1. Introduction
Adenomyosis (A) is a benign uterine disorder defined by the presence of ectopic endometrial glands and stroma within the myometrium. The endometrial glands extend at least 2.5 mm beneath the basal endometrium, frequently leading to uterine enlargement [
1]. Traditionally considered a condition of multiparous women aged 40–50 years, A is increasingly recognized in younger patients, with 5–25% of cases diagnosed before the age of 39 [
2]. The disease frequently coexists with uterine fibroids (35–55%) and endometriosis (65–70%) [
3]. Reported risk factors include multiparity, age over 40 years, and previous uterine surgery such as cesarean section [
4].
Clinically, approximately two-thirds of affected women present with symptoms including dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility [
5]. Beyond its symptomatic burden, A is increasingly recognized as an important factor in reproductive medicine. Evidence suggests detrimental effects on both spontaneous conception and assisted reproductive technology (ART) outcomes [
6]. Several systematic reviews and meta-analyses confirm the negative impact of A on fertility, showing reduced clinical pregnancy rates, higher miscarriage rates (31% vs. 14.1% in controls), and impaired live birth outcomes [
7,
8,
9]. More recently, large cohort analyses have also demonstrated an association between A and adverse perinatal outcomes [
10].
The underlying mechanisms by which A contributes to infertility remain incompletely understood, partly due to the frequent coexistence of endometriosis. Hypothesized pathways include disruption of myometrial architecture, abnormal uterine contractility, impaired uterine peristalsis, elevated intrauterine pressure, altered steroid hormone signaling, chronic inflammation, oxidative stress, and downregulation of implantation-related molecular markers [
11]. Importantly, alterations of the junctional zone (JZ)—the inner myometrium critical for uterine peristalsis and implantation—are believed to play a central role [
12].
The JZ can be accurately assessed in the coronal plane using three-dimensional transvaginal sonography (3D-TVUS). Morphological abnormalities of the JZ observed by 3D ultrasound have been correlated with A and provide high diagnostic accuracy [
12]. According to the Morphological Uterus Sonographic Assessment (MUSA) group, the myometrium is divided into three layers: the JZ (inner myometrium), the middle myometrium (extending to the arcuate vascular plexus), and the outer myometrium adjacent to the serosa [
12]. The JZ was first described as a hypoechoic halo surrounding the endometrium [
13,
14]. While two-dimensional transvaginal ultrasonography (2D-TVUS) provides limited evaluation, 3D-TVUS allows complete multiplanar assessment and superior visualization, especially when combined with volume contrast imaging (VCI) [
15,
16]. JZ visibility may vary according to endometrial thickness, parity, or fibroid presence [
17], although some studies found no correlation with demographic or hormonal factors [
18].
Despite these advances, adequate JZ visualization remains technically challenging. In a study by Rasmussen et al., even in expert hands, JZ was visualized in only 44% of sagittal and 68% of coronal views [
19,
20]. Sonographic features of A include direct signs—such as hyperechoic sub endometrial lines, nodular projections, and myometrial cysts—and indirect signs including myometrial heterogeneity [
12,
15,
21,
22]. However, similar features may also occur in other uterine pathologies, including endometrial carcinoma with myometrial invasion [
23]. Recent consensus statements emphasize that diagnosis should not rely solely on JZ thickness, but rather on the integration of multiple sonographic features [
15,
24,
25,
26].
Although hysterosalpingo-contrast sonography (HyCoSy) is primarily performed for the evaluation of tubal patency in women with infertility, recent evidence suggests it may also provide valuable information regarding uterine morphology. In transvaginal 4D-HyCoSy, A has been associated with a significantly higher incidence of contrast agent reflux, reflecting potential JZ disruption and altered myometrial architecture [
27]. Moreover, HyCoSy has been shown to be a safe, cost-effective, and practical diagnostic tool, with some studies reporting spontaneous pregnancies following the procedure in women with unexplained infertility [
28]. Recent reviews further emphasize its clinical relevance, highlighting both its diagnostic potential and its limitations, such as operator dependency and variability in technique [
29]. When integrated into the infertility work-up, HyCoSy therefore offers a broader perspective, simultaneously addressing tubal and uterine factors, and complementing 2D/3D-TVUS and MRI [
22,
30].
Histologically, A is characterized by the presence of endometrial glands and stroma infiltrating ≥2–2.5 mm into the myometrium [
31,
32]. However, diagnostic thresholds remain debated, as the transition between inner and outer myometrium is gradual and superficial invasion may be physiological [
33,
34]. Structural abnormalities of the JZ include smooth muscle cell (SMC) hyperplasia, nuclear enlargement, altered cell density, and the presence of myofibroblasts indicative of chronic tissue microtrauma [
35,
36,
37]. Immunohistochemistry reveals altered α-smooth muscle actin (α-SMA) expression, increased collagen deposition, and extracellular matrix remodeling, findings consistent with chronic injury and repair [
36,
38,
39].
Histopathological confirmation remains the gold standard for diagnosing A, typically defined by ectopic endometrial glands and stroma located at least 2–2.5 mm beyond the endometrial–myometrial junction [
31,
32,
34]. Microscopic studies have demonstrated smooth muscle cell disorganization, hypertrophy, and extracellular matrix remodeling in the JZ, as well as increased vascular density at this level [
35,
36,
37]. Immunohistochemical analyses further support these findings, with increased α-smooth muscle actin (α-SMA) expression indicating myofibroblast proliferation and CK7 staining highlighting glandular invasion [
38,
39]. Such correlations between imaging and histology may help clarify the mechanisms by which A impairs uterine function and fertility.
Despite advances in histology and imaging, the lack of standardized diagnostic criteria and classification systems leads to variability in prevalence estimates and clinical interpretation [
5,
34,
40]. A major limitation remains the heterogeneity of outcome reporting across studies, underscoring the urgent need for harmonized diagnostic frameworks [
41]. Modern approaches propose phenotype-based classifications linking imaging and histology with clinical outcomes [
34,
40]. Furthermore, emerging perspectives suggest that advanced ultrasound techniques, MRI protocols, and artificial intelligence may enhance reproducibility and diagnostic accuracy in A [
42].
The aim of this study is to evaluate the morphological changes in the endo-myometrial JZ using 3D ultrasound, and to assess endometrial invasion through contrast-enhanced HyCoSy in infertile patients who wish to preserve their fertility. The study further aims to correlate the 3D ultrasound features of myometrial invasion with histopathological characteristics and the depth of glandular invasion into the myometrial structure.
2. Materials and Methods
This retrospective study included 140 women who agreed to participate and were examined between 2018 and 2024 at the Clinical County Hospital of Craiova. The study group comprised 100 patients (71.4%) diagnosed with A based on ultrasound criteria. Within this group, some presented with primary infertility (PI) and others with secondary infertility (SI).
The control group consisted of forty patients (28.6%), of which twenty (14.28%) were young women without uterine pathology who had died in road traffic accidents and whose families provided informed consent for histological analysis, and the other twenty were patients without gynecological pathology who consented to undergo complete transvaginal ultrasound (2D-TVUS and 3D-TVUS) and contrast-enhanced HyCoSy for comparative evaluation. To improve clarity, we specify that the control group included two distinct subgroups with different purposes: (B1) post-mortem controls used exclusively for histopathological comparison, and (B2) living volunteers used exclusively for ultrasound and HyCoSy imaging reference values. These two subgroups were not merged for any direct statistical comparison.
Written informed consent was obtained from all participants or their legal representatives. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics and Scientific Deontology Committee of the University of Medicine and Pharmacy of Craiova (approval no. 88/13 September 2018).
All women with PI or SI and the twenty ultrasound-evaluated controls underwent clinical and sonographic assessment. Only the living volunteers (n = 20) served as imaging controls for ultrasound-based parameters, including uterine dimensions, JZ thickness, myometrial features, and tubal patency assessed by HyCoSy. Diagnosis of A and infertility was based on clinical and ultrasound assessment, transabdominal and transvaginal (2D grayscale and color Doppler) performed with a Voluson E10 ultrasound system (GE Healthcare), equipped with 3–5 MHz transabdominal and 3–9 MHz transvaginal probes. Evaluation followed established sonographic criteria focusing on characteristics of the end myometrial JZ. All ultrasound features were described according to the standardized terminology and definitions of the MUSA consensus, including junctional zone thickness, subendometrial echogenic lines, hypoechoic striations, myometrial cysts, and JZ regularity.
Ultrasound findings were evaluated in accordance with the 2019 adenomyosis classification and reporting recommendations proposed by Van den Bosch et al. [
12], which provide a standardized framework for describing lesion type, location, extent, and junctional zone characteristics. All JZ measurements were obtained using standardized 3D multiplanar reconstruction, and scans were performed by two senior operators with over 10 years of experience in gynecologic ultrasound. Ambiguous cases were reviewed in consensus to ensure consistency and reduce inter-observer variability.
Surgical treatment (total hysterectomy) was performed in 40 patients (40%) who no longer desired fertility preservation. Uterine tissue samples from these patients were fixed in 10% formalin and processed via paraffin embedding for histological analysis.
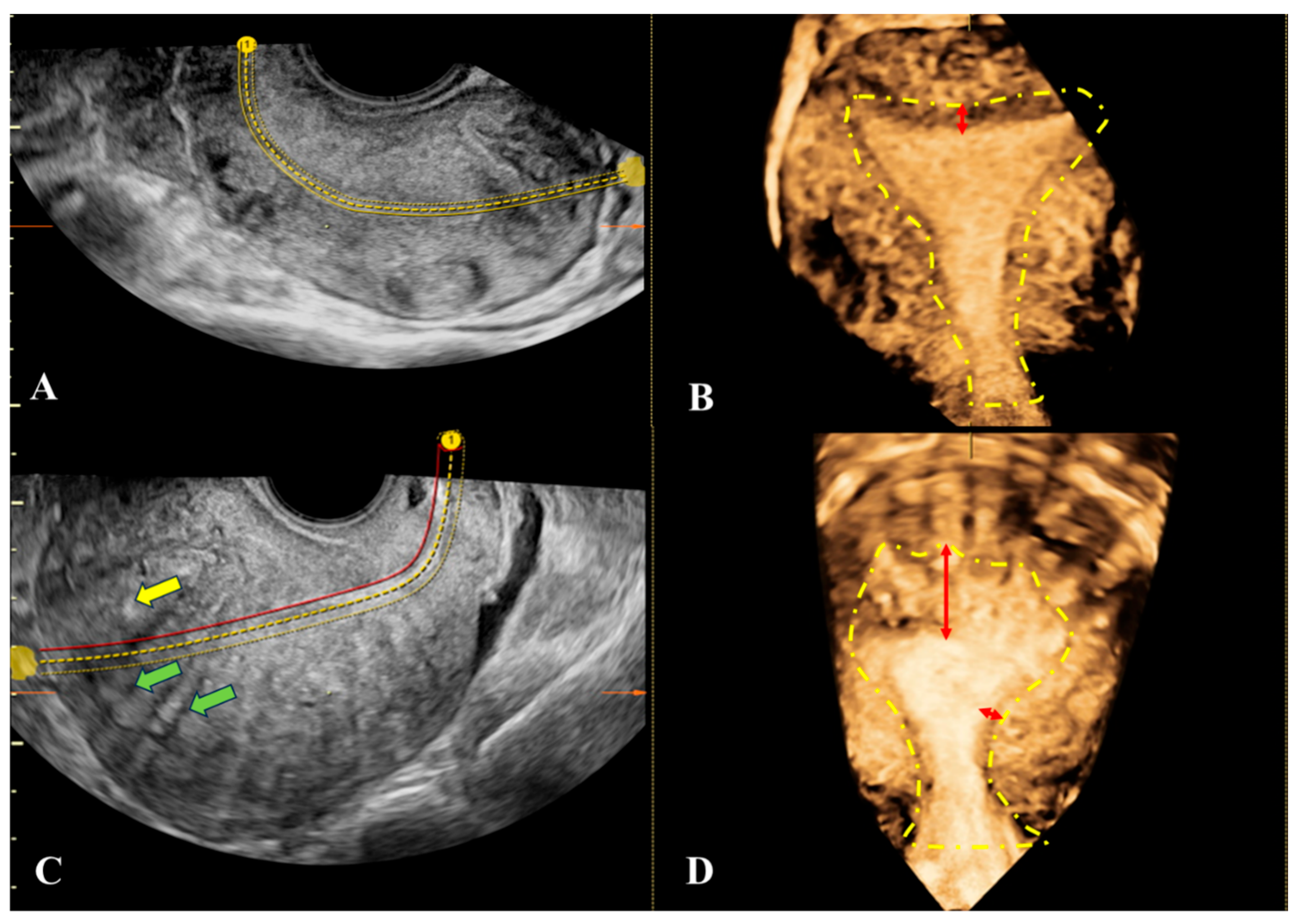
All patients attempting conception underwent additional evaluation of the uterine cavity, fallopian tubes, and tubal patency by HyCoSy using transcervical infusion of SonoVue® contrast agent (8 mL/vial, Bracco, Milan, Italy) through a Foley catheter. The investigation was performed during the early proliferative phase. Prior to contrast administration, 2D- and 3D-TVUS scans were performed with multiplanar reconstructions to evaluate the uterine size, the endometrial thickness, the minimum and maximum JZ thickness (JZmin, JZmax), the asymmetry of myometrial walls, and the presence of myometrial cysts, hyperechoic areas, and hypoechoic striations.
The presence of A was considered when JZmax > 5 mm or JZmax – JZmin > 5 mm in association with other ultrasound features These findings were correlated, when possible, with the etiology of infertility.
Each patient completed a form indicating age and infertility type (PI—group “A1” or SI—group “A2”). All collected data were centralized using Microsoft Excel, and average measurements were determined as follows: age, uterine dimensions, minimum and maximum JZ thickness, endometrial thickness, prevalence (%) of cystic lesions, presence of hyperechoic areas, hypoechoic striations, CD34+ vascular density, and smooth muscle cell density in both cases in group A2 and control cases in group B1. Statistical analysis was performed using GraphPad Prism 10—one-way ANOVA to compare mean values across the four study groups (A1, A2, B1, B2), followed by Tukey HSD post hoc testing for pairwise comparisons. Welch’s t-test (Two-Sample Assuming Unequal Variances) was applied for specific two-group comparisons where appropriate. Histological parameters were compared between adenomyosis cases and the post-mortem control subgroup, whereas ultrasound and HyCoSy parameters were compared only with the living control subgroup. No combined statistical analysis of the two control subgroups was performed. The figures display mean values and standard deviations (SDs). Regarding vascular and cellular densities, an average of 4 images were captured in JZ with a 20× objective from each specimen, using constant manual exposure and illumination settings. All elements (cells, blood vessels) were counted manually using the “manual tag” function in Image ProPlus 6.0, the average was calculated for each slide, then for each subgroup analyzed. The final percentage averages were graphically represented using Microsoft Excel, and for statistical analysis, we used the same comparative statistical tests. All histological and immunohistochemical assessments, including SMC and vascular density quantification, were performed by a senior histopathologist blinded to group allocation and clinical data. Cell counting was conducted on standardized ×200 fields using identical illumination and exposure settings, and values were recorded before unblinding for statistical analysis.
Tissue samples were analyzed in the Department of Pathology, Clinical County Emergency Hospital of Craiova, and in the Research Center for Microscopic Morphology and Immunology, University of Medicine and Pharmacy of Craiova. Tissue specimens were fixed in 4% neutral buffered formaldehyde, embedded in paraffin, and sectioned at 4 μm thickness using a microtome. A diagnosis was established using both histopathological and immunohistochemical methods. Post-mortem control specimens were used only to characterize the normal histological structure of the JZ and myometrium, serving as a reference for histopathological comparisons with A cases. Post-mortem control specimens were included because healthy uterine tissue from young women without gynecological pathology is rarely available from surgical cases. These samples were used exclusively for histopathological reference, whereas all ultrasound and HyCoSy analyses were performed only in living participants.
The immunohistochemical procedure was carried out following a standard protocol, adjusted according to the specific antibodies used. Antigen retrieval was performed by microwave heating in citrate buffer (pH 6.0), in accordance with the manufacturers’ instructions. Subsequently, the slides were incubated for 30 min in 3% hydrogen peroxide to block endogenous peroxidase activity, followed by incubation in a 3% skim milk saline solution to prevent nonspecific antibody binding. Primary antibodies (
Table 1) diluted according to the manufacturers’ recommendations (
Table 1; Dako, Glostrup, Denmark; Abcam, Cambridge, UK), were applied overnight at 4 °C followed by HRP-conjugated secondary antibodies (Nikirei-Bioscience, Tokyo, Japan). Detection was achieved with 3,3′-diaminobenzidine (DAB; Nikirei-Bioscience), and sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared in xylene, and mounted with Canadian balsam for microscopic examination.
4. Discussion
Our study provides new insights into the morphological and structural changes associated with A in women with PI and SI, emphasizing the importance of JZ alterations in diagnostic imaging and histopathology. This study shows that, in patients with A, there is a significantly greater thickening of the JZ area and a higher percentage of alterations compared to patients without A, and these JZ alterations observed by three-dimensional ultrasound are associated with A [
16].
We observed that patients with A (Group “A”) were significantly older compared to those in control group (Group “B”). This finding is consistent with the epidemiological profile described in previous studies, where A is more frequently diagnosed in women aged >40 years, often in multiparous women or those with a history of uterine surgery [
2,
3,
4]. In line with Abu Hashim et al. [
6], who reported a high prevalence of A in infertile populations, our results support the hypothesis that the disease may differentially affect fertility across age groups. JZ alterations in women with A represent an important factor contributing to infertility [
43,
44].
Our study employed modern imaging techniques to evaluate women with infertility and included in the analysis those patients who exhibited sonographic changes characteristic of this pathology. The main focus was placed on the ultrasonographic and histopathological assessment of the JZ.
Direct sonographic signs of A—including hyperechogenic sub endometrial lines, myometrial cysts, and hypoechogenic striations—were consistently observed in our cohorts. These findings align with those of Exacoustos et al. [
16] and Van den Bosch et al. [
22], who emphasized the diagnostic weight of direct sonographic features over indirect signs. In our study, intramyometrial cysts were more prevalent in A1 patients, while hypoechogenic striations were frequent in both groups (A1, A2). This may suggest that specific imaging features could predominate depending on age and reproductive history, as also noted by Tellum et al. [
24,
30].
All uterine diameters were significantly larger in group A patients with A compared to group B patients. Enlargement of the uterus in A has been consistently described in the literature [
1,
45], although the correlation between uterine size and infertility remains debated. Our findings suggest that increased uterine size may be more pronounced in women diagnosed with A.
Although uterine dimensions were significantly larger in women with adenomyosis compared to controls, this finding must be interpreted with caution given the age difference between the groups. Uterine size naturally increases with age, cumulative estrogen exposure, and parity, independently of A. Therefore, part of the observed enlargement in Group (A) may reflect age-related physiological changes rather than A alone. This age-related effect has been noted in previous studies evaluating uterine morphology across reproductive age ranges and represents a potential confounding factor in our analysis.
One of the most relevant findings of our study is the significantly greater maximum and mean JZ thickness in both A1 and A2 group compared to group B2. These results are consistent with the literature describing JZ thickening and irregularity as hallmark sonographic features of A [
12,
15,
16,
21,
22]. Tellum et al. [
24] and Rasmussen et al. [
19] highlighted that JZ > 5 mm is strongly associated with A, while Harmsen et al. [
15] emphasized the diagnostic relevance of irregular or interrupted JZ in the updated MUSA consensus.
The specific parameters used to assess the junctional zone—such as JZmax, JZmin, and the JZmax − JZmin difference—have important clinical relevance. JZmax reflects the degree of focal thickening and is associated with local invasion of endometrial tissue, while JZmin provides a reference for baseline myometrial thickness. The difference between JZmax and JZmin is considered a marker of JZ irregularity and structural distortion, both of which have been linked to impaired uterine peristalsis, altered sperm transport, and disrupted endometrial–myometrial communication. Several studies have shown that increased JZ thickness (>5 mm) or marked asymmetry predicts reduced implantation rates, poorer IVF outcomes, and greater disease severity. Therefore, the detailed evaluation of these parameters provides clinically meaningful information about the functional integrity of the JZ and its potential contribution to infertility.
Tocci et al. suggested that the “endometrial–subendometrial myometrium unit (JZ) disruption disorder” should be regarded as a distinct condition from adenomyosis, mainly characterized by pathological changes or abnormal thickening of the junctional zone (JZ) [
46]. According to other studies, the proliferation and excessive growth of smooth muscle tissue within the JZ may represent early stages that precede the expansion of endometrial cells and the development of adenomyosis [
46,
47,
48].
In our study, we found that both patients with PI (Group A1) and those with SI (Group A2) had a maximal JZ thickness greater than 5 mm, findings consistently associated with A. Additionally, in the A group, the average thickness calculated from the maximum and minimum JZ measurements also exceeded 5 mm. These observations suggest that A can be present even in younger, nulliparous patients, but appears more frequently in women with a history of at least one prior pregnancy reinforcing the role of JZ thickening in patients with SI, suggesting that repeated pregnancies and uterine trauma may accentuate these changes, as also proposed by Kishi et al. [
38].
Although our findings demonstrate significantly greater JZ thickness in women with adenomyosis, these differences must be interpreted with caution. JZ morphology is known to change with age, increasing naturally in thickness and irregularity across the reproductive years. Therefore, part of the JZ alterations observed in our cohort—particularly in the older A2 subgroup—may reflect age-related physiological remodeling rather than adenomyosis-specific changes alone. This age-related variability represents an important confounding factor and should be considered when comparing JZ parameters between groups.
The clinical utility of JZ evaluation is increasingly recognized in reproductive medicine. Beyond its diagnostic value, accumulating evidence suggests that JZ thickness and structural irregularity may also serve as predictors of reproductive outcomes and treatment response. Several studies have reported that an increased or heterogeneous JZ is associated with impaired uterine peristalsis, altered sperm transport, reduced implantation rates, and poorer ART outcomes, indicating that JZ morphology could help identify women at higher risk of subfertility or implantation failure [
7,
48,
49]. Furthermore, emerging data show that medical therapies such as dienogest or GnRH analogs may reduce JZ thickness and improve uterine function, suggesting a potential link between JZ remodeling and therapeutic benefit [
50]. In this context, our findings reinforce the concept that JZ assessment—particularly maximal thickness exceeding 5 mm—may have prognostic relevance in clinical decision-making, providing an opportunity for individualized fertility counseling and tailored treatment strategies.
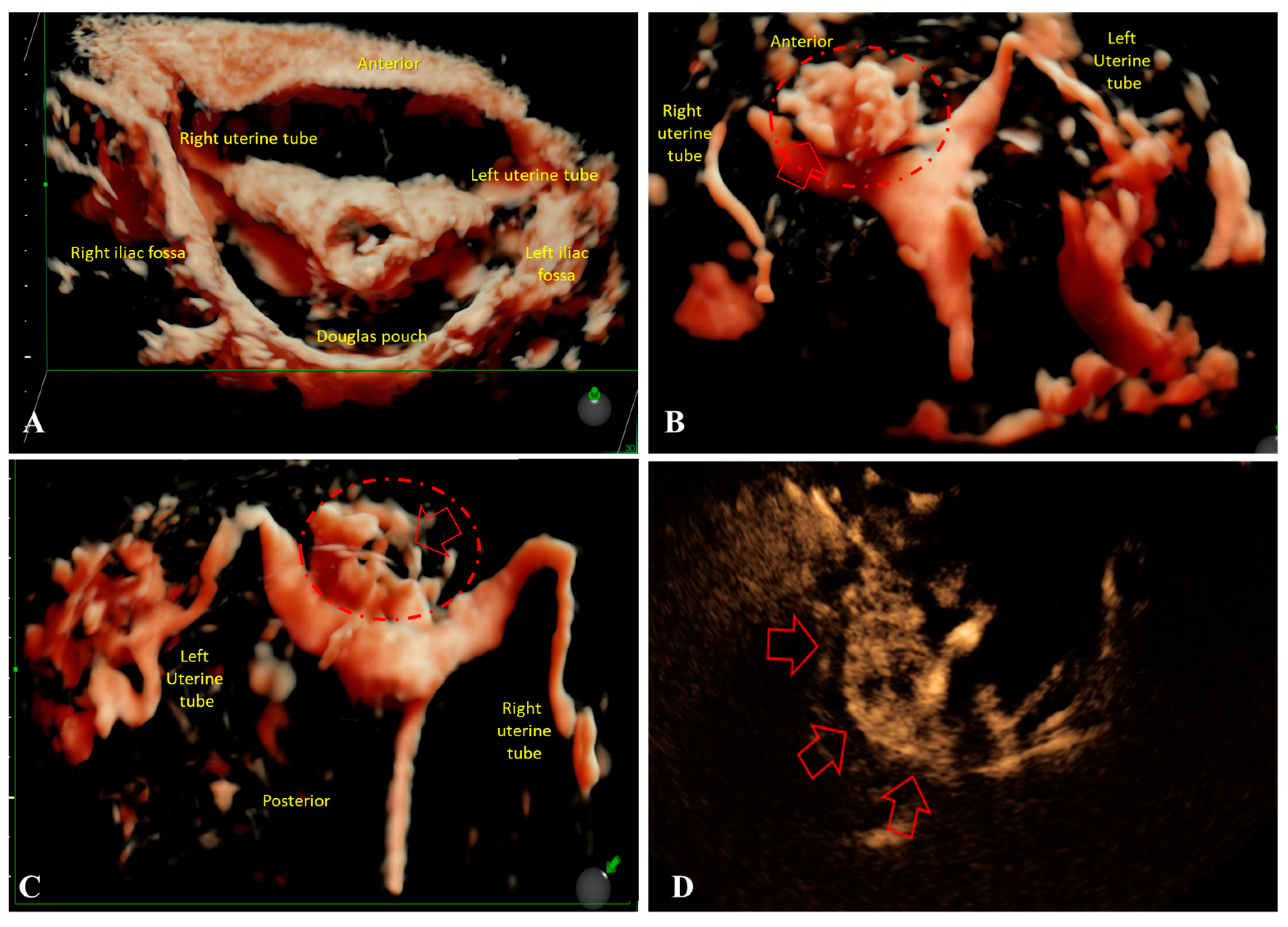
Although contrast-enhanced HyCoSy is primarily designed to evaluate tubal patency, our findings suggest that it can also provide complementary information on uterine morphology in A. In our cohort, HyCoSy revealed both focal and multifocal invasion patterns of the myometrium, which correlated with structural changes detected by 2D/3D-TVUS with similar distribution between A1 and A2 groups. Previous studies have emphasized the role of three-dimensional sonography in assessing JZ and its correlation with histopathology [
16], as well as the importance of standardized sonographic criteria such as those proposed by the MUSA group [
15,
22]. In this context, HyCoSy may serve as an ancillary tool, offering simultaneous assessment of tubal status and uterine structure, particularly relevant in the infertility work-up. Although HyCoSy revealed focal and multifocal patterns of intramyometrial contrast leakage suggestive of adenomyotic invasion, we did not perform quantitative measurements of invasion depth. At present, standardized criteria for quantifying the depth of myometrial infiltration on contrast-enhanced HyCoSy are lacking, and the technique is predominantly used qualitatively to assess the presence and distribution of leakage rather than precise millimetric penetration. In our study, HyCoSy findings were therefore interpreted in correlation with 2D/3D-TVUS morphology rather than as standalone quantitative metrics. We acknowledge this as a limitation, and future methodological developments—such as volumetric contrast analysis or automated tracking of contrast dispersion—may enable more robust quantification of invasion depth.
While HyCoSy provided valuable qualitative information on intramyometrial contrast leakage and its distribution, the diagnostic implications of these findings must be interpreted cautiously. HyCoSy is not validated as a diagnostic tool for A, and in our study, it was not systematically compared with a gold standard such as MRI or histopathology for all participants. Therefore, the observed invasion patterns should be considered complementary observations rather than definitive markers of A. Further studies incorporating direct validation are needed before HyCoSy can be reliably integrated into diagnostic algorithms for A.
Importantly, tubal obstruction—either proximal or distal—was more frequently observed in Group “A1”. This suggests that infertility in younger women may be multifactorial, combining A-related uterine changes with compromised tubal patency. While HyCoSy is not yet included among the standardized imaging criteria for A [
24,
30], its combined use with 3D-TVUS could increase diagnostic accuracy and help identify patients at higher risk of impaired fertility.
Diagnosing A through high-resolution ultrasound and contrast-enhanced HyCoSy has important implications for infertility management. The early identification of JZ thickening, irregularity, or intramyometrial invasion patterns can guide individualized therapeutic strategies aimed at optimizing reproductive outcomes. Several studies have shown that medical pretreatments—such as GnRH agonists or dienogest—may improve implantation conditions and clinical pregnancy rates in women with adeno-myosis undergoing ART [
48,
49]. In selected cases, uterine-sparing interventions, including adenomyomectomy, may further enhance fertility potential, particularly in younger patients with focal disease [
50]. Moreover, identifying adenomyosis on ultrasound can influence IVF planning by guiding decisions on modified stimulation protocols, freeze-all strategies, or delaying embryo transfer to allow adequate endometrial recovery [
51]. Taken together, our findings suggest that three-dimensional transvaginal ultra-sound, when complemented by qualitative information from HyCoSy, may offer useful insights into uterine structural alterations associated with adenomyosis in the infertility work-up. However, given the study’s retrospective design, the limited number of histologically confirmed cases, and the absence of systematic MRI validation, this combined approach should be regarded as a promising exploratory tool rather than a fully established diagnostic method.
Our findings also align with recent efforts to standardize the sonographic evaluation of A. The 2019 ultrasound-based classification proposed by Van den Bosch et al. [
12] provides a structured framework for describing lesion type, extent, and junctional zone abnormalities, reinforcing the importance of consistent terminology in clinical and research settings. Although variability in JZ measurements on 3D-TVUS is acknowledged in the literature, especially regarding reconstruction angle and operator technique, the strong concordance between imaging and histology in our cohort supports the reliability of advanced ultrasound when performed using standardized criteria. These developments highlight the growing movement toward harmonized reporting of A and underscore the need for continued refinement of imaging-based classification systems.
Although this study was not primarily designed to evaluate diagnostic accuracy, the subset of 40 patients who underwent hysterectomy allowed us to make observations regarding the diagnostic performance of ultrasound. All 40 women showed 2D/3D-TVUS features highly suggestive of A—including JZ thickening >5 mm, myometrial cysts, hyperechoic islands, and irregular or disrupted JZ contours—and all were subsequently confirmed to have A on histopathological analysis. This complete concordance indicates a high positive predictive value of 2D/3D ultrasound for detecting moderate-to-severe A in this selected cohort. These findings are consistent with previous studies demonstrating strong agreement between advanced transvaginal ultrasound features and histopathology, particularly in patients with pronounced morphological changes and when examinations are performed by experienced operators [
16,
52]. While the retrospective design and selection bias limit formal sensitivity or specificity estimation, this observation reinforces the clinical reliability of ultrasound in identifying A in symptomatic infertile women.
In the A2 subgroup, hysterectomy was not performed for infertility itself but was indicated due to severe A-related symptoms. Most patients experienced a combination of debilitating Dy, Men, and, in several cases, Met or Hy, all contributing to significant impairment of quality of life. These symptoms persisted despite medical management. Moreover, patients in A2 exhibited markedly reduced ovarian reserve (mean AMH 0.21 ± 0.15 ng/mL), which further limited reproductive potential and influenced the decision toward definitive surgical treatment. Thus, hysterectomy in this group primarily reflected symptom severity rather than infertility management.
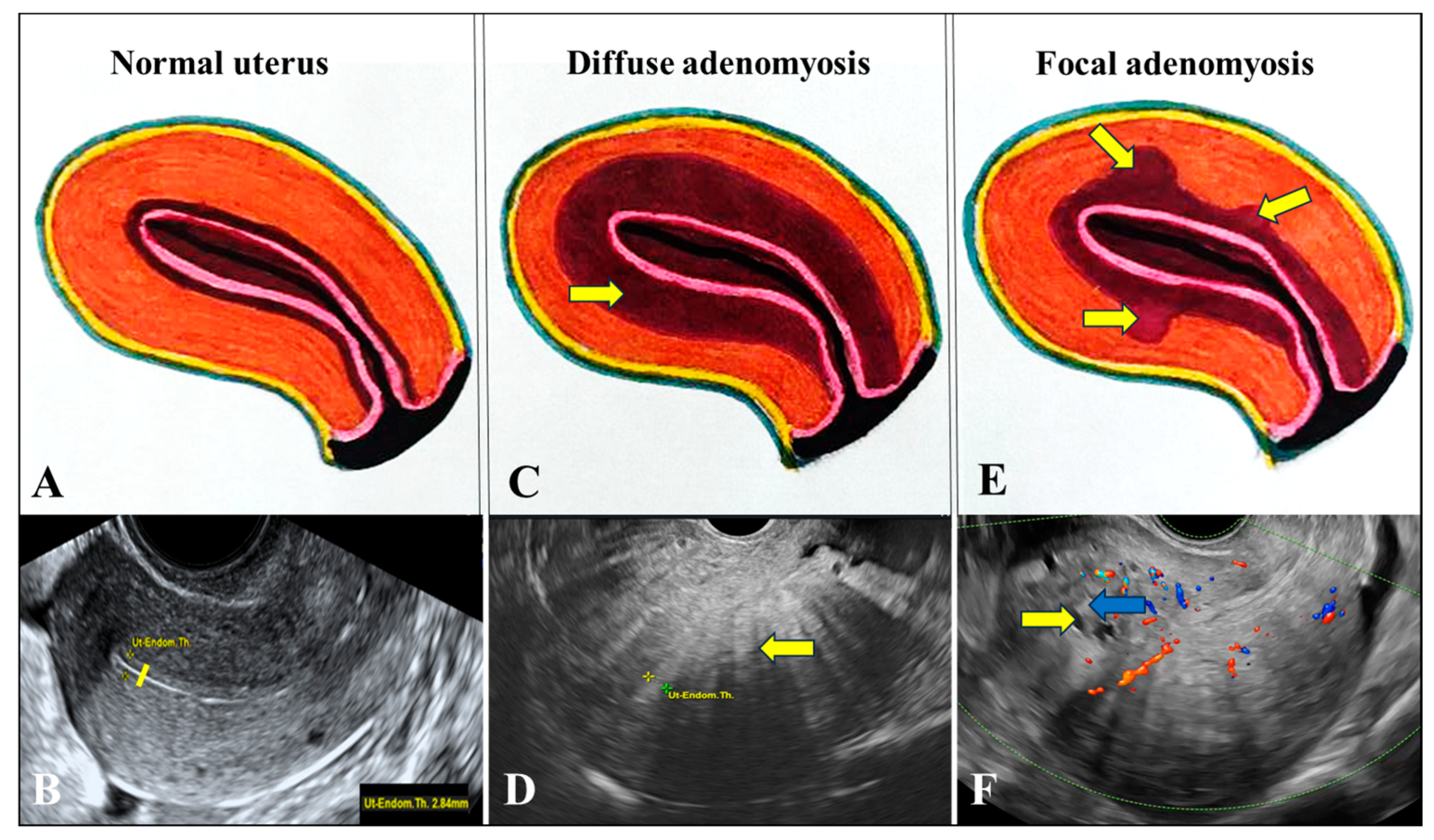
In A, the uterine JZ shows thickening and discontinuities caused by the invasion of endometrial tissue into the myometrium. Normally, the JZ presents a smooth transition between the endometrium and the myometrium, but in A, this boundary becomes irregular and blurred, containing endometrial glands and stroma within the muscle wall. This process results in typical microscopic changes, such as small cystic formations, hemorrhage, and a thickened, uneven appearance visible on imaging [
53].
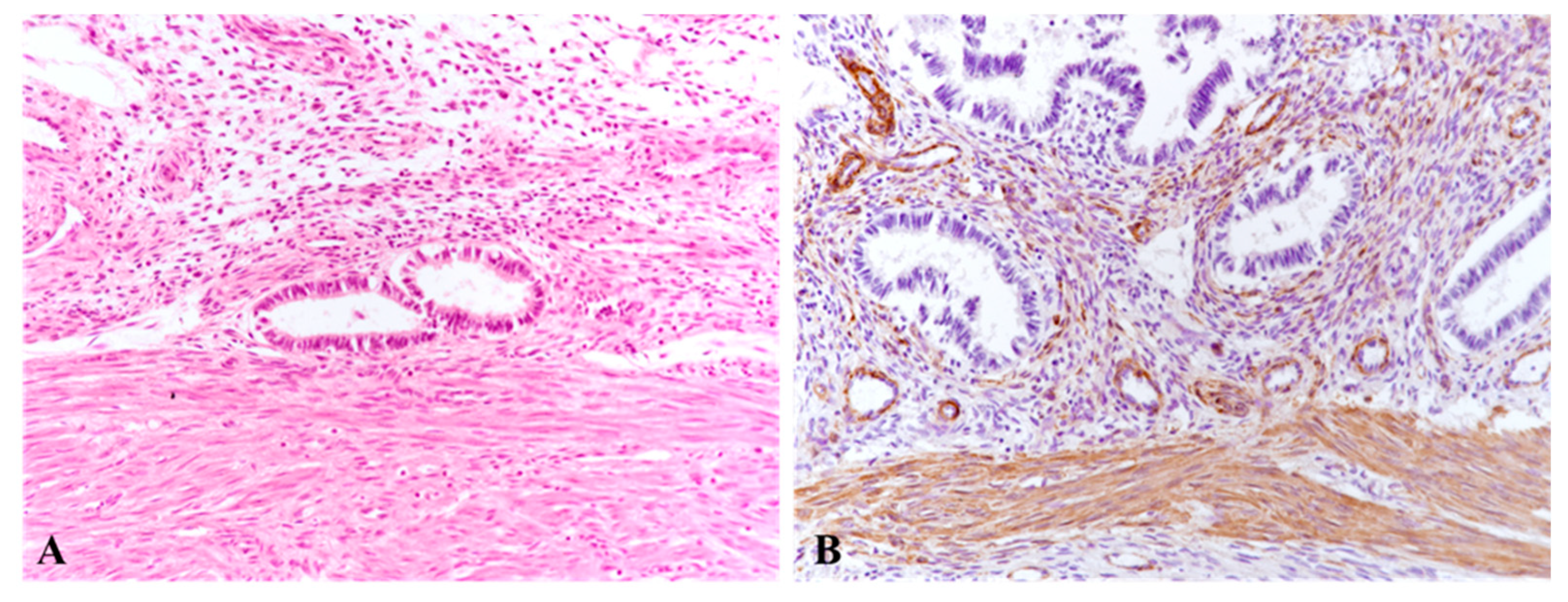
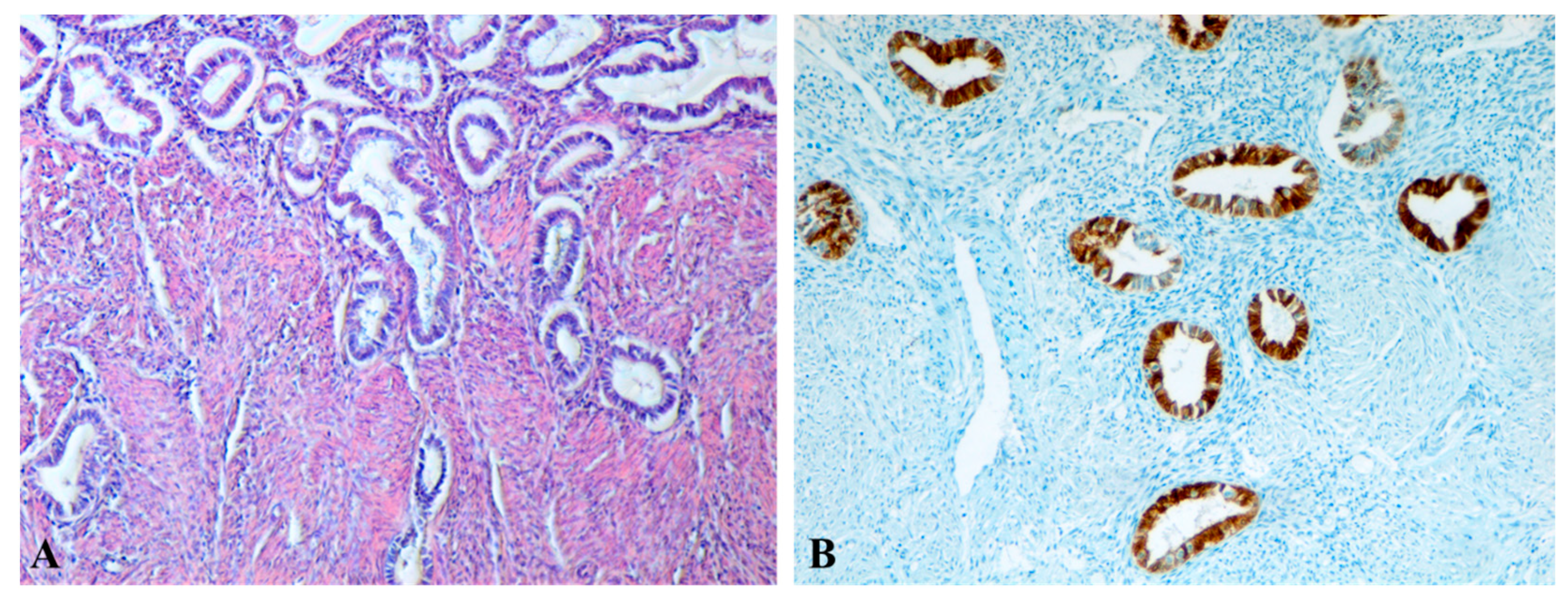
Histology confirmed the presence of ectopic endometrial glands and stroma within the myometrium, associated with SMC disorganization, hypertrophy, and metaplasia.
While two studies reported no difference in α-SMA expression between uteri with and without A [
13,
38], our findings demonstrated a reduction in SMCs density, resulting from cell hypertrophy and structural disorganization due to the invasion of endometrial glands, together with an increase in CD34+ vascular density, indicating intensified angiogenesis and ongoing tissue remodeling processes described by Ibrahim et al. [
36] and Mehasseb et al. [
35]. Collagen remodeling and disruption of the parallel orientation between SMCs and endometrial glands further support the hypothesis that A involves altered myometrial contractility and extracellular matrix remodeling [
31,
38,
39]. These changes may explain impaired uterine peristalsis and suboptimal conditions for implantation, as also discussed by Tamura et al. [
11] and Vercellini et al. [
7].
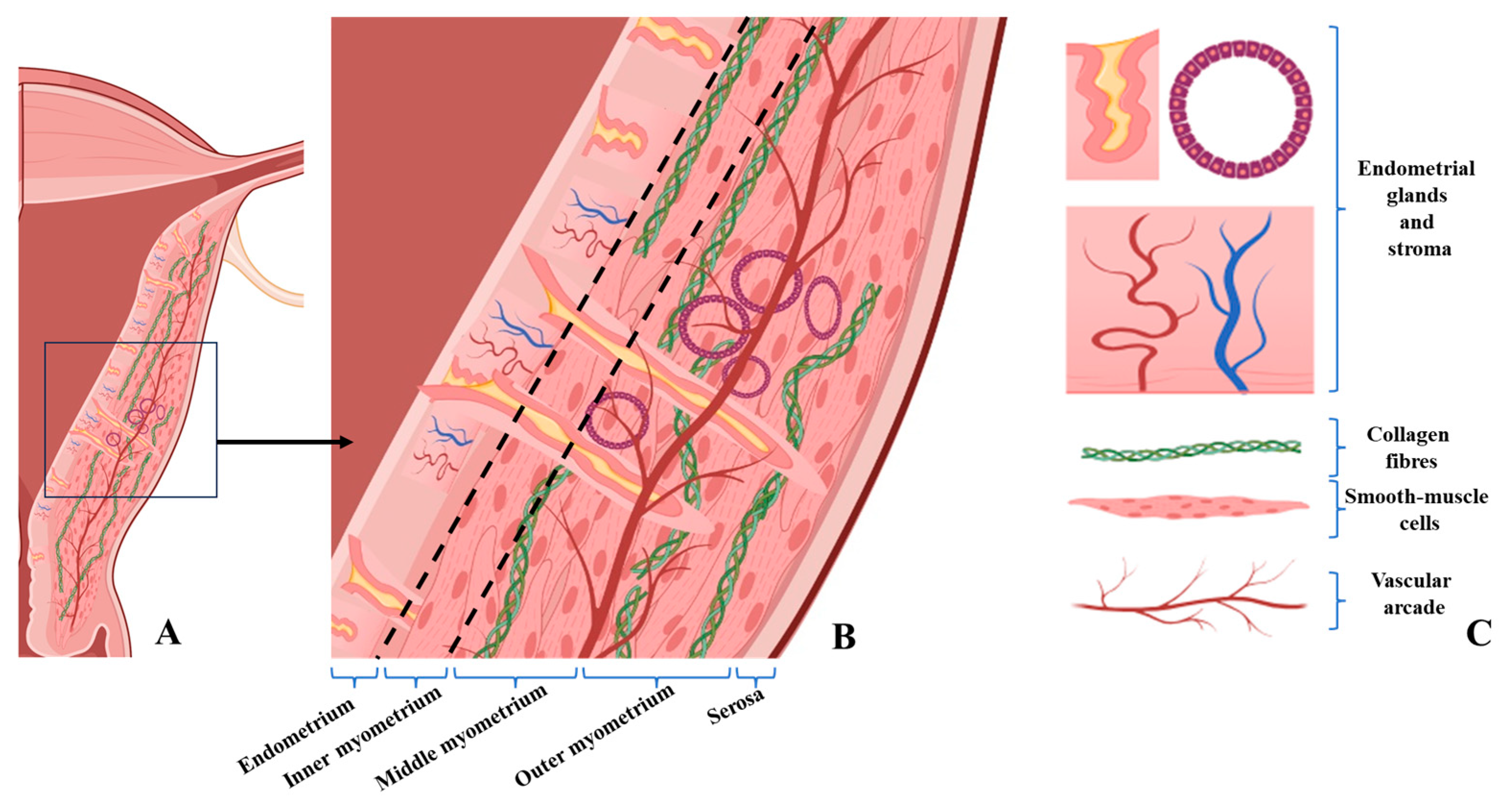
The schematic diagrams were designed to synthesize and visually illustrate the key histopathological findings observed in our study. The depicted features—including glandular invasion beyond the junctional zone, disruption of smooth muscle cell orientation, expansion of loose connective tissue rich in collagen fibers, and the increased vascular network—directly reflect the microscopic changes documented on HE and immunohistochemical staining (α-SMA and CD34). These diagrams therefore serve as an integrative visual summary that complements the histological images by contextualizing how the structural alterations detected microscopically manifest within the broader architecture of the uterine wall.
Our results underline the clinical significance of JZ assessment in A-related infertility. The finding that JZ thickening is more pronounced in group A2 of patients suggests a progressive nature of the disease, with cumulative effects over time and parity. This supports the use of JZ thickness as a prognostic marker in reproductive outcomes, consistent with recent reviews [
8,
9]. Furthermore, the comparable diagnostic performance of 3D-TVUS and MRI reported by Alcázar et al. [
25] highlights the practicality of ultrasound as a first-line tool in infertility work-up.
The present study has several limitations. The sample size was relatively small, and the retrospective design may introduce selection bias. Histological confirmation of A was available only for hysterectomy specimens, using standard Hematoxylin–Eosin staining as well as immunohistochemical staining with the anti-CK7 antibody, which highlighted the presence of endometrial glands within the myometrial structure (Group A2), limiting direct histological–imaging correlation in Group A2. Additionally, the coexistence of tubal obstruction and A complicates the attribution of infertility to A alone. These challenges have been repeatedly emphasized in the literature [
5,
7,
11,
30].
A major limitation of this study is the statistically significant age disparity between the adenomyosis group and the control group. Age is known to influence several reproductive and uterine parameters, including junctional zone thickness, uterine volume, ovarian reserve, and the prevalence and severity of symptoms. Therefore, part of the structural and functional differences observed between groups—such as increased JZ thickness, uterine enlargement, and reduced AMH levels—may be attributable to age-related physiological changes rather than adenomyosis alone. This represents a significant confounding factor, and our findings must be interpreted with caution. Future studies with age-matched control groups are needed to identify how adenomyosis may influence these parameters. Interpretation of the literature concerning the JZ must be approached with caution, given the variability between imaging modalities (such as TVUS) and the absence of a standardized definition. While conventional histology does not reveal the functional characteristics of the JZ, imaging studies increasingly suggest a significant functional role of this structure in uterine pathologies, including A.
Future research should focus on validating our findings in larger, prospective, and preferably multicenter cohorts to reduce the impact of sample size and selection bias. Standardization of diagnostic criteria, particularly through the integration of updated MUSA definitions, is needed to improve the reproducibility of imaging assessments. The correlation between ultrasound phenotypes of A and reproductive outcomes should be further explored, ideally by incorporating detailed information on embryo implantation and assisted reproductive technology (ART) success rates.
There is a pressing need to develop a unified definition of A that includes clear criteria for both histopathological and imaging evaluation. Translational studies combining imaging findings with molecular and immunohistochemical profiling could help refine phenotype-based classifications of A and clarify the underlying mechanisms linking JZ alterations to infertility.
Moreover, the integration of artificial intelligence and machine learning algorithms into ultrasound image analysis has the potential to enhance diagnostic accuracy and reduce inter-observer variability.
Furthermore, longitudinal studies starting from adolescence are warranted to monitor progressive changes in the JZ and their potential evolution toward A.