Clinical Characteristics of Patients with Neovascular Age-Related Macular Degeneration and Responses to Anti-VEGF Therapy: Four-Group Stratification Based on Drusen and Punctate Hyperfluorescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment Method and Data Collection
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Twelve-Month Outcomes of IVA Therapy
3.3. MDS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Iida, T.; Gomi, F.; Yasukawa, T.; Yamashiro, K.; Honda, S.; Maruko, I.; Kataoka, K. Japanese clinical guidelines for neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2025, 69, 639–660. [Google Scholar] [CrossRef]
- Ferris, F.L., III; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid pigment epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.T.; Yannuzzi, L.A.; Freund, K.B. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [PubMed]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Spaide, R.F.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.; Orlach, D.A. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995, 15, 100–110. [Google Scholar] [CrossRef]
- Miyake, M.; Ooto, S.; Yamashiro, K.; Takahashi, A.; Yoshikawa, M.; Akagi-Kurashige, Y.; Ueda-Arakawa, N.; Oishi, A.; Nakanishi, H.; Tamura, H.; et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci. Rep. 2015, 5, 16204. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hiroe, T.; Morimoto, M.; Mimura, K.; Ito, A.; Akiyama, H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2018, 62, 144–150. [Google Scholar] [CrossRef]
- Kuranami, A.; Maruko, R.; Maruko, I.; Hasegawa, T.; Iida, T. Pachychoroid neovasculopathy has clinical properties that differ from conventional neovascular age-related macular degeneration. Sci. Rep. 2023, 13, 7379. [Google Scholar] [CrossRef]
- Inoda, S.; Takahashi, H.; Inoue, Y.; Tan, X.; Tampo, H.; Arai, Y.; Yanagi, Y.; Kawashima, H. Cytokine profiles of macular neovascularization in the elderly based on a classification from a pachychoroid/drusen perspective. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 747–758. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Ojima, Y.; Yamashiro, K.; Ooto, S.; Tamura, H.; Nakagawa, S.; Yoshimura, N. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina 2010, 30, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, B.H.; Park, K.H.; Woo, S.J. Punctate hyperfluorescence spot as a common choroidopathy of central serous chorioretinopathy and polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2014, 158, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Goto, K.; Date, Y.; Hiraki, R.; Mizukawa, K.; Miki, A. Clinical characteristics of punctate hyperfluorescence spots in the fellow eye of patients with unilateral macular neovascularization with no drusen. J. Clin. Med. 2024, 13, 5394. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Goto, K.; Mizukawa, K.; Hiraki, R.; Miki, A.; Kimura, S. Punctate hyperfluorescence as a favorable predictive factor for treatment response following a switch to brolucizumab for patients with aflibercept-refractory neovascular age-related macular degeneration. J. Clin. Med. 2025, 14, 5141. [Google Scholar] [CrossRef]
- Kamao, H.; Goto, K.; Mito, Y.; Miki, A.; Kiryu, J. Effects of smoking on outcomes of antivascular endothelial growth factor therapy in patients with neovascular age-related macular degeneration smoking and anti-VEGF Therapy in nAMD. J. Ophthalmol. 2018, 2018, 2353428. [Google Scholar] [CrossRef]
- Kamao, H.; Mitsui, E.; Date, Y.; Goto, K.; Mizukawa, K.; Miki, A. Clinical characteristics of unilateral macular neovascularization patients with pachydrusen in the fellow eye. J. Clin. Med. 2024, 13, 3757. [Google Scholar] [CrossRef]
- Klein, R.; Davis, M.D.; Magli, Y.L.; Segal, P.; Klein, B.E.K.; Hubbard, L. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991, 98, 1128–1134. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Wong, D.W.K.; Scassellati Sforzolini, B.; Goldbaum, M.; Tang, K.C.; Spaide, R.F.; Freund, K.B.; Slakter, J.S.; Guyer, D.R.; Sorenson, J.A.; et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch. Ophthalmol. 1999, 117, 1503–1510. [Google Scholar] [CrossRef]
- Maruko, I.; Iida, T.; Saito, M.; Nagayama, D.; Saito, K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am. J. Ophthalmol. 2007, 144, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Leys, A.; Herrmann-Delemazure, B.; Stalmans, P.; Tittl, M.; Yannuzzi, L.A.; Burke, K.M.; Fisher, Y.L.; Freund, K.B.; Guyer, D.R.; et al. Radiation-associated choroidal neovasculopathy. Ophthalmology 1999, 106, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Wise, K.; Kingsley, R.M. Idiopathic polypoidal choroidal vasculopathy and sickle cell retinopathy. Am. J. Ophthalmol. 2000, 129, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Mauget-Faÿsse, M.; Cornut, P.L.; Quaranta El-Maftouhi, M.; Leys, A. Polypoidal choroidal vasculopathy in tilted disk syndrome and high myopia with staphyloma. Am. J. Ophthalmol. 2006, 142, 970–975. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kishi, S.; Akiyama, H. Chronic choriocapillaris ischemia in dilated vortex vein region in pachychoroid neovasculopathy. Sci. Rep. 2021, 11, 16274. [Google Scholar] [CrossRef]
- Tomany, S.C.; Wang, J.J.; van Leeuwen, R.; Klein, R.; Mitchell, P.; Vingerling, J.R.; Klein, B.E.K.; Smith, W.; de Jong, P.T.V.M. Risk factors for incident age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2004, 111, 1280–1287. [Google Scholar] [CrossRef]
- Hyman, L.; Schachat, A.P.; He, Q.; Leske, M.C. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch. Ophthalmol. 2000, 118, 351–358. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Jensen, S.C. The relation of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 1997, 104, 1804–1812. [Google Scholar] [CrossRef]
- Tan, J.S.L.; Mitchell, P.; Smith, W.; Wang, J.J. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2007, 114, 1143–1150. [Google Scholar] [CrossRef]
- Choi, J.K.; Lym, Y.L.; Moon, J.W.; Shin, H.J.; Cho, B. Diabetes mellitus and early age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 196–199. [Google Scholar] [CrossRef] [PubMed]
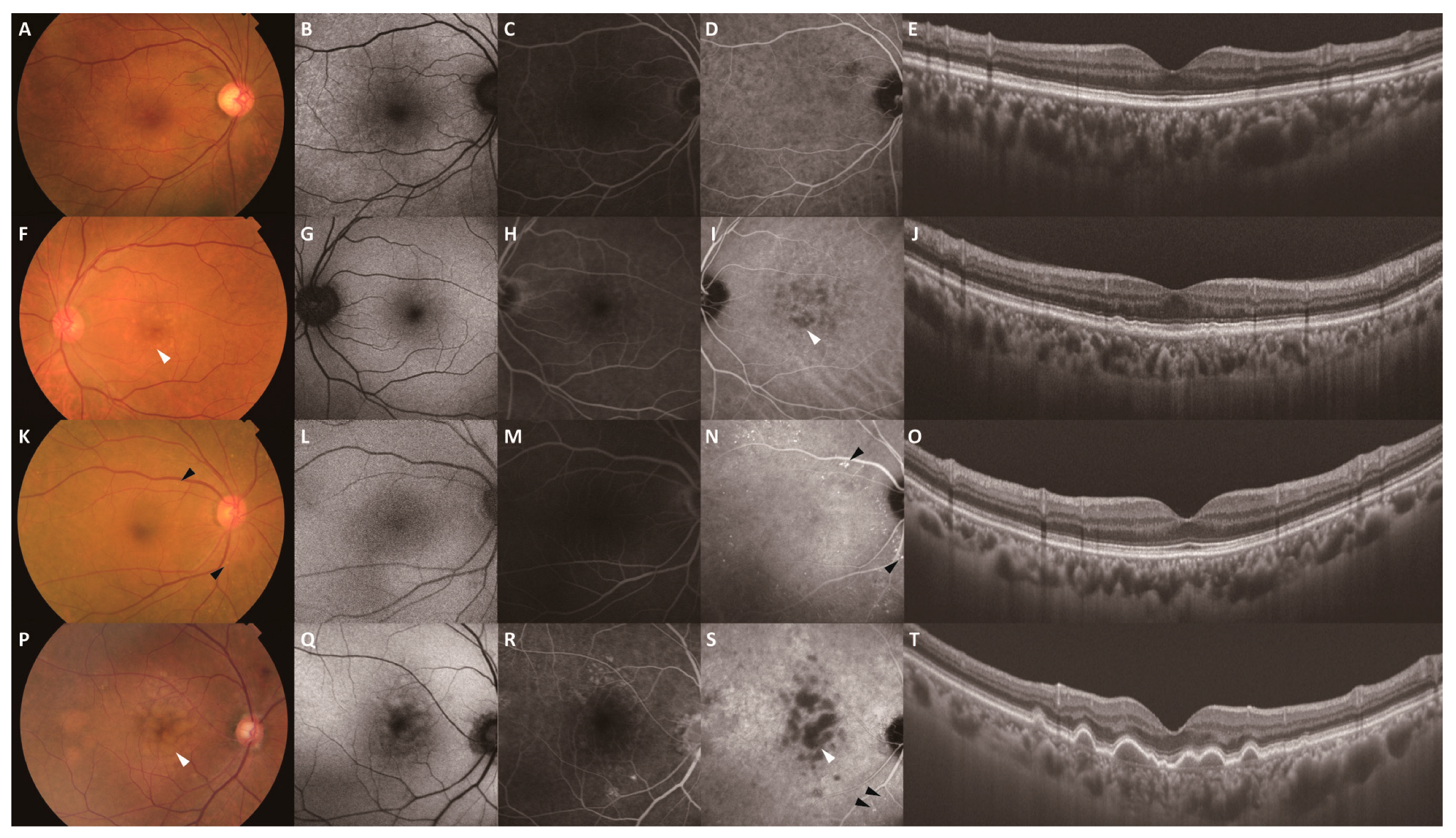
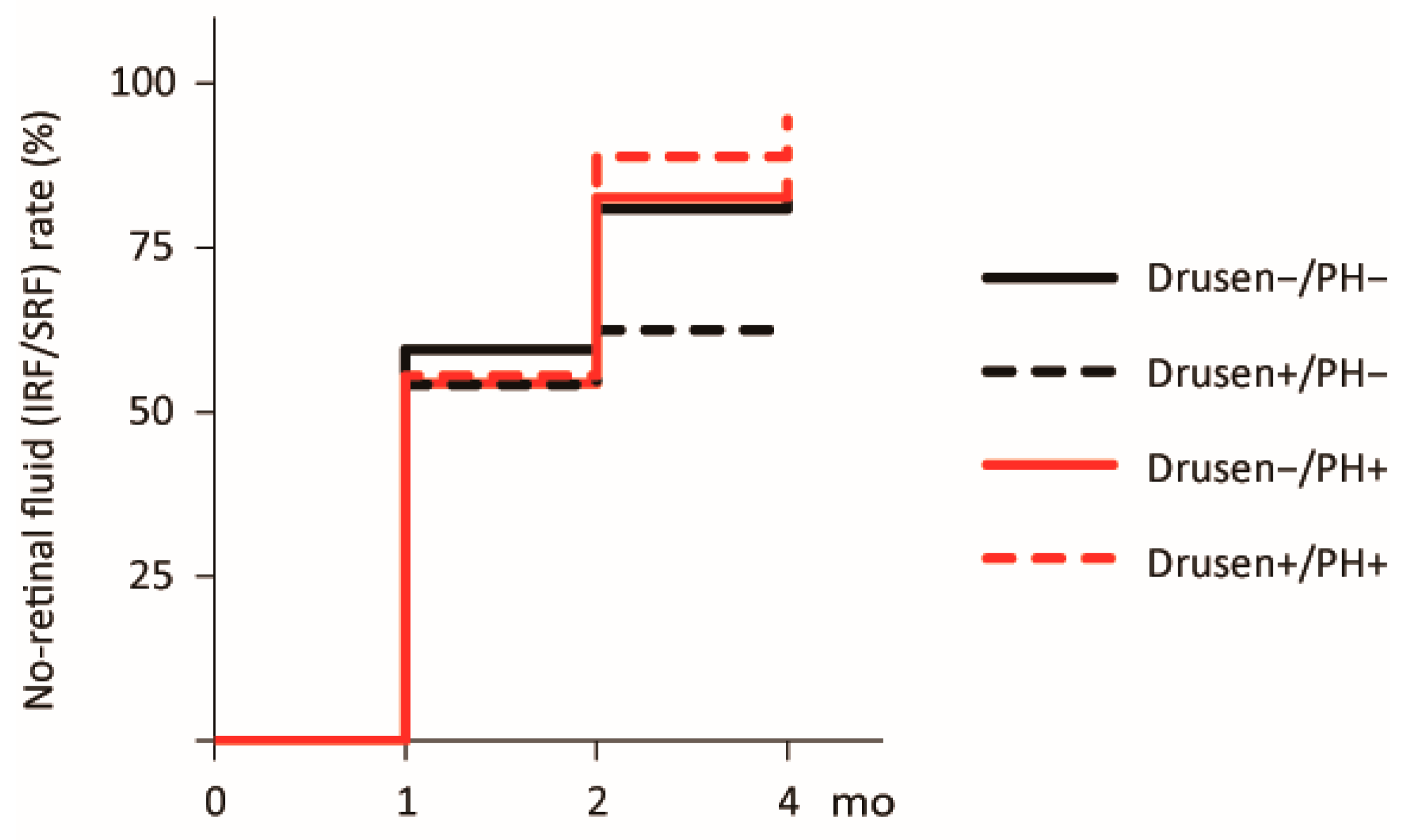
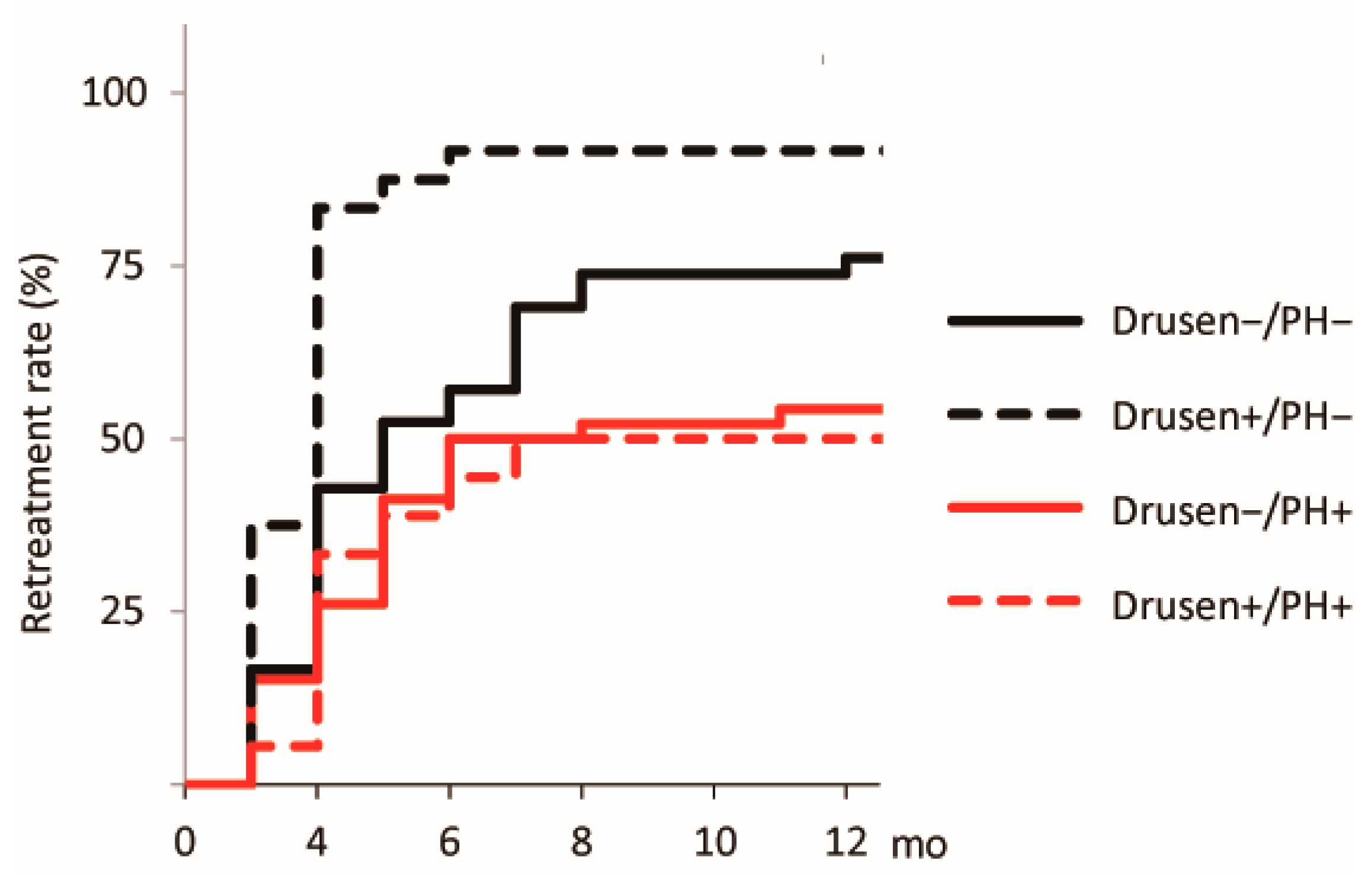
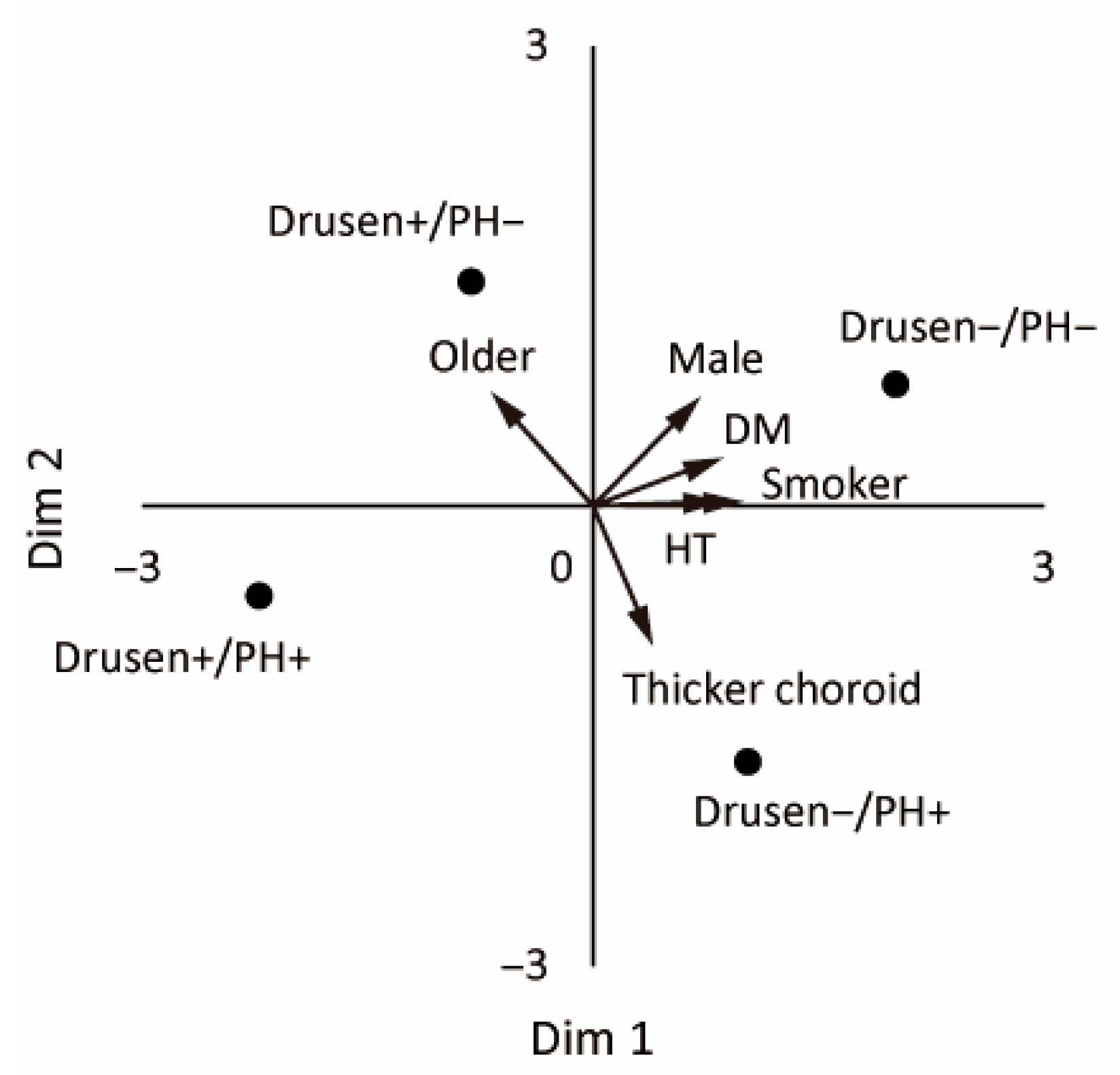
| Characteristic | Drusen−/PH− (n = 42) | Drusen+/PH− (n = 24) | Drusen−/PH+ (n = 46) | Drusen+/PH+ (n = 18) | p |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 72.9 (7.6) | 77.8 (8.0) | 70.0 (9.0) | 75.6 (7.6) | <0.001 |
| Sex (female), no. (%) | 8 (19.0) | 6 (25.0) | 14 (30.4) | 6 (33.3) | 0.56 |
| Hypertension, no. (%) | 23 (54.8) | 12 (50.0) | 27 (58.7) | 5 (27.8) | 0.15 |
| Diabetes, no. (%) | 18 (42.9) | 4 (16.7) | 9 (19.6) | 2 (11.1) | 0.02 |
| Smoking habits (ever-smokers), No. (%) | 34 (81.0) | 16 (66.7) | 35 (76.1) | 10 (55.6) | 0.20 |
| Presence of polypoidal lesion, No. (%) | 20 (47.6) | 3 (12.5) | 24 (52.2) | 9 (50.0) | 0.01 |
| Presence of IRF, No. (%) | 4 (9.5) | 2 (8.3) | 5 (10.9) | 2 (11.1) | 0.99 |
| Presence of SRF, No. (%) | 42 (100.0) | 24 (100.0) | 45 (97.8) | 16 (88.9) | 0.10 |
| Presence of SHRM, No. (%) | 0.67 | ||||
| Exudation | 5 (11.9) | 4 (16.7) | 8 (17.4) | 4 (22.2) | |
| Hemorrhage | 10 (23.8) | 2 (8.3) | 10 (21.7) | 3 (16.7) | |
| Neovascular tissue | 1 (2.4) | 1 (4.2) | 0 (0.0) | 0 (0.0) | |
| No SHRM | 26 (61.9) | 17 (70.8) | 28 (60.9) | 10 (55.6) |
| Characteristic | Drusen−/PH− (n = 42) | Drusen+/PH− (n = 24) | Drusen−/PH+ (n = 46) | Drusen+/PH+ (n = 18) | F, p |
|---|---|---|---|---|---|
| Baseline, LSMean (95%CI) | |||||
| logMAR | 0.32 (0.21–0.43) | 0.32 (0.18–0.47) | 0.20 (0.10–0.31) | 0.22 (0.04–0.39) | 1.04, 0.38 |
| CRT (μm) | 310.0 (277.8–342.1) | 305.1 (262.6–347.7) | 307.8 (277.1–338.6) | 320.9 (271.8–370.0) | 0.09, 0.97 |
| SFCT (μm) | 230.9 (202.2–259.6) | 173.3 (135.3–211.3) | 282.2 (254.8–309.6) | 230.6 (186.7–274.4) | 7.30, <0.001 |
| Month 12, LSMean (95%CI) | |||||
| logMAR | 0.17 (0.05–0.29) | 0.34 (0.18–0.50) | 0.11 (−0.01–0.22) | 0.07 (−0.11–0.26) | 2.33, 0.08 |
| CRT (μm) | 216.3 (199.7–232.9) | 245.3 (223.4–267.3) | 218.5 (202.7–234.4) | 231.4 (206.1–256.8) | 1.78, 0.15 |
| SFCT (μm) | 200.7 (171.2–230.3) | 142.8 (103.7–181.9) | 248.2 (220.0–276.5) | 199.1 (154.0–244.2) | 6.40, <0.001 |
| Number of injections, LSMean (95%CI) | 5.8 (5.0–6.5) | 7.2 (6.2–8.2) | 4.9 (4.1–5.6) | 5.1 (3.9–6.3) | 4.71, 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamao, H.; Goto, K.; Mizukawa, K.; Hiraki, R.; Miki, A.; Kimura, S. Clinical Characteristics of Patients with Neovascular Age-Related Macular Degeneration and Responses to Anti-VEGF Therapy: Four-Group Stratification Based on Drusen and Punctate Hyperfluorescence. J. Clin. Med. 2025, 14, 8593. https://doi.org/10.3390/jcm14238593
Kamao H, Goto K, Mizukawa K, Hiraki R, Miki A, Kimura S. Clinical Characteristics of Patients with Neovascular Age-Related Macular Degeneration and Responses to Anti-VEGF Therapy: Four-Group Stratification Based on Drusen and Punctate Hyperfluorescence. Journal of Clinical Medicine. 2025; 14(23):8593. https://doi.org/10.3390/jcm14238593
Chicago/Turabian StyleKamao, Hiroyuki, Katsutoshi Goto, Kenichi Mizukawa, Ryutaro Hiraki, Atsushi Miki, and Shuhei Kimura. 2025. "Clinical Characteristics of Patients with Neovascular Age-Related Macular Degeneration and Responses to Anti-VEGF Therapy: Four-Group Stratification Based on Drusen and Punctate Hyperfluorescence" Journal of Clinical Medicine 14, no. 23: 8593. https://doi.org/10.3390/jcm14238593
APA StyleKamao, H., Goto, K., Mizukawa, K., Hiraki, R., Miki, A., & Kimura, S. (2025). Clinical Characteristics of Patients with Neovascular Age-Related Macular Degeneration and Responses to Anti-VEGF Therapy: Four-Group Stratification Based on Drusen and Punctate Hyperfluorescence. Journal of Clinical Medicine, 14(23), 8593. https://doi.org/10.3390/jcm14238593







