0.9 | 0.054 | 11.2 ± 0.9 | 11.2 ± 1.0 | 0.982 |
| HbA1c g (%) | 5.2 (5.1–5.4) | 5.3 (5.1–5.4) | 5.1 (5.0–5.4) | 0.248 | 5.3 (5.1–5.5) | 5.2 (5.0–5.3) | 0.065 |
| Glycated albumin | 12.7 ± 1.0 | 12.9 ± 0.9 | 12.7 ± 1.0 | 0.358 | 12.91 ± 1.03 | 12.64 ± 0.78 | 0.153 |
| SAF h (AU) | 1.8 (1.7–2.1) | 1.8 (1.6–2.1) | 1.9 (1.7–2.0) | 0.262 | 1.8 (1.7–2.0) | 1.9 (1.7–2.1) | 0.788 |
| d-ROMs i (U.CARR) j | 642 ± 133 | 633 ± 127 | 648 ± 141 | 0.628 | 649 ± 141 | 628 ± 126 | 0.437 |
| 75-g oral glucose tolerance test | |||||||
| Fasting PG k (mg/dL) | 81 ± 7 | 81 ± 7 | 81 ± 7 | 0.972 | 81 ± 7 | 82 ± 6 | 0.651 |
| 30-min PG l (mg/dL) | 140 ± 18 | 134 ± 19 | 140 ± 18 | 0.846 | 138 ± 18 | 145 ± 18 | 0.061 |
| 60-min PG m (mg/dL) | 162 ± 28 | 165 ± 24 | 159 ± 31 | 0.303 | 160 ± 30 | 167 ± 24 | 0.179 |
| 120-min PG n (mg/dL) | 136 ± 25 | 136 ± 25 | 136 ± 25 | 0.922 | 135 ± 27 | 138 ± 20 | 0.482 |
| Fasting IRI o (mU/mL) | 6.4(4.5–9.1) | 5.9(4.3–8.9) | 6.6 (5.1–9.4) | 0.317 | 6.3 (4.5–8.9) | 6.4 (5.2–9.4) | 0.663 |
| IRI 30-min p (mU/mL) | 50.2 (34.1–68.4) | 49.9 (34.1–71.3) | 50.2 (34.1–64.9) | 0.748 | 49.6 (34.1–68.4) | 50.2 (34.1–73.3) | 0.834 |
| IRI 60 min q (mU/mL) | 62.7 (47.4–88.9) | 63.5 (48.8–87.1) | 60.4 (45.3–91.4) | 0.499 | 63.5 (39.2–82.0) | 60.2 (50.3–92.5) | 0.598 |
| IRI 120 min r (mU/mL) | 54.9 (41.1–69.4) | 54.2 (42.8–71.3) | 55.9 (38.8–69.4) | 0.645 | 49.3 (39.5–67.9) | 61.2 (46.7–73.7) | 0.110 |
| Insulinogenic index | 0.74 (0.55–1.1) | 0.78 (0.56–1.09) | 0.7 (0.52–1.11) | 0.514 | 0.73 (0.56–1.11) | 0.75 (0.48–1.00) | 0.442 |
| HOMA-R s | 1.24 (0.92–1.92) | 1.18 (0.83–1.93) | 1.32 (0.96–1.88) | 0.434 | 1.23 (0.85–1.88) | 1.31 (0.97–1.94) | 0.558 |
| ISI t | 5.74 (4.28–7.98) | 5.79 (4.23–8.14) | 5.74 (4.57–7.98) | 0.992 | 6.43 (4.58–8.26) | 5.37 (4.12–7.33) | 0.325 |
| Maternal Adverse Events (−) (n = 22) | Maternal Adverse Events (+) (n = 20) | p-Value | |
|---|---|---|---|
| Age (years) | 37(32–40) | 38 (34–41) | 0.528 |
| Body mass index (kg/m2) | 21.0 (18.5–22.3) | 21.6 (19.6–23.1) | 0.147 |
| OGTT-diagnosed GDM, n (%) | 18(81.8) | 17(85) | 0.782 |
| GA at CGM start (weeks) | 29(29–30) | 29(28–29) | 0.068 |
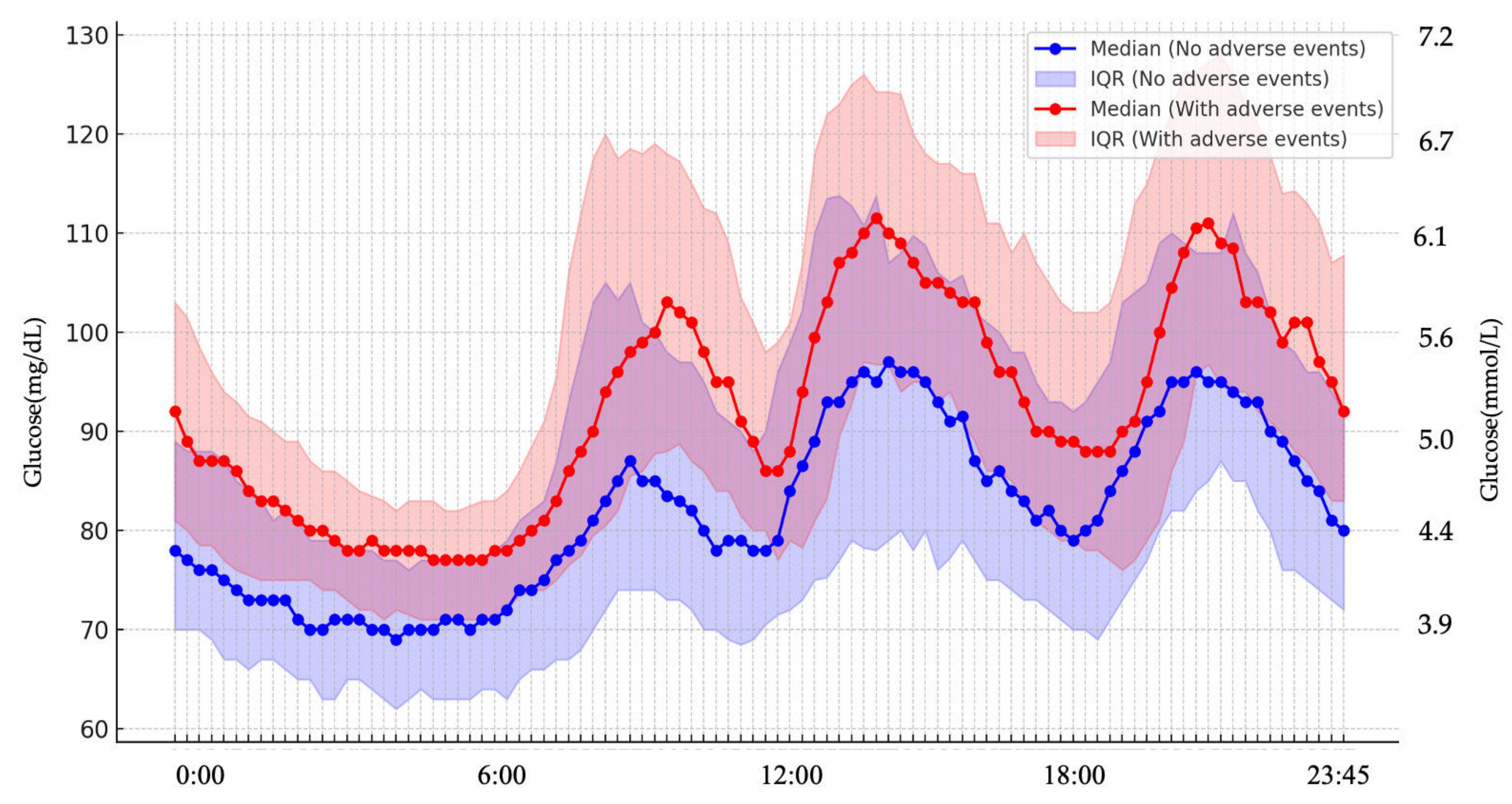
| Mean glucose level (mg/dL) | 85.6 ± 7.7 | 93.8 ± 5.9 | <0.001 * |
| Mean glucose level (mg/dL) 6:00–24:00 | 90.9 ± 7.9 | 101.1 ± 6.3 | <0.001 * |
| Mean glucose level (mg/dL) 0:00–06:00 | 78.1 ± 9.2 | 86.1 ± 8.4 | 0.006 * |
| Markers of glucose variability | |||
| SD b (mg/dL) | 19.7 (17.9–22.4) | 20.8 (18.8–25.9) | 0.162 |
| CV c (%) | 23 (21.0–26.5) | 22 (19.2–25.7) | 0.173 |
| MAGE d (mg/dL) | 48.7 (44.9–56.1) | 51.1 (46.7–62.0) | 0.148 |
| MODD e (mg/dL) | 16.3 ± 3.4 | 16.6 ± 2.5 | 0.749 |
| Time above range (%) | 1.4 (0.3–2.5) | 2.9 (1.9–5.6) | 0.003 * |
| Time in range (%) | 94.0 (86.5–96.9) | 96.5 (90.8–97.3) | 0.385 |
| Time below range (%) | 4.5 (0.6–11.6) | 0.6 (0.0–3.4) | 0.015 * |
| Without GDM (n = 70) | GDM (n = 45) | p-Value | |
|---|---|---|---|
| Age (years) | 35 (31–38) | 38 (33–40) | 0.046 * |
| Body weight (kg) | 54 (50–60) | 52 (48–58) | 0.186 |
| Body mass index (kg/m2) | 21.3 (19.9–23.5) | 21.3 (19–23.1) | 0.498 |
| Weight gain (kg) at diagnosis | 6.3 (3.6–8.0) | 6.0 (4.2–8.0) | 0.977 |
| Total weight gain (kg) | 10.3 (7.6–12.2) | 9.0 (6.7–12.1) | 0.236 |
| IVF b, n (%) | 20 (28.5) | 18 (40) | 0.227 |
| Primiparous, n (%) | 39 (55.7) | 28 (62.2) | 0.563 |
| Plasma glucose levels at 60 min after 50g-GCT c (mg/dL) | 154 (144–160) | 160 (150–169) | 0.015 * |
| Family history of diabetes, n (%) | 28 (40.0) | 23 (51.1) | 0.255 |
| HDL-C d (mg/dL) | 74.9 ± 15.3 | 78.3 ± 14.5 | 0.274 |
| LDL-C e (mg/dL) | 151.6 ± 43.5 | 158.1 ± 42.2 | 0.469 |
| Triglycerides (mg/dL) | 206 (166–271) | 195 (165–234) | 0.739 |
| HbA1c f (%) at diagnosis | 5.1 (5.0–5.4) | 5.3 (5.1–5.4) | 0.127 |
| Glycated albumin (mg/dL) | 12.7 ± 0.9 | 12.9 ± 1.0 | 0.278 |
| SAF g (AU) | 1.8 (1.6–2.0) | 1.9 (1.7–2.3) | 0.002 * |
| SAF(AU) adjusted for maternal age | 1.8 (1.6–2.0) | 1.9 (1.7–2.3) | 0.001 * |
| d-ROMs h (U.CARR) i | 620 ± 140 | 676 ± 116 | 0.027 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakuto, Y.; Ohara, M.; Koide, K.; Osamura, A.; Matsuura, R.; Yamagishi, S.-i.; Sekizawa, A. Skin Autofluorescence and Perinatal Outcomes in Pregnant Women with a Positive Glucose Challenge Test: A Prospective Study with Exploratory Analyses of Oxidative Stress and CGM Metrics. J. Clin. Med. 2025, 14, 8796. https://doi.org/10.3390/jcm14248796
Kakuto Y, Ohara M, Koide K, Osamura A, Matsuura R, Yamagishi S-i, Sekizawa A. Skin Autofluorescence and Perinatal Outcomes in Pregnant Women with a Positive Glucose Challenge Test: A Prospective Study with Exploratory Analyses of Oxidative Stress and CGM Metrics. Journal of Clinical Medicine. 2025; 14(24):8796. https://doi.org/10.3390/jcm14248796
Chicago/Turabian StyleKakuto, Yuri, Makoto Ohara, Keiko Koide, Anna Osamura, Rei Matsuura, Sho-ichi Yamagishi, and Akihiko Sekizawa. 2025. "Skin Autofluorescence and Perinatal Outcomes in Pregnant Women with a Positive Glucose Challenge Test: A Prospective Study with Exploratory Analyses of Oxidative Stress and CGM Metrics" Journal of Clinical Medicine 14, no. 24: 8796. https://doi.org/10.3390/jcm14248796
APA StyleKakuto, Y., Ohara, M., Koide, K., Osamura, A., Matsuura, R., Yamagishi, S.-i., & Sekizawa, A. (2025). Skin Autofluorescence and Perinatal Outcomes in Pregnant Women with a Positive Glucose Challenge Test: A Prospective Study with Exploratory Analyses of Oxidative Stress and CGM Metrics. Journal of Clinical Medicine, 14(24), 8796. https://doi.org/10.3390/jcm14248796






