Rare Localization of Extramammary Paget’s Disease in the Axilla: A Case Report and Literature Review
Abstract
1. Introduction
2. Methodology
2.1. The Review of Literature
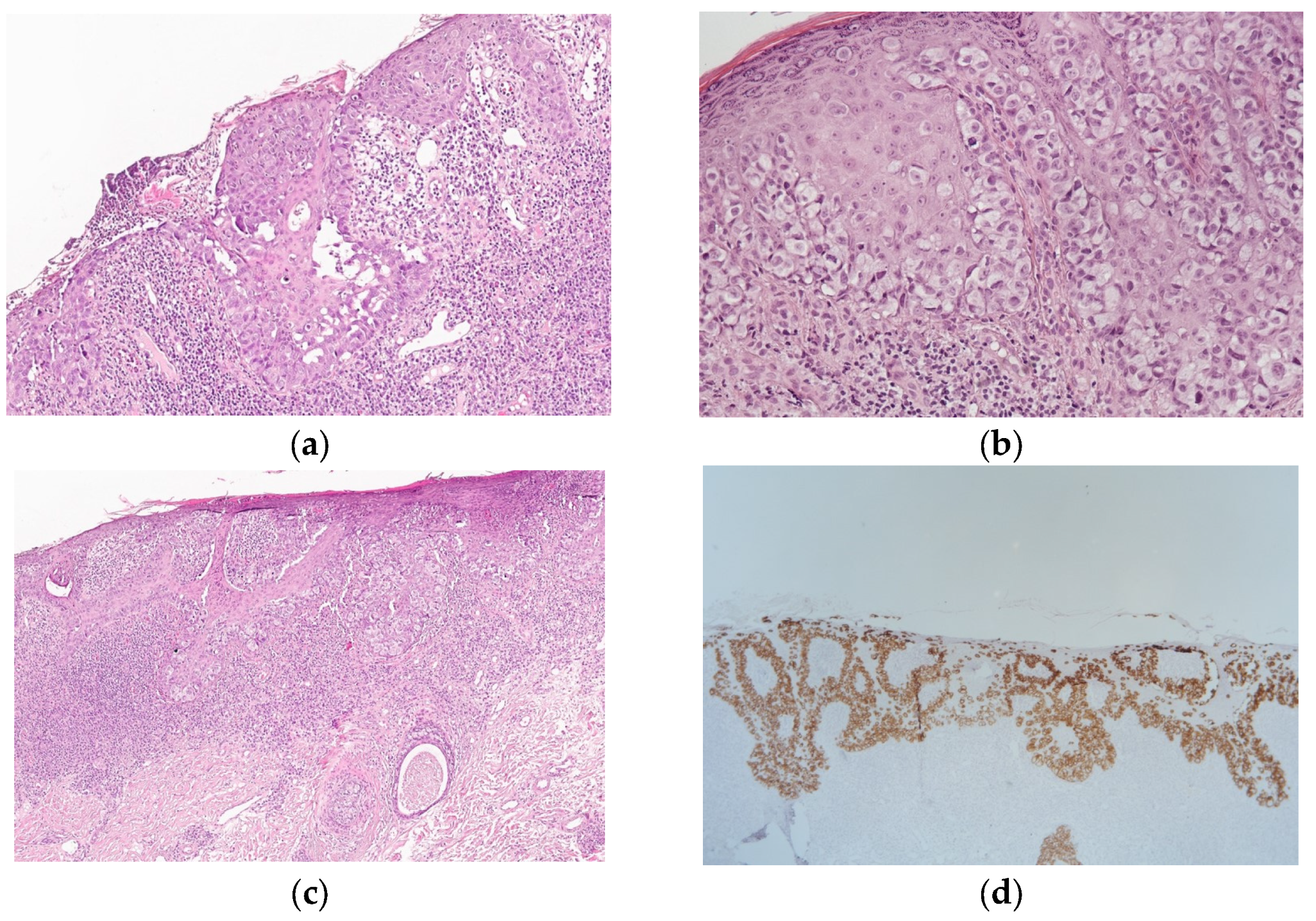
2.2. Morphology and Immunohistochemistry
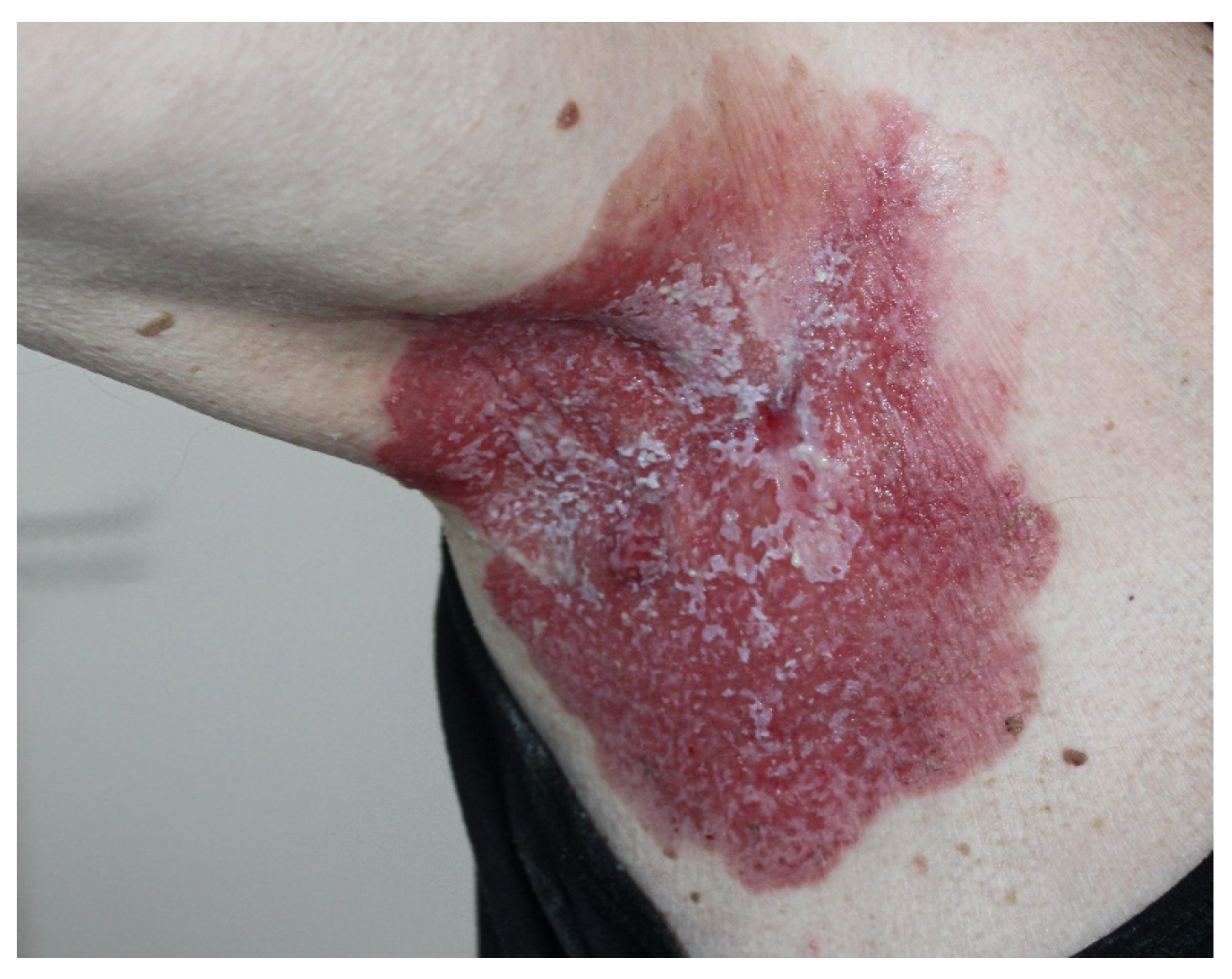
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Androgen receptors |
| EMPD | Extramammary Paget’s disease |
| ER | Estrogen receptors |
| ICF | Informed consent form |
| IHC | Immunohistochemical |
| PRLR | Transmembrane prolactin receptor |
References
- Crocker, H. Paget’s disease affecting the scrotum and penis. Trans. Pathol. Soc. Lond. 1889, 40, 187–191. [Google Scholar]
- Zhao, C.; Li, Y.; Zhang, C.; Zhang, G.; Li, H. Extramammary Paget’s disease involving the axilla: Case series and literature review. Int. J. Dermatol. 2023, 62, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Li, G.K.; Chung, J.H.; Chow, V.L. Extramammary Paget’s Disease: 20 Years of Experience in Chinese Population. Int. J. Surg. Oncol. 2012, 2012, 416418. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P. Ceruminous Neoplasms of the Ear. Head Neck Pathol. 2018, 12, 350–361. [Google Scholar] [CrossRef]
- Siesling, S.; Elferink, M.A.; van Dijck, J.A.; Pierie, J.P.; Blokx, W.A. Epidemiology and treatment of extramammary Paget disease in the Netherlands. Eur. J. Surg. Oncol. 2007, 33, 951–955. [Google Scholar] [CrossRef]
- Pérez, J.C.; Salgado, A.C.; Pérez-Mies, B.; Rullán, J.A.D.; Ajuria-Illarramendi, O.; Alia, E.M.G.; Serrano Domingo, J.J. Extramammary Paget Disease: A Therapeutic Challenge, for a Rare Entity. Curr. Oncol. Rep. 2023, 25, 1081–1094. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, J.; Tao, X.; Xu, C. Intraepithelial extramammary Paget’s disease of the vulva: The clinicopathological characteristics, management, and outcome in a study of 18 female patients. Dermatol. Surg. 2016, 42, 1142–1146. [Google Scholar] [CrossRef]
- Cho, A.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Park, J.Y. Outcomes and prognostic factors of surgically treated extramammary Paget’s disease of the vulva. J. Gynecol. Oncol. 2023, 34, e76. [Google Scholar] [CrossRef]
- Zollo, J.D.; Zeitouni, N.C. The roswell park cancer institute experience with extramammary paget’s disease. Br. J. Dermatol. 2000, 142, 59–65. [Google Scholar] [CrossRef]
- Hirata, Y.; Aoyagi, S.; Mizuno, O.; Homma, E.; Hata, H.; Shimizu, H. Bilateral axillary Paget’s disease: Diagnostic pitfalls of dermoscopic features. Int. J. Dermatol. 2014, 53, e126–e127. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Dong, J.; Cao, Y.; Li, W.; Zhang, F.; Zeng, X. Axillary masses as clinical manifestations of male sweat gland carcinoma associated with extramammary Paget’s disease and accessory breast carcinoma: Two cases report and literature review. World J. Surg. Oncol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Miyamoto, T.; Adachi, K.; Fujishima, M. Axillary apocrine carcinoma with Paget’s disease and apocrine naevus. Clin. Exp. Dermatol. 2009, 34, e110–e113. [Google Scholar] [CrossRef]
- Goto, H.; Adachi, K.; Yamada, N.; Shiomi, T.; Kiyohara, Y.; Yoshida, Y.; Yamamoto, O. Concurrent occurrence of axillary extramammary Paget’s disease and squamous cell carcinoma. Eur. J. Dermatol. 2016, 26, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choe, Y.S.; Jung, H.D.; Ahn, S.K.; Cha, Y.C.; Cho, K.H.; Choi, H.Y.; Chung, K.Y.; Huh, C.H.; Kim, I.H.; et al. Korean Society for Skin Cancer and Korean Dermatopathology Research Group. A multicenter study on extramammary Paget’s disease in Korea. Int. J. Dermatol. 2011, 50, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Funaro, D.; Krasny, M.; Lam, C.; Desy, D.; Sauthier, P.; Bouffard, D. Extramammary Paget disease: Epidemiology and association to cancer in a Quebec-based population. J. Low Genit. Tract. Dis. 2013, 17, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Omori, M.; Sakai, A. Invasive extramammary Paget’s disease with lymph node metastases and high-grade B-cell lymphoma. An. Bras. Dermatol. 2023, 98, 414–418. [Google Scholar] [CrossRef]
- Lloyd, J.; Flanagan, A.M. Mammary and extramammary Paget’s disease. J. Clin. Pathol. 2000, 53, 742–749. [Google Scholar] [CrossRef]
- Ito, T.; Kaku, Y.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Oda, Y.; Hagihara, A.; Furue, M.; Uchi, H. Tumor thickness as a prognostic factor in extramammary Paget’s disease. J. Dermatol. 2015, 42, 269–275. [Google Scholar] [CrossRef]
- Kiniwa, Y.; Yasuda, J.; Saito, S.; Saito, R.; Motoike, I.N.; Danjoh, I.; Kinoshita, K.; Fuse, N.; Yamamoto, M.; Okuyama, R. Identification of genetic alterations in extramammary Paget disease using whole exome analysis. J. Dermatol. Sci. 2019, 94, 229–235. [Google Scholar] [CrossRef]
- Cheng, P.S.; Lu, C.L.; Cheng, C.L.; Lai, F.J. Significant male predisposition in extramammary Paget disease: A nationwide population-based study in Taiwan. Br. J. Dermatol. 2014, 171, 191–193. [Google Scholar] [CrossRef]
- Wolf, K.; Stewart, L.; Rapini, R.; Mutyambizi, K. Multifocal extramammary Paget’s disease-associated adenocarcinoma: A rare condition of flexoral skin of multiple sites. Dermatol. Online J. 2016, 22, 13030/qt8bp3138w. [Google Scholar] [CrossRef]
- Mun, J.H.; Park, S.M.; Kim, G.W.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Clinical and dermoscopic characteristics of extramammary Paget disease: A study of 35 cases. Br. J. Dermatol. 2016, 174, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.Y.; Luo, B.; Wang, X.W.; Xiao, Y.; Tian, J.Y. Axillary Paget disease with a visible satellite: A case report and literature review. Diagn. Pathol. 2021, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, B.; Wu, X.; Wei, W. Case report: A case of Paget disease outside the axillary breast. Medicine 2024, 103, e37541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, G.; Li, Y.; Zhang, C.; Yang, S.; Li, H. Concurrent extramammary Paget’s disease involving the genitalia and axilla: Case report and literature review. Australas. J. Dermatol. 2022, 63, e226–e230. [Google Scholar] [CrossRef]
- Chuchvara, N.; Reilly, C.; Haroon, A.; Wassef, C.; Maghari, A.; Rao, B. Atypical cells on reflectance confocal microscopy may not represent melanoma: A case of axillary pigmented extramammary Paget disease. J. Cutan. Pathol. 2020, 47, 1170–1174. [Google Scholar] [CrossRef]
- Dai, C.; Baird, B.A.; Lyon, T.D.; Sokumbi, O.; Degesys, C.A. Extramammary Paget disease of the axilla and scrotum in a Caucasian man. JAAD Case Rep. 2022, 22, 87–89. [Google Scholar] [CrossRef]
- Felts, C.; Durkin, V.; Kerkvliet, A. A Rare Case of Axillary Extramammary Paget’s Disease with an Underlying Adenocarcinoma. South Dak. Med. 2024, 77, 152–156. [Google Scholar]
- Kimura, R.; Yoshida, Y.; Shiomi, T.; Yanagihara, S.; Tsutsumi, R.; Sugita, K.; Yamamoto, O. Multiorgan involvement of extramammary Paget’s disease with mucinous carcinoma-like components. Eur. J. Dermatol. 2016, 26, 615–617. [Google Scholar] [CrossRef]
- Imai, S.; Nitto, H.; Imai, R. Axillary Paget’s disease. J. Dermatol. 1981, 8, 245–252. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hagari, Y.; Inoue, S.; Watanabe, T.; Yoshino, T. Axillary apocrine carcinoma with benign apocrine tumours: A case report involving a pathological and immunohistochemical study and review of the literature. J. Clin. Pathol. 2005, 58, 757–761. [Google Scholar] [CrossRef]
- Scalvenzi, M.; Cappello, M.; Russo, D.; Mascolo, M.; Monfrecola, G.; Costa, C. Double extramammary Paget’s disease. G. Ital. Dermatol. Venereol. 2018, 153, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.B.; Kim, S.E.; Kim, Y.A.; Choi, H.R. Co-occurrence of apocrine adenocarcinoma and invasive mammary-type ductal carcinoma in extramammary Paget disease of the axilla. Arch. Plast Surg. 2020, 47, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Hirai, I.; Tanese, K.; Nakamura, Y.; Otsuka, A.; Fujisawa, Y.; Yamamoto, Y.; Hata, H.; Fujimura, T.; Matsushita, S.; Yoshino, K.; et al. Assessment of the methods used to detect HER2-positive advanced extramammary Paget’s disease. Med. Oncol. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C.; Kunitake, H.; Stafford, C.; Bordeianou, L.G.; Francone, T.D.; Ricciardi, R. High Risk of Proximal and Local Neoplasms in 2206 Patients with Anogenital Extramammary Paget’s Disease. Dis. Colon. Rectum. 2019, 62, 1283–1293. [Google Scholar] [CrossRef]
- Lam, C.; Funaro, D. Extramammary Paget’s disease: Summary of current knowledge. Dermatol. Clin. 2010, 28, 807–826. [Google Scholar] [CrossRef]
- Smith, A.A. Pre-Paget cells: Evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis. Res. 2019, 8, 203–205. [Google Scholar] [CrossRef]
- Willman, J.H.; Golitz, L.E.; Fitzpatrick, J.E. Vulvar clear cells of Toker: Precursors of extramammary Paget’s disease. Am. J Dermatopathol. 2005, 27, 185–188. [Google Scholar] [CrossRef]
- Baker, G.M.; Bret-Mounet, V.C.; Xu, J.; Fein-Zachary, V.J.; Tobias, A.M.; Bartlett, R.A.; Clohessy, J.G.; Vlachos, I.S.; Massicott, E.S.; Wulf, G.M.; et al. Toker Cell Hyperplasia in the Nipple-Areolar Complex of Transmasculine Individuals. Mod. Pathol. 2023, 36, 100121. [Google Scholar] [CrossRef]
- Wong, J.; Pina, A.; Mayrand, M.H.; Rahimi, K. Pagetoid Squamous Intraepithelial Neoplasia of the Vulva as a Mimicker of Vulvar Extramammary Paget Disease: Two Cases with Basal Layer Sparing. Int. J. Surg. Pathol. 2023, 31, 1302–1307. [Google Scholar] [CrossRef]
- Kibbi, N.; Owen, J.L.; Worley, B.; Wang, J.X.; Harikumar, V.; Downing, M.B.; Aasi, S.Z.; Aung, P.P.; Barker, C.A.; Bolotin, D.; et al. Evidence-Based Clinical Practice Guidelines for Extramammary Paget Disease. JAMA Oncol. 2022, 8, 618–628. [Google Scholar] [CrossRef]
- Nabavizadeh, R.; Vashi, K.B.; Nabavizadeh, B.; Narayan, V.M.; Master, V.A. Extramammary Paget’s disease: Updates in the workup and management. Asian J. Urol. 2022, 9, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Watanabe, S. The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget’s disease. Br. J. Dermatol. 2000, 142, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kakiuchi, N.; Yoshida, K.; Inoue, Y.; Irie, H.; Kataoka, T.R.; Hirata, M.; Funakoshi, T.; Matsushita, S.; Hata, H.; et al. Unbiased Detection of Driver Mutations in Extramammary Paget Disease. Clin. Cancer Res. 2021, 15, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Mazzeo, R.; De Scordilli, M.; Del Fabro, A.; Vitale, M.G.; Bortot, L.; Nicoloso, M.S.; Corsetti, S.; Bonotto, M.; Scalone, S.; et al. Human epidermal growth factor receptor-2 (HER2) is a potential therapeutic target in extramammary Paget’s disease of the vulva. Int. J. Gynecol. Cancer 2020, 30, 1672–1677. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Li, Q.; Liang, J.; Sun, L.; Yi, X.; Chen, Z.; Yan, R.; Xie, G.; Li, W.; et al. Nucleation of DNA repair factors by FOXA1 links DNA demethylation to transcriptional pioneering. Nat. Genet. 2016, 48, 1003–1013. [Google Scholar] [CrossRef]
- Lin, J.R.; Liang, J.; Zhang, Q.A.; Huang, Q.; Wang, S.S.; Qin, H.H.; Chen, L.J.; Xu, J.H. Microarray-based identification of differentially expressed genes in extramammary Paget’s disease. Int. J. Clin. Exp. Med. 2015, 15, 7251–7260. [Google Scholar]
- Clevenger, C.V.; Gadd, S.L.; Zheng, J. New mechanisms for PRLr action in breast cancer. Trends Endocrinol. Metab. 2009, 20, 223–229. [Google Scholar] [CrossRef]
- Scarbrough, C.A.; Vrable, A.; Carr, D.R. Definition, Association with Malignancy, Biologic Behavior, and Treatment of Ectopic Extramammary Paget’s Disease: A Review of the Literature. J. Clin. Aesthet Dermatol. 2019, 12, 40–44. [Google Scholar]
- Ren, F.; Zhao, S.; Yang, C.; Liu, J.; Shang, Q.; Feng, K.; Kang, X.; Zhang, R.; Wang, X.; Wang, X. Applications of photodynamic therapy in extramammary Paget’s disease. Am. J. Cancer Res. 2023, 13, 4492–4507. [Google Scholar]
- Chang, K.; Li, G.X.; Kong, Y.Y.; Shen, X.X.; Qu, Y.Y.; Jia, Z.W.; Wang, Y.; Dai, B.; Ye, D.W. Chemokine Receptors CXCR4 and CXCR7 are Associated with Tumor Aggressiveness and Prognosis in Extramammary Paget Disease. J. Cancer 2017, 8, 2471–2477. [Google Scholar] [CrossRef]
- Kibbi, N.; Owen, J.L.; Worley, B.; Wang, J.X.; Harikumar, V.; Aasi, S.Z.; Chandra, S.; Choi, J.N.; Fujisawa, Y.; Iavazzo, C.; et al. Anatomic Subtype Differences in Extramammary Paget Disease: A Meta-Analysis. JAMA Dermatol. 2024, 160, 417–424. [Google Scholar] [CrossRef]
- Ortuz Lessa, C.; Fernández Varela Gómez, F.; Garzón Ortega, V.H.; Sandoval García, A.; López Soto, K.; Brito Brito, N.R. Insights Into Mammary and Extramammary Paget’s Disease: Diagnosis, Management, and Recent Advances. Cureus. 2025, 17, e80531. [Google Scholar] [CrossRef]
- Chung, P.H.; Leong, J.Y.; Voelzke, B.B. Surgical Experience with Genital and Perineal Extramammary Paget’s Disease. Urology. 2019, 128, 90–95. [Google Scholar] [CrossRef]
- Hendi, A.; Brodland, D.G.; Zitelli, J.A. Extramammary Paget’s disease: Surgical treatment with Mohs micrographic surgery. J. Am. Acad. Dermatol. 2004, 51, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Hatta, N.; Yamada, M.; Hirano, T.; Fujimoto, A.; Morita, R. Extramammary Paget’s disease: Treatment, prognostic factors and outcome in 76 patients. Br. J. Dermatol. 2008, 158, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Koike, I.; Wada, H.; Miyagi, E.; Kasuya, T.; Kaizu, H.; Matsui, T.; Mukai, Y.; Ito, E.; Inoue, T. Radiation therapy for extramammary Paget’s disease: Treatment outcomes and prognostic factors. Ann. Oncol. 2014, 25, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Koike, I.; Wada, H.; Miyagi, E.; Kasuya, T.; Kaizu, H.; Mukai, Y.; Inoue, T. Postoperative radiation therapy for extramammary Paget’s disease. Br. J. Dermatol. 2015, 172, 1014–1020. [Google Scholar] [CrossRef]
- Sawada, M.; Kato, J.; Yamashita, T.; Yoneta, A.; Hida, T.; Horimoto, K.; Sato, S.; Uhara, H. Imiquimod 5% cream as a therapeutic option for extramammary Paget’s disease. J. Dermatol. 2018, 45, 216–219. [Google Scholar] [CrossRef]
- Fontanelli, R.; Papadia, A.; Martinelli, F.; Lorusso, D.; Grijuela, B.; Merola, M.; Solima, E.; Ditto, A.; Raspagliesi, F. Photodynamic therapy with M-ALA as non-surgical treatment option in patients with primary extramammary Paget’s disease. Gynecol. Oncol. 2013, 130, 90–94. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Fontanelli, R.; Rossi, G.; Ditto, A.; Solima, E.; Hanozet, F.; Kusamura, S. Photodynamic therapy using a methyl ester of 5-aminolevulinic acid in recurrent Paget’s disease of the vulva: A pilot study. Gynecol. Oncol. 2006, 103, 581–586. [Google Scholar] [CrossRef]
- Ishizuki, S.; Nakamura, Y. Extramammary Paget’s Disease: Diagnosis, Pathogenesis, and Treatment with Focus on Recent Developments. Curr. Oncol. 2021, 28, 2969–2986. [Google Scholar] [CrossRef]
- Phyo, A.K.; Mun, K.S.; Kwan, K.C.; Ann, C.C.; Kuppusamy, S. Genitourinary extramammary Paget’s disease: Review and outcome in a multidisciplinary setting. Int. J. Clin. Exp. Pathol. 2020, 13, 2369–2376. [Google Scholar] [PubMed]
- Hata, H.; Kitamura, S.; Inamura, Y.; Imafuku, K.; Homma, E.; Muramatsu, K.; Natsuga, K.; Abe, R.; Shimizu, H. mTOR expression correlates with invasiveness and progression of extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Takeuchi, S.; Moroi, Y.; Hayashida, S.; Kido, M.; Tomoeda, H.; Uenotsuchi, T.; Tu, Y.T.; Furue, M.; Urabe, K. Concordant overexpression of phosphorylated ATF2 and STAT3 in extramammary Paget’s disease. J. Cutan. Pathol. 2009, 36, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Xu, F.; Zhang, Q.A.; Lin, J.; Wu, Z.; Zhang, X.; Luo, Y.; Xu, J.; Guan, M. Correlation of DLC1 gene methylation with oncogenic PIK3CA mutations in extramammary Paget’s disease. Mod. Pathol. 2012, 25, 1160–1168. [Google Scholar] [CrossRef]
- Kang, Z.; Xu, F.; Zhang, Q.A.; Wu, Z.; Zhang, X.; Xu, J.; Luo, Y.; Guan, M. Oncogenic mutations in extramammary Paget’s disease and their clinical relevance. Int. J. Cancer 2013, 132, 824–831. [Google Scholar] [CrossRef]
- Hatta, N. Prognostic Factors of Extramammary Paget’s Disease. Curr. Treat. Options Oncol. 2018, 19, 47. [Google Scholar] [CrossRef]
- Plaza, J.A.; Torres-Cabala, C.; Ivan, D.; Prieto, V.G. HER-2/neu expression in extramammary Paget disease: A clinicopathologic and immunohistochemistry study of 47 cases with and without underlying malignancy. J. Cutan. Pathol. 2009, 36, 729–733. [Google Scholar] [CrossRef]
- Smith, K.J.; Tuur, S.; Corvette, D.; Lupton, G.P.; Skelton, H.G. Cytokeratin 7 staining in mammary and extramammary Paget’s disease. Mod. Pathol. 1997, 10, 1069–1074. [Google Scholar]
- Matin, R.N.; Gibbon, K.; Rizvi, H.; Harwood, C.A.; Cerio, R. Cutaneous mucinous carcinoma arising in extramammary Paget disease of the perineum. Am. J. Dermatopathol. 2011, 33, 705–709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolyannikova, V.; Krot, M.; Filatov, A.; Mordovina, A.; Kharitonova, M.; Gafurova, O.; Kudryashova, V.; Lutokhina, Y.; Pirozhkov, S.; Vukolova, M. Rare Localization of Extramammary Paget’s Disease in the Axilla: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 8581. https://doi.org/10.3390/jcm14238581
Smolyannikova V, Krot M, Filatov A, Mordovina A, Kharitonova M, Gafurova O, Kudryashova V, Lutokhina Y, Pirozhkov S, Vukolova M. Rare Localization of Extramammary Paget’s Disease in the Axilla: A Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(23):8581. https://doi.org/10.3390/jcm14238581
Chicago/Turabian StyleSmolyannikova, Vera, Marina Krot, Andrey Filatov, Alina Mordovina, Maria Kharitonova, Olga Gafurova, Valentina Kudryashova, Yulia Lutokhina, Sergey Pirozhkov, and Marina Vukolova. 2025. "Rare Localization of Extramammary Paget’s Disease in the Axilla: A Case Report and Literature Review" Journal of Clinical Medicine 14, no. 23: 8581. https://doi.org/10.3390/jcm14238581
APA StyleSmolyannikova, V., Krot, M., Filatov, A., Mordovina, A., Kharitonova, M., Gafurova, O., Kudryashova, V., Lutokhina, Y., Pirozhkov, S., & Vukolova, M. (2025). Rare Localization of Extramammary Paget’s Disease in the Axilla: A Case Report and Literature Review. Journal of Clinical Medicine, 14(23), 8581. https://doi.org/10.3390/jcm14238581






