Clinical Efficacy of One Short Course of Mannan-Conjugated Birch Pollen Allergoid Immunotherapy: A Comparative Evaluation After Prior Placebo Treatment
Abstract
1. Introduction
2. Materials and Methods
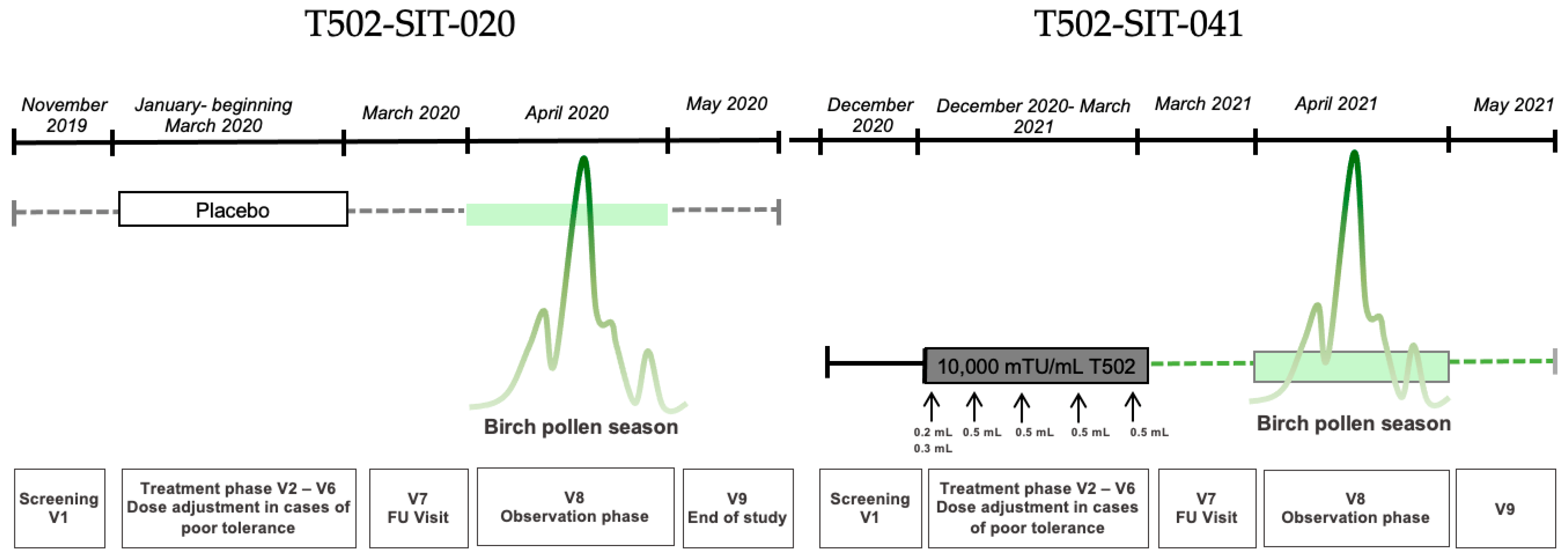
2.1. T502-SIT-020 Study (EudraCT Number: 2018-002522-23) [28]
2.2. T502-SIT-041 Study (EudraCT Number: 2020-004126-32)
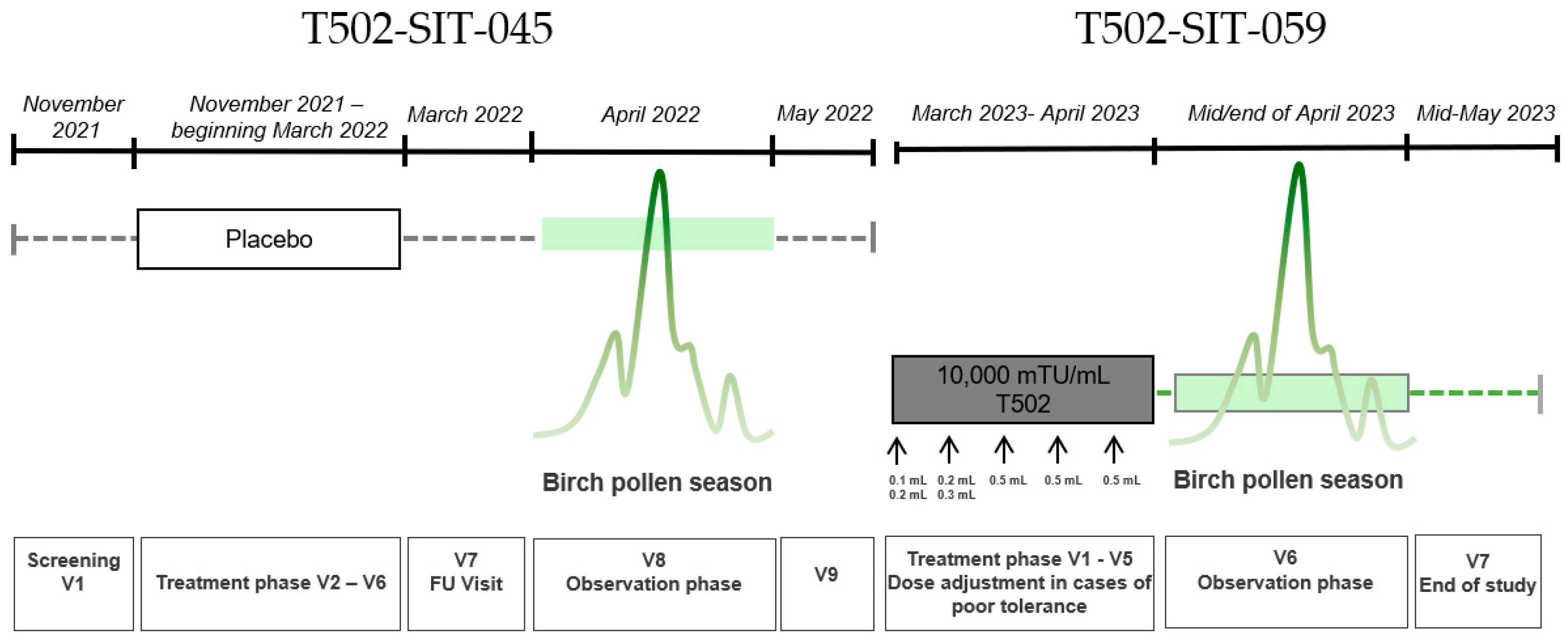
2.3. T502-SIT-045-Study (EudraCT-Number: 2021-002252-36)
2.4. T502-SIT-059-Study (EudraCT-Number: 2022-004082-20)
2.5. Endpoints
- 0 = no symptoms;
- 1 = mild symptoms (clearly present but minimal perception; easy to tolerate);
- 2 = moderate symptoms (clearly bothersome but tolerable);
- 3 = severe symptoms (difficult to tolerate; interferes with daily activities and/or sleep).
- The scores of the six symptoms were summed and divided by six to calculate the dSS, resulting in a score between 0 and 3.
- 0 = no medication;
- 1 = use of antihistamine tablets;
- 2 = use of nasal corticosteroids (with or without antihistamines);
- 3 = use of oral corticosteroids (with or without nasal corticosteroids and antihistamines).
- The CSMS was calculated by adding the dSS and dMS as follows: CSMS = dSS (0–3) + dMS (0–3), resulting in a score in the range of 0 to 6 [29].
- 0 = no medication;
- 0.5 = use of antihistamine eye drops;
- 1 = use of antihistamine tablets;
- 1.5 = use of nasal corticosteroids.
2.6. Patient Groups
2.7. Statistical Analysis
3. Results
3.1. Patients
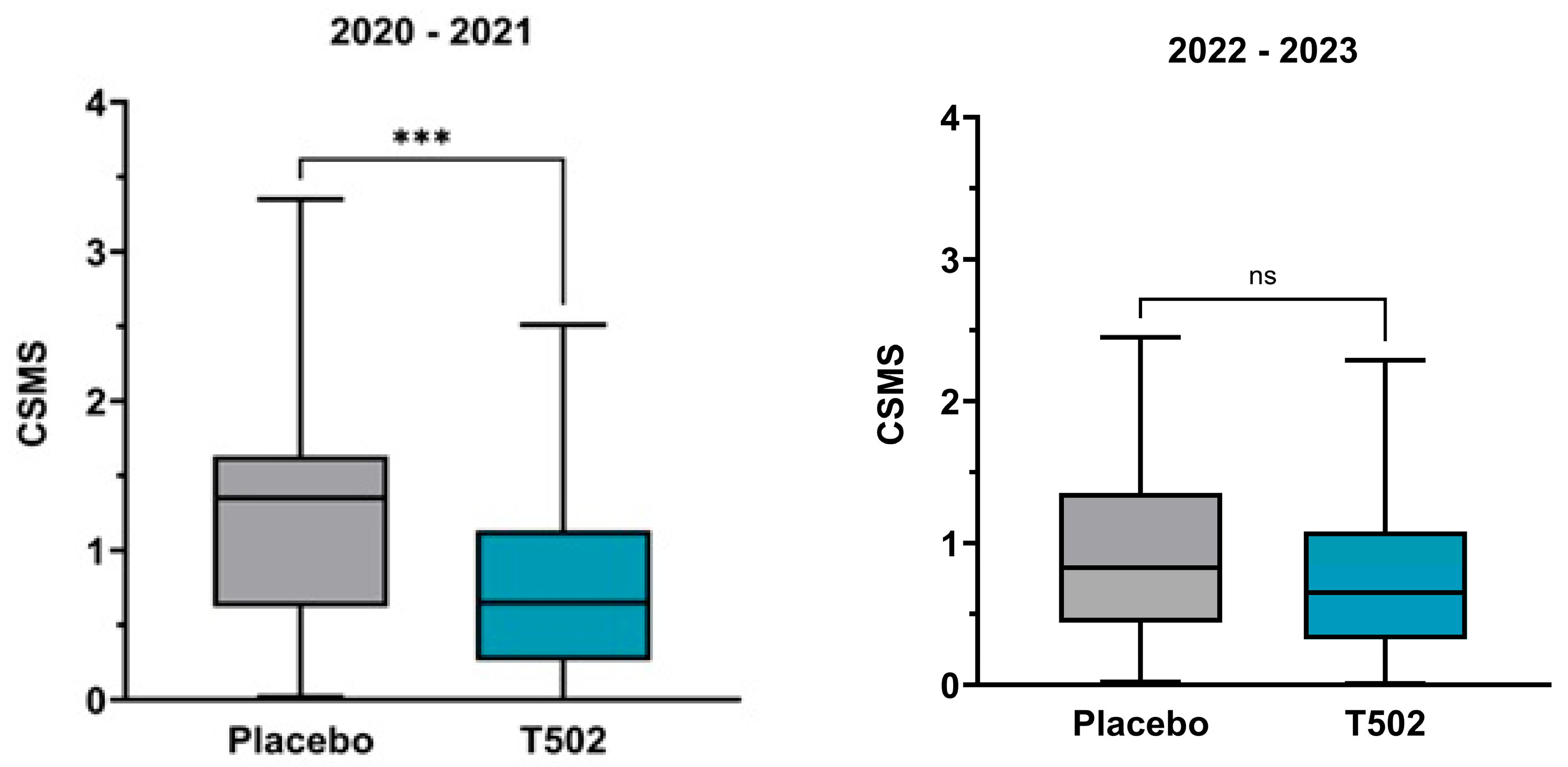
3.2. Combined Symptom and Medication Score
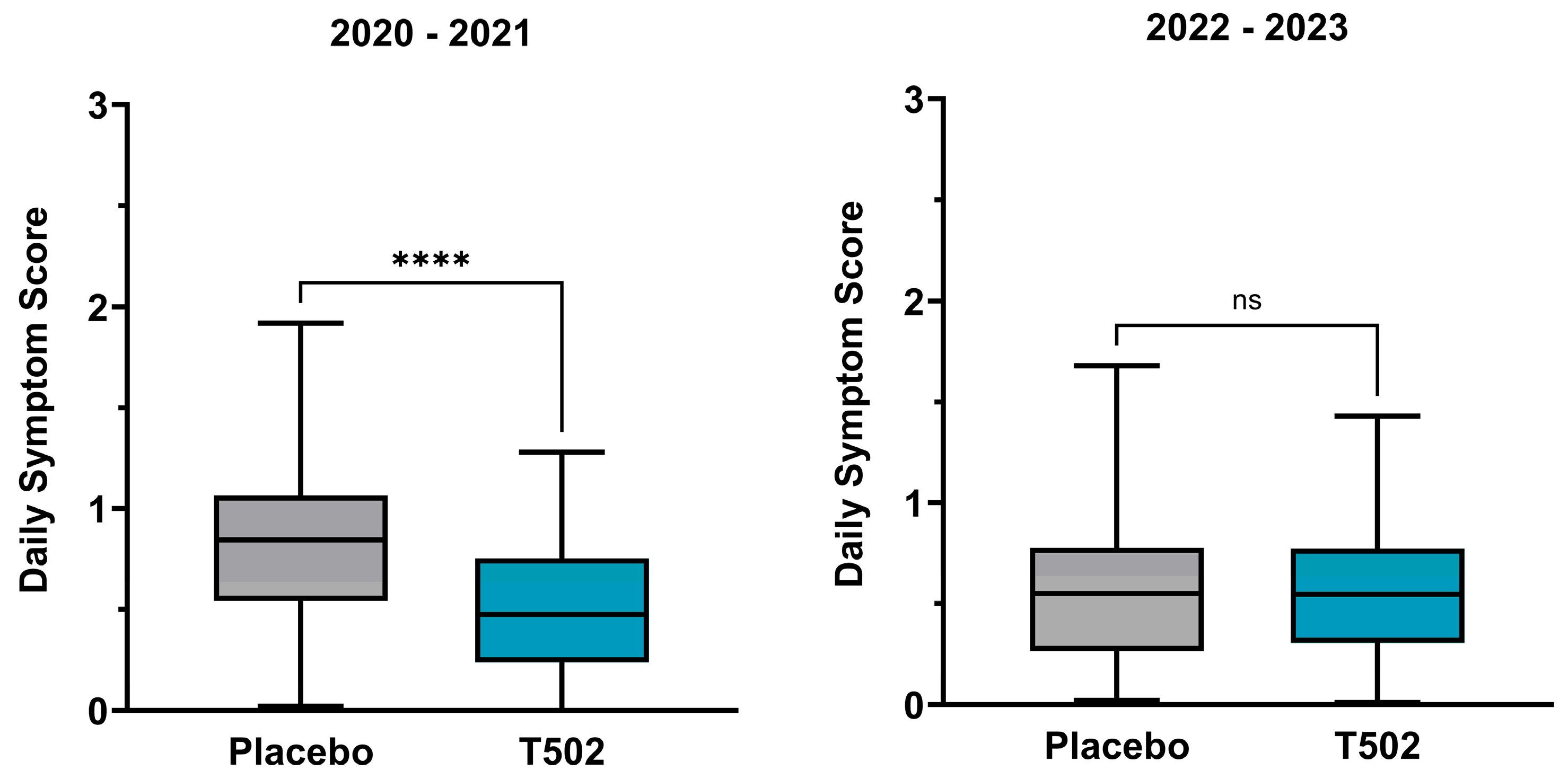
3.3. Daily Symptom Score
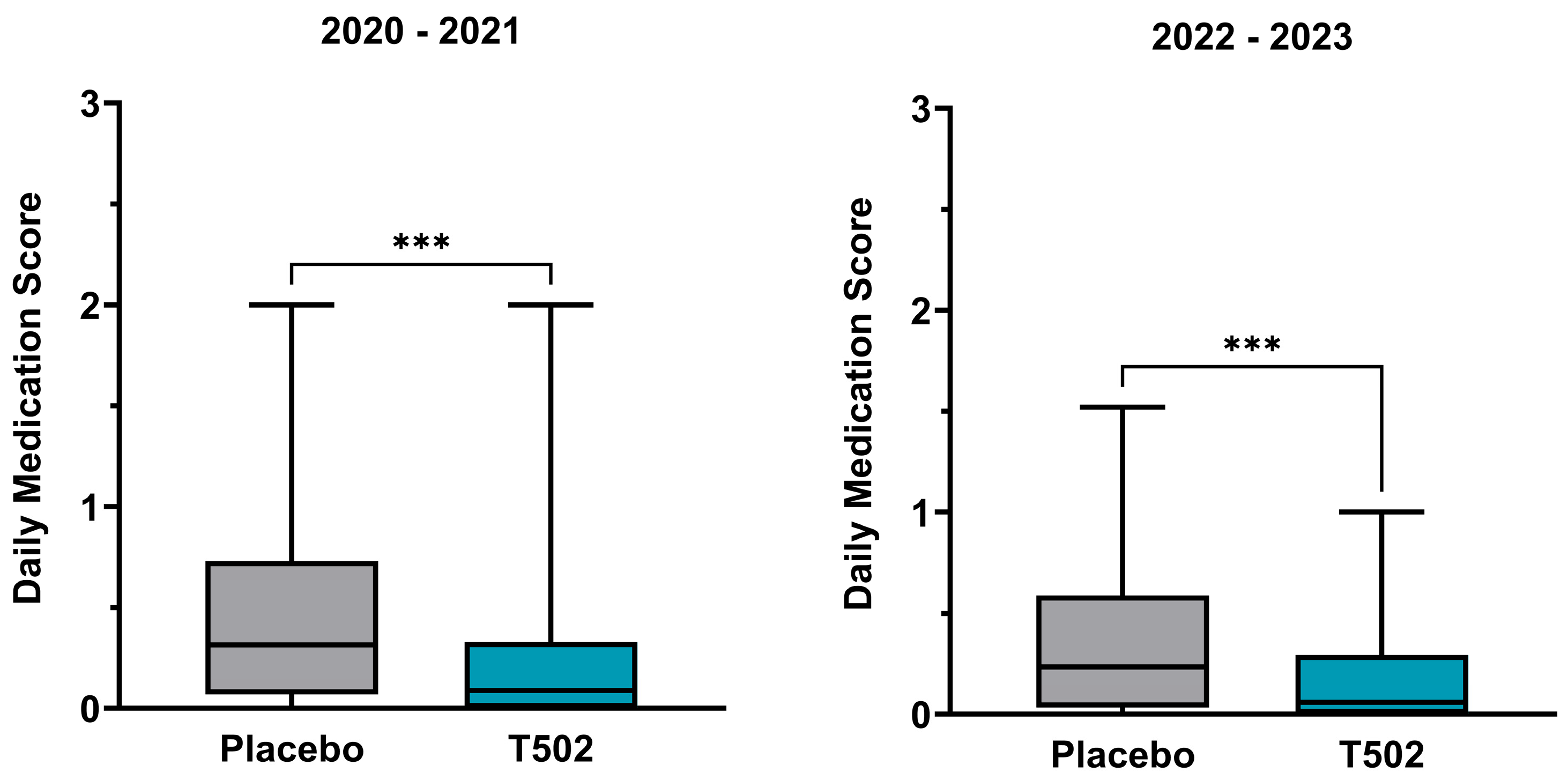
3.4. Daily Medication Score
4. Discussion
4.1. Limitations
4.2. COVID-19
4.3. Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIT | Allergen-specific immunotherapy |
| AR | Allergic rhinitis |
| ARC | Allergic rhinoconjunctivitis |
| COVID-19 | Coronavirus disease 2019 |
| CSMS | Combined symptom and medication score |
| dMS | Daily medication score |
| dSS | Daily symptom score |
| IgE | Immunoglobulin E |
| mTU | Mannan therapeutic units |
| p | (p-value) value for significance |
| SCIT | Subcutaneous immunotherapy |
| SLIT | Sublingual immunotherapy |
| SPSS | Statistical Package for the Social Sciences |
| V | Visit |
| WAO | World Allergy Organization |
References
- Novak, N.; Worm, M.; Staubach, P.; Jutel, M.; Sager, A.; Pfaar, O. Subcutaneous birch pollen allergen immunotherapy with a depigmented polymerized extract shows only sustained and long-term efficacy in a subgroup of monosensitized adults and adolescents with allergic rhinitis. Clin. Transl. Allergy 2022, 12, e12185. [Google Scholar] [CrossRef]
- Biedermann, T.; Winther, L.; Till, S.J.; Panzner, P.; Knulst, A.; Valovirta, E. Birch pollen allergy in Europe. Allergy 2019, 74, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Yang, J.; Zhang, J.; Yan, Y.; Sun, S.; Wang, J.; Li, X.; Chen, R.; Zhang, L. Airborne pollen exposure and risk of hospital admission for allergic rhinitis in Beijing: A time-stratified case-crossover study. Clin. Transl. Allergy 2024, 14, e12380. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Rosewich, M.; Lee, D.; Zielen, S. Pollinex Quattro: An innovative four injections immunotherapy in allergic rhinitis. Hum. Vaccines Immunother. 2013, 9, 1523–1531. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, C.; Zhang, L. Recent developments and highlights in allergic rhinitis. Allergy 2019, 74, 2320–2328. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E. International consensus statement on allergy and rhinology: Allergic rhinitis–2023. Int. Forum Allergy Rhinol. 2023, 13, 293–859. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.; Canonica, G.; Van Weel, C. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Baena-Cagnani, C.E.; Bonini, S.; Canonica, G.W.; Casale, T.B.; van Wijk, R.G.; Ohta, K.; Zuberbier, T.; Schünemann, H.J. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J. Allergy Clin. Immunol. 2010, 126, 466–476. [Google Scholar] [CrossRef]
- Penagos, M.; Durham, S.R. Allergen Immunotherapy: Optimal Duration for Respiratory Allergy. Curr. Treat. Options Allergy 2024, 11, 245–256. [Google Scholar] [CrossRef]
- Wallace, D.V.; Dykewicz, M.S.; Bernstein, D.I.; Blessing-Moore, J.; Cox, L.; Khan, D.A.; Lang, D.M.; Nicklas, R.A.; Oppenheimer, J.; Portnoy, J.M. The diagnosis and management of rhinitis: An updated practice parameter. J. Allergy Clin. Immunol. 2008, 122, S1–S84. [Google Scholar] [CrossRef]
- Biedermann, T.; Kuna, P.; Panzner, P.; Valovirta, E.; Andersson, M.; de Blay, F.; Thrane, D.; Jacobsen, S.H.; Stage, B.S.; Winther, L. The SQ tree SLIT-tablet is highly effective and well tolerated: Results from a randomized, double-blind, placebo-controlled phase III trial. J. Allergy Clin. Immunol. 2019, 143, 1058–1066.e6. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M. Evolution of Immunotherapy Against Pollen Allergy. Curr. Protein Pept. Sci. 2023, 24, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Sager, A.; Mosges, R.; Worm, M. A high-dose, depigmented polymerized birch pollen extract for subcutaneous allergen immunotherapy has a favourable efficacy/safety ratio. Clin. Transl. Allergy 2023, 13, e12315. [Google Scholar] [CrossRef]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; van Wijk, R.G.; Halken, S.; Larenas-Linnemann, D.; Pawankar, R.; Pitsios, C.; et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy 2018, 73, 765–798. [Google Scholar] [CrossRef]
- Pfaar, O.; Bachert, C.; Kuna, P.; Panzner, P.; Džupinová, M.; Klimek, L.; van Nimwegen, M.J.; Boot, J.D.; Yu, D.; Opstelten, D.J.E.; et al. Sublingual allergen immunotherapy with a liquid birch pollen product in patients with seasonal allergic rhinoconjunctivitis with or without asthma. J. Allergy Clin. Immunol. 2019, 143, 970–977. [Google Scholar] [CrossRef]
- Vogelberg, C.; Brüggenjürgen, B.; Richter, H.; Jutel, M. House dust mite immunotherapy in Germany: Real-world adherence to a subcutaneous allergoid and a sublingual tablet. Allergo J. Int. 2021, 30, 183–191. [Google Scholar] [CrossRef]
- Penagos, M.; Eifan, A.O.; Durham, S.R.; Scadding, G.W. Duration of Allergen Immunotherapy for Long-Term Efficacy in Allergic Rhinoconjunctivitis. Curr. Treat. Options Allergy 2018, 5, 275–290. [Google Scholar] [CrossRef]
- Scadding, G.W.; Calderon, M.A.; Shamji, M.H.; Eifan, A.O.; Penagos, M.; Dumitru, F.; Sever, M.L.; Bahnson, H.T.; Lawson, K.; Harris, K.M.; et al. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years Among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial. J. Am. Med. Assoc. 2017, 317, 615–625. [Google Scholar] [CrossRef]
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Pavón-Romero, G.F.; Parra-Vargas, M.I.; Ramírez-Jiménez, F.; Melgoza-Ruiz, E.; Serrano-Pérez, N.H.; Teran, L.M. Allergen Immunotherapy: Current and Future Trends. Cells 2022, 11, 212. [Google Scholar] [CrossRef]
- Benito-Villalvilla, C.; Soria, I.; Subiza, J.L.; Palomares, O. Novel vaccines targeting dendritic cells by coupling allergoids to mannan. Allergo J. Int. 2018, 27, 256–262. [Google Scholar] [CrossRef]
- Pfaar, O.; Becker, S.; Calabria, C.; Hartenstein, D.; Jung, J.; Zimmer, J.; Ponda, P. Comparison of allergen immunotherapy practice patterns in inhalant allergies in the United States of America and Europe: Similarities and differences 2023. World Allergy Organ. J. 2023, 16, 100766. [Google Scholar] [CrossRef]
- Casanovas, M.; Gómez, M.; Carnés, J.; Fernández-Caldas, E. Skin tests with native, depigmented and glutaraldehyde polymerized allergen extracts. J. Investig. Allergol. Clin. Immunol. 2005, 15, 30–36. [Google Scholar] [PubMed]
- Sirvent, S.; Soria, I.; Cirauqui, C.; Cases, B.; Manzano, A.I.; Diez-Rivero, C.M.; Reche, P.A.; López-Relaño, J.; Martínez-Naves, E.; Cañada, F.J.; et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J. Allergy Clin. Immunol. 2016, 138, 558–567.e11. [Google Scholar] [CrossRef] [PubMed]
- Manzano, A.I.; Javier Cañada, F.; Cases, B.; Sirvent, S.; Soria, I.; Palomares, O.; Fernández-Caldas, E.; Casanovas, M.; Jiménez-Barbero, J.; Subiza, J.L. Structural studies of novel glycoconjugates from polymerized allergens (allergoids) and mannans as allergy vaccines. Glycoconj. J. 2016, 33, 93–101. [Google Scholar] [CrossRef]
- Vinay, T.N.; Park, C.S.; Kim, H.Y.; Jung, S.J. Toxicity and dose determination of quillaja saponin, aluminum hydroxide and squalene in olive flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2014, 158, 73–85. [Google Scholar] [CrossRef]
- Mosges, R.; Zeyen, C.; Raskopf, E.; Acikel, C.; Sahin, H.; Allekotte, S.; Cuevas, M.; Shamji, M.H.; Subiza, J.L.; Casanovas, M. A randomized, double-blind, placebo-controlled trial with mannan-conjugated birch pollen allergoids. Allergy 2023, 79, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Demoly, P.; van Wijk, R.G.; Bonini, S.; Bousquet, J.; Canonica, G.W.; Calderon, M.A. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: An EAACI Position Paper. Allergy 2014, 69, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Mösges, R.; Raskopf, E.; Klimek, L.; Pfaar, O.; Zielen, S.; Xenofontos, E.; Decker, L.; Neuhof, C.; Rybachuk, A.; Acikel, C. Short-course subcutaneous treatment with birch pollen allergoids greatly improves symptom and medication scores in birch allergy. Allergy 2024, 80, 817–826. [Google Scholar] [CrossRef]
- Canonica, G.; Baena-Cagnani, C.; Bousquet, J.; Bousquet, P.; Lockey, R.; Malling, H.J.; Passalacqua, G.; Potter, P.; Valovirta, E. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy 2007, 62, 317–324. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satitsuksanoa, P.; Angelina, A.; Palomares, O.; Akdis, M. Mechanisms in AIT: Insights 2021. Allergol Sel. 2022, 6, 259–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zielen, S.; Bernstein, J.A.; Sturm, G.J.; Jutel, M.; Pfaar, O.; RESONATE Investigator Group; de Kam, P.J. Six Injections of Modified Adjuvanted PQ Grass Is Effective and Well-Tolerated in a Pivotal Phase III Trial. Allergy 2025, 80, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Biedermann, T.; Klimek, L.; Sager, A.; Robinson, D. Depigmented–polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy 2013, 68, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.T.; Iraola, V.; Himly, M.; Robinson, D.S.; Badiola, C.; García-Robaina, J.C.; Briza, P.; Carnés, J. Depigmented and polymerised house dust mite allergoid: Allergen content, induction of IgG4 and clinical response. Int. Arch. Allergy Immunol. 2010, 153, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Raskopf, E.; Shah-Hosseini, K.; Zadoyan, G.; Mösges, R. A review of allergoid immunotherapy: Is cat allergy a suitable target? Immunotherapy 2016, 8, 331–349. [Google Scholar] [CrossRef]
- Pfaar, O.; Agache, I.; de Blay, F.; Bonini, S.; Chaker, A.M.; Durham, S.R.; Gawlik, R.; Hellings, P.W.; Jutel, M.; Kleine-Tebbe, J.; et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy 2019, 74 (Suppl. S108), 3–25. [Google Scholar] [CrossRef]
- Bousquet, J.; Sousa-Pinto, B.; Anto, J.M.; Bedbrook, A.; Czarlewski, W.; Ansotegui, I.J.; Bergmann, K.C.; Braido, F.; Brussino, L.; Cecchi, L.; et al. Concurrent validity, cut-offs and ability to change of patient-reported outcome measures for rhinitis and asthma in MASK-air®. Clin. Transl. Allergy 2024, 14, e12390. [Google Scholar] [CrossRef]
- Dayal, A.K.; Sinha, V. Trend of Allergic Rhinitis Post COVID-19 Pandemic: A Retrospective Observational Study. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 50–52. [Google Scholar] [CrossRef]
- Dror, A.A.; Eisenbach, N.; Marshak, T.; Layous, E.; Zigron, A.; Shivatzki, S.; Morozov, N.G.; Taiber, S.; Alon, E.E.; Ronen, O.; et al. Reduction of allergic rhinitis symptoms with face mask usage during the COVID-19 pandemic. J. Allergy Clin. Immunol. Pr. 2020, 8, 3590–3593. [Google Scholar] [CrossRef]
- Mengi, E.; Kara, C.O.; Alptürk, U.; Topuz, B. The effect of face mask usage on the allergic rhinitis symptoms in patients with pollen allergy during the COVID-19 pandemic. Am. J. Otolaryngol. 2022, 43, 103206. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Inomata, T.; Iwagami, M.; Sung, J.; Nagino, K.; Adachi, T.; Morita, H.; Tamari, M.; Kainuma, K.; Kan-O, K.; et al. The impact of COVID-19 on hay fever treatment in Japan: A retrospective cohort study based on the Japanese claims database. Clin. Transl. Allergy 2024, 14, e12394. [Google Scholar] [PubMed]
- Korematsu, S.; Fujisawa, T.; Saito, N.; Tezuka, J.; Miura, K.; Kobayashi, I.; Miyata, I.; Kosugi, Y.; Gohda, Y.; Koike, Y.; et al. Suppressed pediatric asthma hospitalizations during the COVID-19 pandemic in Japan, from a national survey. Clin. Transl. Allergy 2024, 14, e12330. [Google Scholar] [CrossRef] [PubMed]
- Palathumpattu, B.; Pieper-Fürst, U.; Acikel, C.; Sahin, H.; Allekotte, S.; Singh, J.; Hess, M.; Sager, A.; Müller, T.; Mösges, R. Correlation of the combined symptom and medication score with quality of life, symptom severity and symptom control in allergic rhinoconjunctivitis. Clin. Transl. Allergy 2022, 12, e12191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Pozo, S.; Wahrhusen, N.; Pfaar, O.; Raskopf, E.; Rybachuk, A.; Acikel, C.; Sahin, H.; Allekotte, S.; Subiza, J.L.; Casanovas, M.; et al. Clinical Efficacy of One Short Course of Mannan-Conjugated Birch Pollen Allergoid Immunotherapy: A Comparative Evaluation After Prior Placebo Treatment. J. Clin. Med. 2025, 14, 8565. https://doi.org/10.3390/jcm14238565
del Pozo S, Wahrhusen N, Pfaar O, Raskopf E, Rybachuk A, Acikel C, Sahin H, Allekotte S, Subiza JL, Casanovas M, et al. Clinical Efficacy of One Short Course of Mannan-Conjugated Birch Pollen Allergoid Immunotherapy: A Comparative Evaluation After Prior Placebo Treatment. Journal of Clinical Medicine. 2025; 14(23):8565. https://doi.org/10.3390/jcm14238565
Chicago/Turabian Styledel Pozo, Sandra, Natascha Wahrhusen, Oliver Pfaar, Esther Raskopf, Anna Rybachuk, Cengizhan Acikel, Hacer Sahin, Silke Allekotte, José Luis Subiza, Miguel Casanovas, and et al. 2025. "Clinical Efficacy of One Short Course of Mannan-Conjugated Birch Pollen Allergoid Immunotherapy: A Comparative Evaluation After Prior Placebo Treatment" Journal of Clinical Medicine 14, no. 23: 8565. https://doi.org/10.3390/jcm14238565
APA Styledel Pozo, S., Wahrhusen, N., Pfaar, O., Raskopf, E., Rybachuk, A., Acikel, C., Sahin, H., Allekotte, S., Subiza, J. L., Casanovas, M., Cuevas, M., & Mösges, R. (2025). Clinical Efficacy of One Short Course of Mannan-Conjugated Birch Pollen Allergoid Immunotherapy: A Comparative Evaluation After Prior Placebo Treatment. Journal of Clinical Medicine, 14(23), 8565. https://doi.org/10.3390/jcm14238565





