Chronic Hydroxyurea Therapy in Children with Sickle Cell Anemia: Mechanisms of Action, Systemic Effects, and Long-Term Safety
Abstract
1. Background
2. Methods
3. Clinical Indications of Hydroxyurea Therapy in Children with Sickle Cell Anemia
4. Mechanism of Action of Hydroxyurea
5. Non-Hematological Effects of Hydroxyurea Therapy in Children with Sickle Cell Anemia
5.1. Neurological Effects
5.2. Immunological Effects
5.3. Endocrinological Effects
6. Adverse and Toxic Effects of Hydroxyurea Therapy in Children with Sickle Cell Anemia
7. Conflicting Evidence and Sources of Heterogeneity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piel, F.B.; Rees, D.C.; DeBaun, M.R.; Nnodu, O.; Ranque, B.; A Thompson, A.; E Ware, R.; Abboud, M.R.; Abraham, A.; Ambrose, E.E.; et al. Defining global strategies to improve outcomes in sickle cell disease: A Lancet Haematology Commission. Lancet Haematol. 2023, 10, e633–e686. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18011. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- GBD 2021 Sickle Cell Disease Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- Colombatti, R.; Perrotta, S.; Samperi, P.; Casale, M.; Masera, N.; Palazzi, G.; Sainati, L.; Russo, G.; on behalf of the Italian Association of Pediatric Hematology-Oncology (AIEOP) Sickle Cell Disease Working Group. Organizing national responses for rare blood disorders: The Italian experience with sickle cell disease in childhood. Orphanet J. Rare Dis. 2013, 8, 169. [Google Scholar] [CrossRef]
- Kavanagh, P.L.; Fasipe, T.A.; Wun, T. Sickle cell disease: A review. JAMA 2022, 328, 57–68. [Google Scholar] [CrossRef]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Brandow, A.M.; Liem, R.I. Advances in the diagnosis and treatment of sickle cell disease. J. Hematol. Oncol. 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; McGowan, V.; Machado, R.F.; Little, J.A.; Taylor, J.; Morris, C.R.; Nichols, J.S.; Wang, X.; Poljakovic, M.; Morris, S.M.; et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood 2005, 106, 3188. [Google Scholar] [CrossRef]
- Nouraie, M.; Lee, J.S.; Zhang, Y.; Kanias, T.; Zhao, X.; Xiong, Z.; Oriss, T.B.; Zeng, Q.; Kato, G.J.; Gibbs, J.S.R.; et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013, 98, 464–472. [Google Scholar] [CrossRef]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of sickle cell disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef]
- Steinberg, M.H.; Sebastiani, P. Genetic modifiers of sickle cell disease. Am. J. Hematol. 2012, 87, 795–803. [Google Scholar] [CrossRef]
- Sebastiani, P.; Ramoni, M.F.; Nolan, V.; Baldwin, C.T.; Steinberg, M.H. Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia. Nat. Genet. 2005, 37, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Gavins, F.N.E. Ischemia-reperfusion injury in sickle cell disease: From basics to therapeutics. Am. J. Pathol. 2019, 189, 706–718. [Google Scholar] [CrossRef]
- Darbari, D.S.; Sheehan, V.A.; Ballas, S.K. The vaso-occlusive pain crisis in sickle cell disease: Definition, pathophysiology, and management. Eur. J. Haematol. 2020, 105, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Turhan, A.; Weiss, L.A.; Mohandas, N.; Coller, B.S.; Frenette, P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proc. Natl. Acad. Sci. USA 2002, 99, 3047–3051. [Google Scholar] [CrossRef]
- Kohne, E. Hemoglobinopathies: Clinical manifestations, diagnosis, and treatment. Dtsch. Arztebl. Int. 2011, 108, 532–540. [Google Scholar] [PubMed]
- Egesa, W.I.; Nakalema, G.; Waibi, W.M.; Turyasiima, M.; Amuje, E.; Kiconco, G.; Odoch, S.; Kumbakulu, P.K.; Abdirashid, S.; Asiimwe, D. Sickle cell disease in children and adolescents: A review of the historical, clinical, and public health perspective of sub-Saharan Africa and beyond. Int. J. Pediatr. 2022, 2022, 3885979. [Google Scholar] [CrossRef]
- McCavit, T.L. Sickle cell disease. Pediatr. Rev. 2012, 33, 195–204. [Google Scholar] [CrossRef]
- Casale, M.; Casciana, M.L.; Ciliberti, A.; Colombatti, R.; Del Vecchio, G.C.; Fasoli, S.; Scacco, C.F.; Giordano, P.; Kiren, V.; Ladogana, S.; et al. Linee-Guida per la Gestione della Malattia Drepanocitica in eta’ Pediatrica in Italia; Associazione Italiana Ematologia Oncologia Pediatrica: Bologna, Italy, 2023. [Google Scholar]
- Quinn, C.T.; Ware, R.E. The modern use of hydroxyurea for children with sickle cell anemia. Haematologica 2025, 110, 1061–1073. [Google Scholar] [CrossRef]
- Wang, W.; Zou, P.; Hwang, S.; Kang, G.; Ding, J.; Heitzer, A.; Schreiber, J.E.; Helton, K.; Hankins, J.S. Effects of Hydroxyurea on Brain Function in Children with Sickle Cell Anemia; Authorea, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Nottage, K.A.; Ware, R.E.; Aygun, B.; Smeltzer, M.; Kang, G.; Moen, J.; Wang, W.C.; Hankins, J.S.; Helton, K.J. Hydroxycarbamide treatment and brain MRI/MRA findings in children with sickle cell anaemia. Br. J. Haematol. 2016, 175, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.E.; Guilliams, K.P.; Ragan, D.; Binkley, M.M.; Mirro, A.; Fellah, S.; Hulbert, M.L.; Blinder, M.; Eldeniz, C.; Vo, K.; et al. Hydroxyurea reduces cerebral metabolic stress in patients with sickle cell anemia. Blood 2019, 133, 2436–2444. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Zou, P.; da Rocha, J.L.D.; Heitzer, A.M.; Patni, T.; Li, Y.; Scoggins, M.A.; Sharma, A.; Wang, W.C.; Helton, K.J.; et al. Hydroxyurea maintains working memory function in pediatric sickle cell disease. PLoS ONE 2024, 19, e0296196. [Google Scholar] [CrossRef]
- Abdullahi, S.U.; Jibir, B.W.; Bello-Manga, H.; Gambo, S.; Inuwa, H.; Tijjani, A.G.; Idris, N.; Galadanci, A.; Hikima, M.S.; Galadanci, N.; et al. Hydroxyurea for primary stroke prevention in children with sickle cell anaemia in Nigeria (SPRING): A double-blind, multicentre, randomised, phase 3 trial. Lancet Haematol. 2022, 9, e26–e37. [Google Scholar] [CrossRef]
- Brandling-Bennett, E.M.; White, D.A.; Armstrong, M.M.; Christ, S.E.; DeBaun, M. Patterns of verbal long-term and working memory performance reveal deficits in strategic processing in children with frontal infarcts related to sickle cell disease. Dev. Neuropsychol. 2003, 24, 423–434. [Google Scholar] [CrossRef]
- King, A.A.; Strouse, J.J.; Rodeghier, M.J.; Compas, B.E.; Casella, J.F.; McKinstry, R.C.; Noetzel, M.J.; Quinn, C.T.; Ichord, R.; Dowling, M.M.; et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am. J. Hematol. 2014, 89, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, F.; Verlhac, S.; Arnaud, C.; Kamdem, A.; Hau, I.; Leveillé, E.; Vasile, M.; Kasbi, F.; Madhi, F.; Fourmaux, C.; et al. Long-term treatment follow-up of children with sickle cell disease monitored with abnormal transcranial Doppler velocities. Blood 2016, 127, 1814–1822. [Google Scholar] [CrossRef]
- Heitzer, A.M.; Longoria, J.; Okhomina, V.; Wang, W.C.; Raches, D.; Potter, B.; Jacola, L.M.; Porter, J.; Schreiber, J.E.; King, A.A.; et al. Hydroxyurea treatment and neurocognitive functioning in sickle cell disease from school age to young adulthood. Br. J. Haematol. 2021, 195, 256–266. [Google Scholar] [CrossRef]
- Partanen, M.; Kang, G.; Wang, W.C.; Krull, K.; King, A.A.; Schreiber, J.E.; Porter, J.S.; Hodges, J.; Hankins, J.S.; Jacola, L.M. Association between hydroxycarbamide exposure and neurocognitive function in adolescents with sickle cell disease. Br. J. Haematol. 2020, 189, 1192–1203. [Google Scholar] [CrossRef]
- Ware, R.E.; Davis, B.R.; Schultz, W.H.; Brown, R.C.; Aygun, B.; Sarnaik, S.; Odame, I.; Fuh, B.; George, A.; Owen, W.; et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia—TCD With Transfusions Changing to Hydroxyurea (TWiTCH): A multicentre, open-label, phase 3, non-inferiority trial. Lancet 2016, 387, 661–670. [Google Scholar] [CrossRef]
- Emokpae, M.A.; Aruomaren, A.; Osime, E. Relationship between neutrophil-to-lymphocyte ratio and inflammatory markers in sickle cell anaemia patients with proteinuria. Med. Sci. 2016, 4, 11. [Google Scholar] [CrossRef]
- Chou, S.T.; Jackson, T.; Vege, S.; Smith-Whitley, K.; Friedman, D.F.; Westhoff, C.M. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood 2013, 122, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Elsayh, K.I.; Saad, K.; Embaby, M.M.; Youssef, M.A.M.; Abdel-Raheem, Y.F.; Sror, S.M.; Galal, S.M.; Hetta, H.F.; Aboul-Khair, M.D.; et al. Circulating microparticles in children with sickle cell anemia in a tertiary center in upper Egypt. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619828839. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Saad, K.; Elsayh, K.I.; Khalaf, S.M.; Mahmoud, K.H.; Elhoufey, A.; Hetta, H.F. Regulatory T-cell phenotypes in children with sickle cell disease. Pediatr. Res. 2022, 91, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, M.J.B.M.; da Silva, R.R.; Pereira, M.C.; Araújo, A.d.S.; Pitta, I.d.R.; Falcão, D.A.; Bezerra, M.A.C.; Pitta, M.G.d.R. Evaluation of CD4+CD25+FoxP3+ T cell populations, IL-10 production, and their correlation with clinical and biochemical parameters in sickle cell anemia patients with leg ulcers. Cytokine 2015, 75, 310–315. [Google Scholar] [CrossRef]
- Zahran, A.M.; Nafady, A.; Saad, K.; Hetta, H.F.; Abdallah, A.-E.M.; Abdel-Aziz, S.M.; Embaby, M.M.; Elgheet, A.M.A.; Darwish, S.F.; Abo-Elela, M.G.M.; et al. Effect of hydroxyurea treatment on the inflammatory markers among children with sickle cell disease. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029619895111. [Google Scholar] [CrossRef]
- Rhodes, M.; Akohoue, S.A.; Shankar, S.M.; Fleming, I.; An, A.Q.; Yu, C.; Acra, S.; Buchowski, M.S. Growth patterns in children with sickle cell anemia during puberty. Pediatr. Blood Cancer 2009, 53, 635–641. [Google Scholar] [CrossRef]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Adel, A. Growth and growth hormon-insulin like growth factor-I (GH-IGF-I) axis in chronic anemias. Acta Biomed. 2017, 88, 101–111. [Google Scholar]
- Wang, W.C.; Helms, R.W.; Lynn, H.S.; Redding-Lallinger, R.; Gee, B.E.; Ohene-Frempong, K.; Smith-Whitley, K.; Waclawiw, M.A.; Vichinsky, E.P.; Styles, L.A.; et al. Effect of hydroxyurea on growth in children with sickle cell anemia: Results of the HUG-KIDS Study. J. Pediatr. 2002, 140, 225–229. [Google Scholar] [CrossRef]
- Nagalapuram, V.M.; Kulkarni, V.M.; Leach, J.; Aban, I.; Sirigaddi, K.M.; Lebensburger, J.D.D.; Iyer, P. Effect of sickle cell anemia therapies on the natural history of growth and puberty patterns. J. Pediatr. Hematol. Oncol. 2019, 41, 606–611. [Google Scholar] [CrossRef]
- Colombatti, R.; Palazzi, G.; Masera, N.; Notarangelo, L.D.; Bonetti, E.; Samperi, P.; Barone, A.; Perrotta, S.; Facchini, E.; Miano, M.; et al. Hydroxyurea prescription, availability and use for children with sickle cell disease in Italy: Results of a National Multicenter survey. Pediatr. Blood Cancer 2018, 65, e26774. [Google Scholar] [CrossRef] [PubMed]
- Namazzi, R.; Bond, C.; Conroy, A.L.; Datta, D.; Tagoola, A.; Goings, M.J.; Jang, J.H.; Ware, R.E.; Opoka, R.; John, C.C. Hydroxyurea reduces infections in children with sickle cell anemia in Uganda. Blood 2024, 143, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; E Ware, R.; Miller, S.T.; Iyer, R.V.; Casella, J.F.; Minniti, C.P.; Rana, S.; Thornburg, C.D.; Rogers, Z.R.; Kalpatthi, R.V.; et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (BABY HUG). Lancet 2011, 377, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Ehr, V.; Leborgne, G.; Vilque, J.P.; Potron, G.; Bernard, P. Secondary cutaneous effects of hydroxyurea: Prospective study of 26 patients from a dermatologic consultation. Rev. Med. Interne 2000, 21, 30–34. [Google Scholar] [CrossRef]
- Halsey, C.; Roberts, I.A.G. The role of hydroxyurea in sickle cell disease. Br. J. Haematol. 2003, 120, 177–186. [Google Scholar] [CrossRef]
- Berthaut, I.; Guignedoux, G.; Kirsch-Noir, F.; de Larouziere, V.; Ravel, C.; Bachir, D.; Galactéros, F.; Ancel, P.-Y.; Kunstmann, J.-M.; Levy, L.; et al. Influence of sickle cell disease and treatment with hydroxyurea on sperm parameters and fertility of human males. Haematologica 2008, 93, 988–993. [Google Scholar] [CrossRef]
- Al Sulaimani, R.; Zitoun, N.; Alothman, H.; Hutson, J.R.; Garcia-Bournissen, F. Safety of Hydroxyurea in Pregnancy: A Systematic Review of the Literature. J. Obstet. Gynaecol. Can. 2025, 47, 102924. [Google Scholar] [CrossRef]
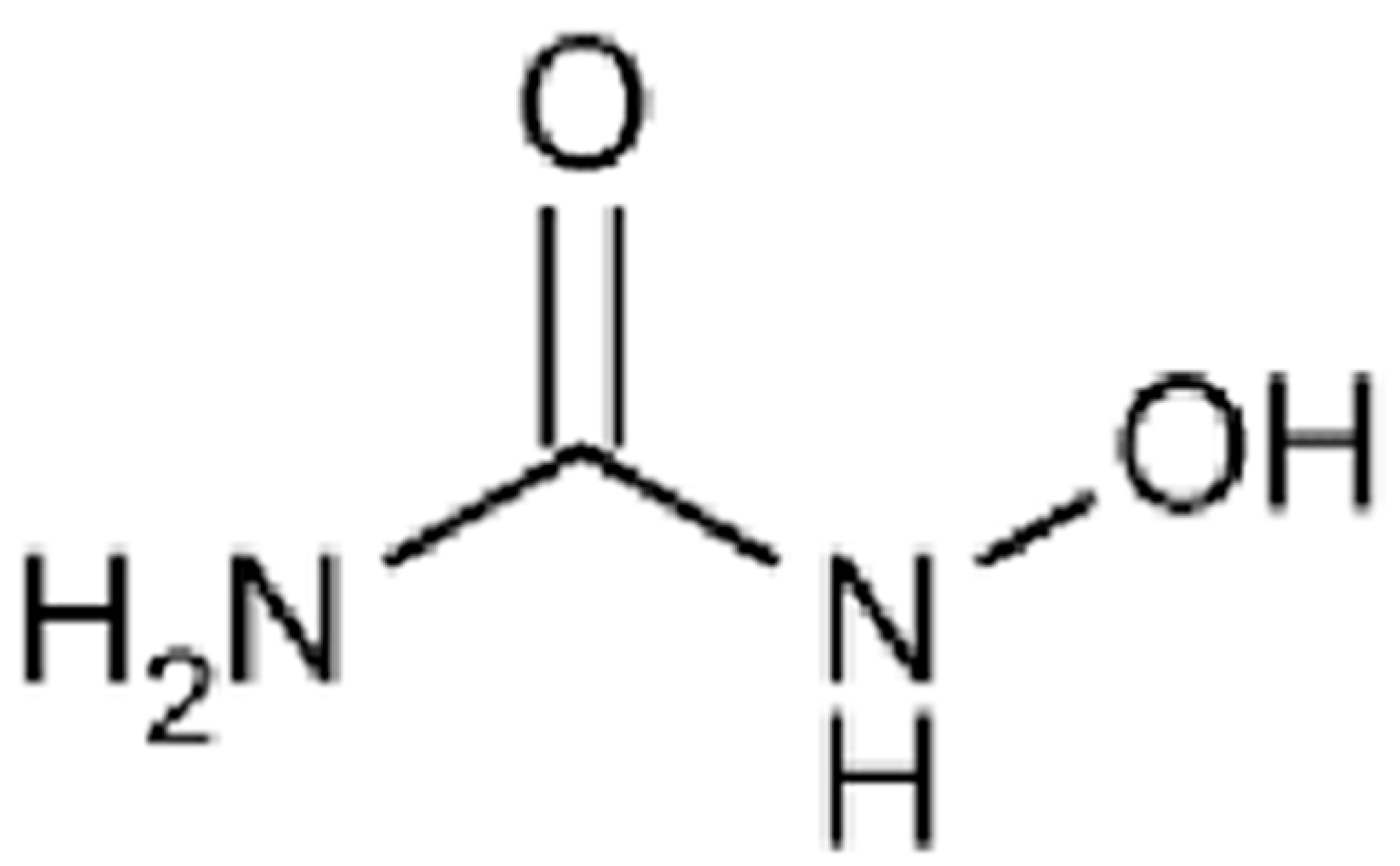
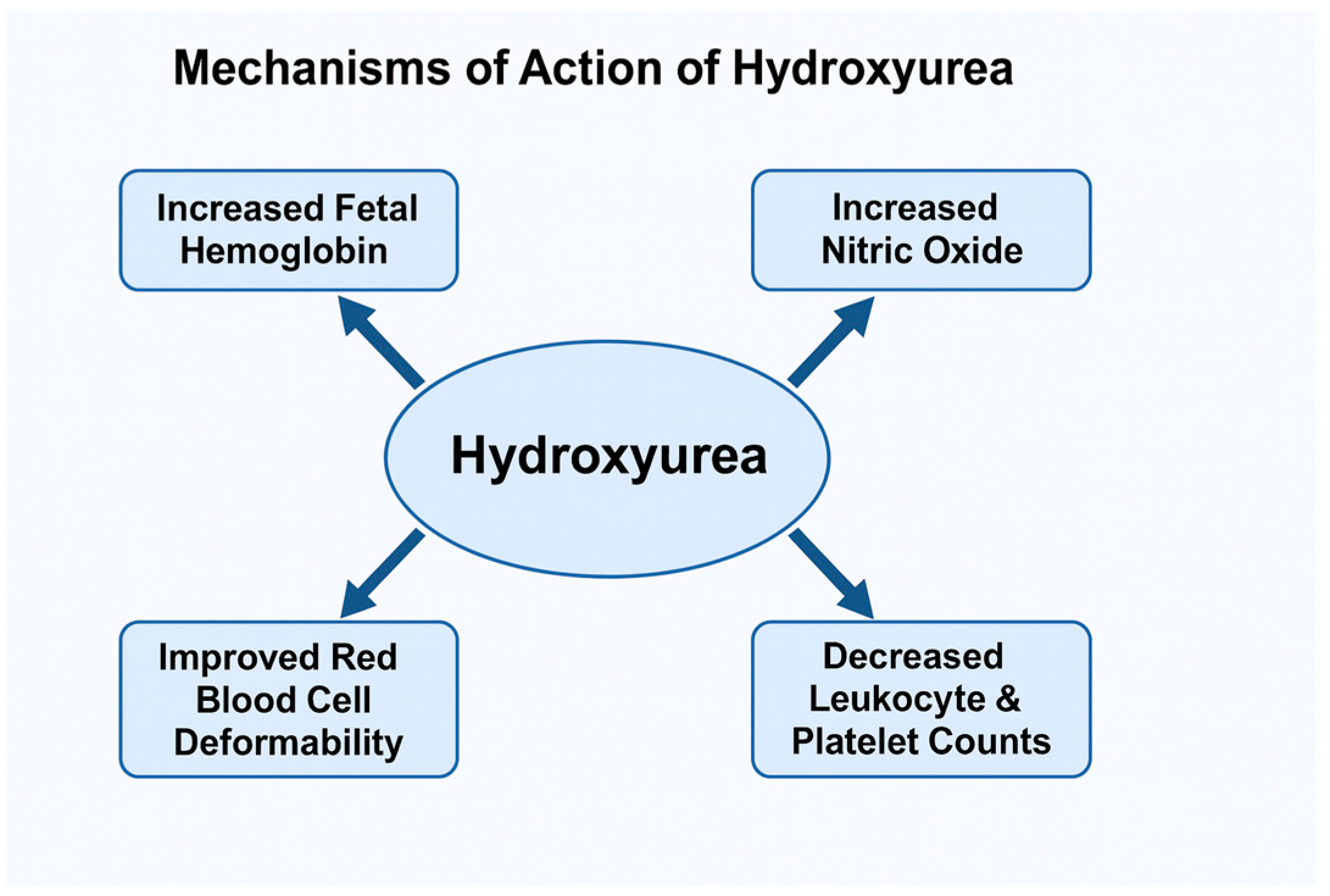
| Outcome | Evidence Summary | Clinical Impact | Evidence Strength (Trial vs. Cohort) + Key Sources |
|---|---|---|---|
| Reduction in vaso-occlusive crises | HU increases HbF and improves RBC rheology, reducing sickling and microvascular obstruction | Fewer pain crises and hospitalizations | Strong evidence from randomized trials and prospective cohorts [22,32,42] |
| Reduction in acute chest syndrome | HU reduces hemolysis, inflammation, and leukocyte adhesion | Lower ACS incidence and respiratory complications | Randomized controlled trials and long-term observational cohorts [21,22,32] |
| Prevention of stroke and silent cerebral infarction | HU maintains normal TCD velocities and improves cerebral oxygenation | Effective alternative to chronic transfusion for primary stroke prevention | Phase III randomized controlled trials and real-world cohorts [25,32,33] |
| Improved anemia and hemolysis markers | HU increases total Hb and reduces reticulocytes, LDH, and bilirubin | Improved oxygen delivery and lower hemolysis burden | Prospective trials and laboratory follow-up cohorts [21,22,30,42] |
| Reduced transfusion requirements | HU reduces VOC/ACS frequency and improves baseline Hb | Lower transfusion exposure and reduced iron overload risk | Randomized controlled trials and real-world cohort studies [22,32,33] |
| Neurocognitive preservation | Improved cerebral perfusion and reduced silent infarct progression | Better neurodevelopmental and cognitive outcomes | Prospective neurocognitive and neuroimaging studies [26,27,28,29,30,31,33] |
| Reduced inflammatory burden | HU decreases leukocytosis, cytokine activation, and endothelial adhesion | Lower systemic inflammation and vascular injury | Observational immunologic and inflammatory marker cohorts [21,34,39] |
| Normal growth and puberty | Improved oxygenation and metabolic balance | Supports normal growth velocity and pubertal development | Prospective pediatric trials and longitudinal cohorts [40,41,42,43] |
| Adverse Effect | Frequency | Management | Notes |
|---|---|---|---|
| Bone marrow suppression (neutropenia, thrombocytopenia, low reticulocytes) | Common, dose-dependent | Temporary drug interruption and dose adjustment | Fully reversible with monitoring |
| Gastrointestinal symptoms (nausea, anorexia) | Occasional | Symptomatic treatment; take with food | Mild and transient |
| Cutaneous changes (hyperpigmentation, xerosis, nail changes) | Occasional | Emollients or dose modification if needed | Usually reversible |
| Renal or hepatic laboratory alterations | Rare | Periodic renal and liver monitoring | Usually mild and transient |
| Oligospermia in males | Occasional | Monitor if fertility concerns | Effects reversible after discontinuation |
| Aplastic crisis (e.g., parvovirus B19) | Rare | Suspend HU until count recovery | Not directly caused by HU |
| Teratogenic risk (pregnancy) | Rare | Avoid in pregnancy; use contraception | Contraindicated during pregnancy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogliazza, F.; Berzieri, M.; Carbone, G.; Ciriaco, D.; Esposito, S. Chronic Hydroxyurea Therapy in Children with Sickle Cell Anemia: Mechanisms of Action, Systemic Effects, and Long-Term Safety. J. Clin. Med. 2025, 14, 8599. https://doi.org/10.3390/jcm14238599
Fogliazza F, Berzieri M, Carbone G, Ciriaco D, Esposito S. Chronic Hydroxyurea Therapy in Children with Sickle Cell Anemia: Mechanisms of Action, Systemic Effects, and Long-Term Safety. Journal of Clinical Medicine. 2025; 14(23):8599. https://doi.org/10.3390/jcm14238599
Chicago/Turabian StyleFogliazza, Federica, Martina Berzieri, Giulia Carbone, Davide Ciriaco, and Susanna Esposito. 2025. "Chronic Hydroxyurea Therapy in Children with Sickle Cell Anemia: Mechanisms of Action, Systemic Effects, and Long-Term Safety" Journal of Clinical Medicine 14, no. 23: 8599. https://doi.org/10.3390/jcm14238599
APA StyleFogliazza, F., Berzieri, M., Carbone, G., Ciriaco, D., & Esposito, S. (2025). Chronic Hydroxyurea Therapy in Children with Sickle Cell Anemia: Mechanisms of Action, Systemic Effects, and Long-Term Safety. Journal of Clinical Medicine, 14(23), 8599. https://doi.org/10.3390/jcm14238599







