Anorganic Bovine Bone vs. Biphasic Calcium Phosphate in a Large Series of Maxillary Sinus Floor Elevations—A Non-Randomized Clinico-Morphological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Protocol
2.3. Data Recorded
2.4. Histopathological Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Variables
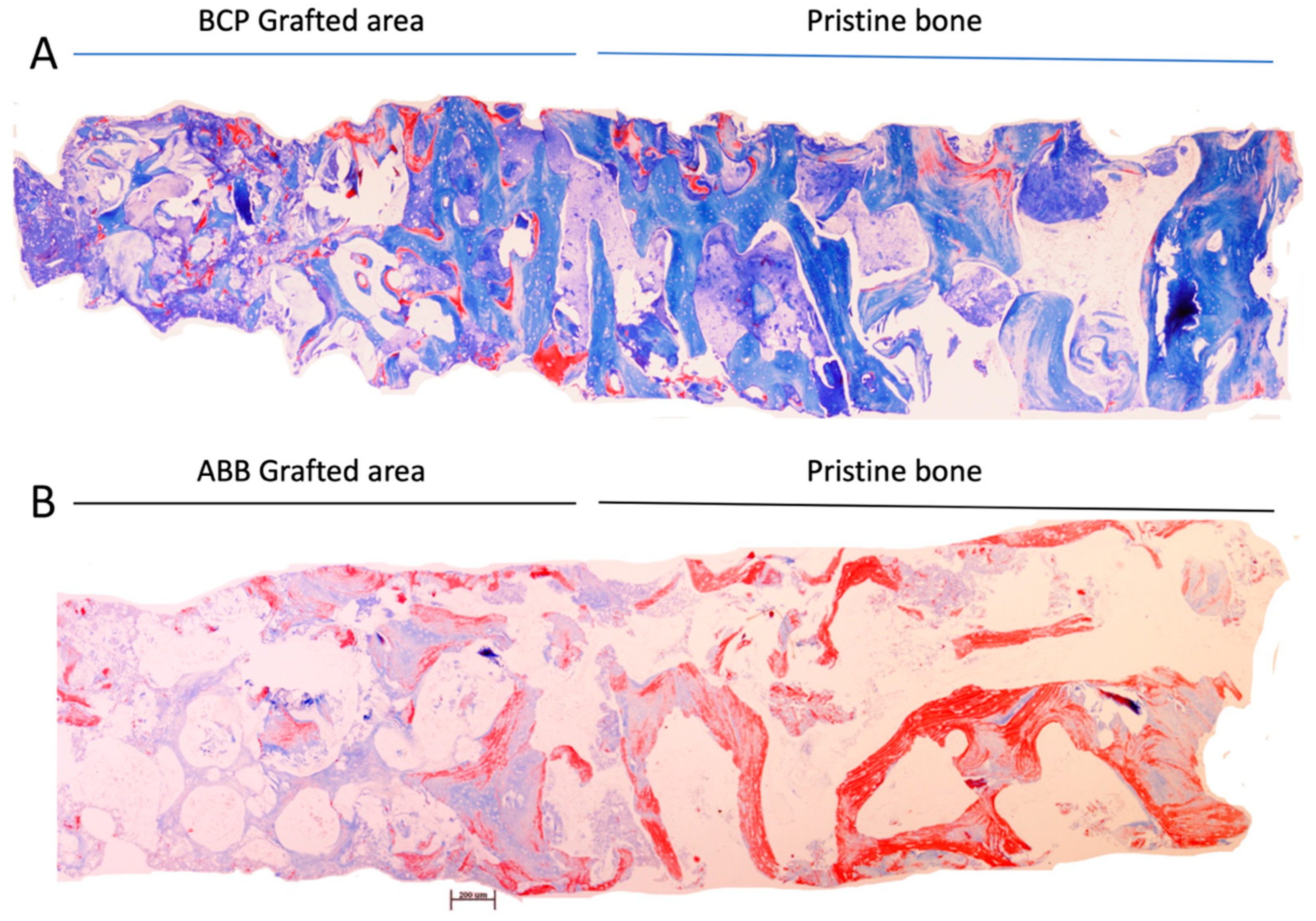
3.2. Histopathological Results
4. Discussion
- Both biomaterials have the same capacity to form mineral bone structure. This is to be expected, since bone formation depends on the genetics and function of the new bone to be formed, very much conditioned by the location where the graft is placed, and not on the action of the biomaterial itself, since this is usually only a scaffold, necessary for cell apposition and the promotion of osteoconduction and later osteogenesis. Avila–Ortiz et al. reported that no biomaterial or combination of biomaterials is able to overcome the characteristics and quantity of pristine bone typical of the grafted area [1]. In this sense, it is important not only to consider that the percentage of new mineralized bone tissue formed by both biomaterials is similar, but also that the osteogenic activity, assessed by the number of new osteoid lines, in both grafts (2.52 (2.67) vs. 2.93 (2.25); p = 0.326; ABB vs. BCP, respectively; Mann–Whitney U test).
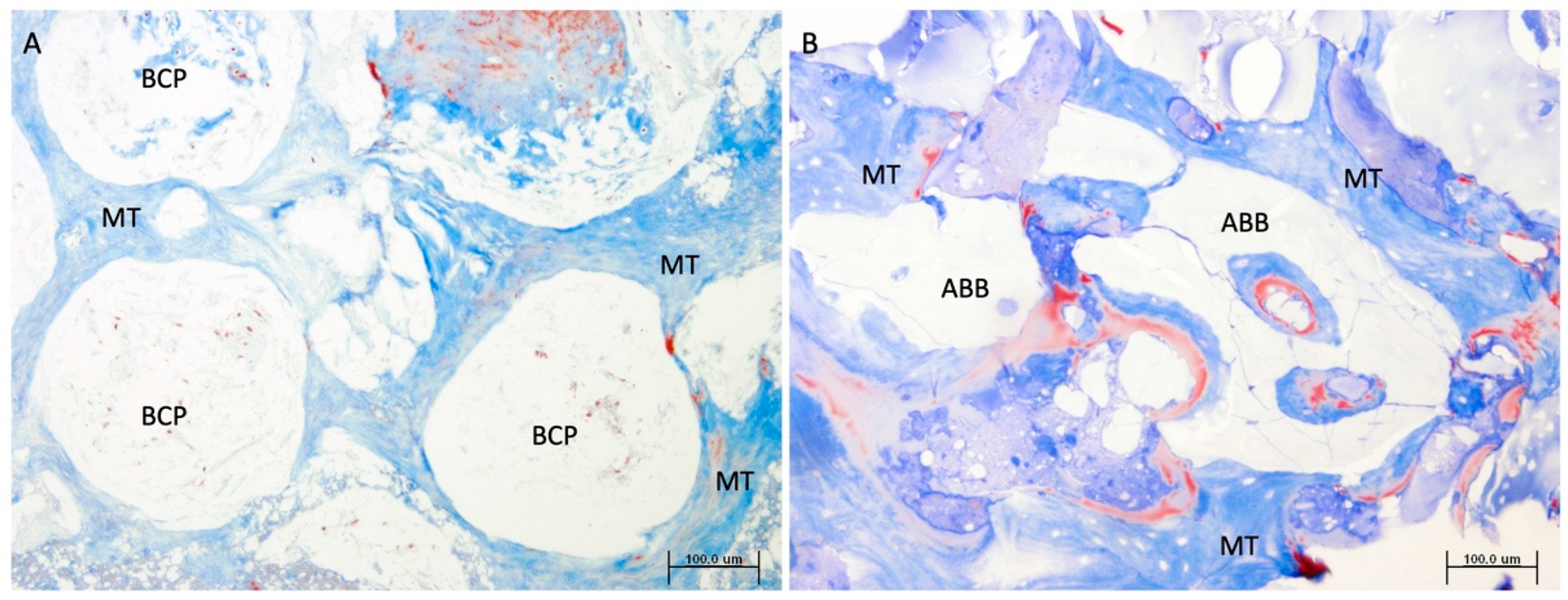
- Regarding the presence of remnant biomaterial, BCP disappears earlier in the newly formed bone, perhaps due to a different resorption mechanism than ABB or a different response to the osteoconduction mechanism. From the histological observation of these samples, it can be clearly seen how the pattern of formation of new mineral structure appears different between the two types of biomaterial particles. While the anorganic bovine bone particles seem to establish an intimate contact with the new bone mineral structure, the beta tricalcium-phosphate (beta-TCP) granules seem to allow bone formation in a more eccentric way, maintaining the porosity between the granules in a more intact way, which could facilitate the earlier physico-chemical action of the fluids and proteins of the tissue microenvironment. On the other hand, comparatively, anorganic bovine bone is much more crystalline in nature and somewhat more rigid in structure, which may slow the rate of bone resorption [15]. As mentioned above, the manufacturing process of each biomaterial can change its morphological, structural, or chemical properties, even if they are of the same origin, which may change the clinical results [16]. For example, Trajkovski et al. determined that the sintering process performed on Cerabone® leads to an increase in crystalline size, which would provide better long-term volume stability of this biomaterial compared to low-temperature or chemically treated bovine biomaterials [6].
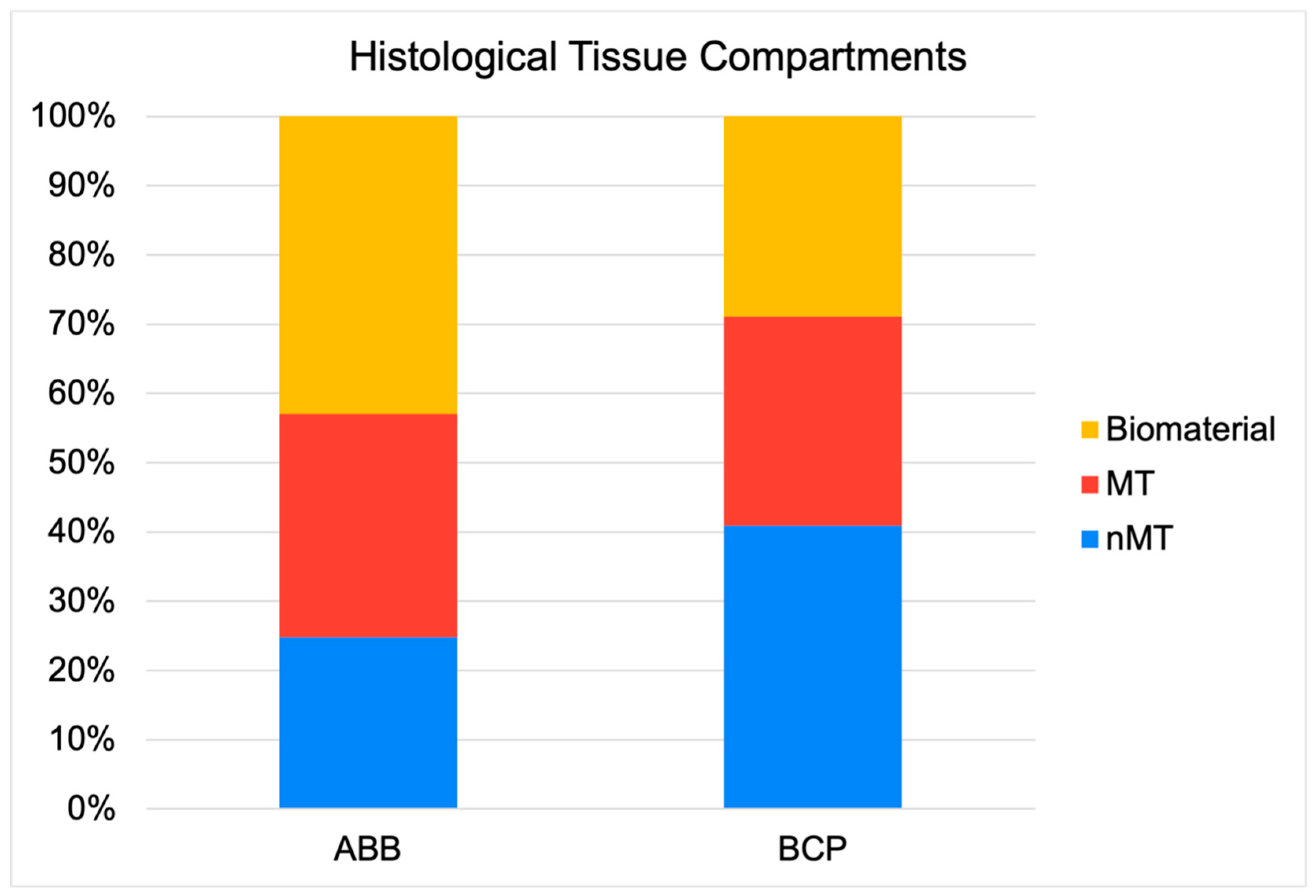
- The sum of the new mineral bone structure and the remaining biomaterial obviously determines the amount of non-mineralized vital bone, also known in the literature as ‘soft connective tissue’. Therefore, in this comparative study, the same as the new bone mineral structure, the amount of remaining biomaterial inversely conditions the amount of non-mineralized bone tissue, this component being greater in the graft composed of Easy Graft+® than in the graft composed of ABB. The function of this tissue component is depreciated in Implant Dentistry, since grafts with more non-mineralized structure are attributed with a lesser primary stability or insertion torque of implants, suggesting lower clinical mechanical retention. However, it is worth noting that it is in this component where stem cells and micro-vascularity accumulate and enhance the bone healing around implants and homeostasis phenomena of this resultant bone. Consequently, due to a greater quantity of non-mineralized bone component in the grafts made with Easy Graft+®, it is also logical to observe in them a greater osteoblastic cellularity, measured in a greater significant number of osteoblasts, and finally a greater osteocyte density, as has occurred in our comparison. The presence of polylactic-co-glycolic acid (PLGA) on the surface of Easy Graft+® particles may also contribute to the increased cellularity found in comparison to grafts made with anorganic bovine bone. In a previous study, we demonstrated that the presence of PLGA, an osteoconductor that facilitates the adhesion of preosteoblasts and osteoblasts to the matrix surface [17], also promoted an increase in the number of mesenchymal stem cells and microvascular density in the non-mineralized tissue of grafts made of these biomaterials, compared to similar grafts without the presence of PLGA [9].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araujo-Pires, A.C.; Mendes, V.C.; Ferreira-Junior, O.; Carvalho, P.S.P.; Guan, L.; Davies, J.E. Investigation of a Novel PLGA/CaP Scaffold in the Healing of Tooth Extraction Sockets to Alveolar Bone Preservation in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Vegh, D.; Mukaddam, K.; Galindo-Moreno, P.; Pjetursson, B.; Payer, M. Treatment alternatives for the rehabilitation of the posterior edentulous maxilla. Periodontology 2000 2023, 93, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Cinar, I.C.; Zboun, M.; Gultekin, B.A.; Saglanmak, A.; Akay, A.S. Retrospective analysis of three different xenografts in maxillary sinus augmentation: Histologic and three-dimensional radiologic study. Quintessence Int. 2023, 54, 640–649. [Google Scholar] [PubMed]
- Flichy-Fernández, A.J.; Blaya-Tárraga, J.A.; O’Valle, F.; Padial-Molina, M.; Peñarrocha-Diago, M.; Galindo-Moreno, P. Sinus floor elevation using particulate PLGA-coated biphasic calcium phosphate bone graft substitutes: A prospective histological and radiological study. Clin. Implant Dent. Relat. Res. 2019, 21, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Mesa, F.; Carranza, N.; Juodzbalys, G.; Aguilar, M.; O’Valle, F. Slow resorption of anorganic bovine bone by osteoclasts in maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2013, 15, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Klassmann, F.-A.; Ervolino, E.; Kluppel, L.-E.; Theodoro, L.-H.; Mulinari-Santos, G.; Garcia, V.-G. A randomised trial of the bone formation after maxillary sinus floor augmentation with bovine hydroxyapatite (Cerabone®) and Photobiomodulation: Histomorphometric and immunohistochemical analysis. J. Clin. Exp. Dent. 2023, 15, e542–e550. [Google Scholar] [CrossRef] [PubMed]
- Konermann, A.; Staubwasser, M.; Dirk, C.; Keilig, L.; Bourauel, C.; Götz, W.; Jäger, A.; Reichert, C. Bone substitute material composition and morphology differentially modulate calcium and phosphate release through osteoclast-like cells. Int. J. Oral Maxillofac. Surg. 2014, 43, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; O’Valle, F.; Monje-Gil, F.; Ortega-Oller, I.; Mesa, F.; Wang, H.-L.; Galindo-Moreno, P. Cellular, Vascular, and Histomorphometric Outcomes of Solvent-Dehydrated vs Freeze-Dried Allogeneic Graft for Maxillary Sinus Augmentation: A Randomized Case Series. Int. J. Oral Maxillofac. Implant. 2017, 32, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.S.; de Carvalho, M.V.N.B.; Hochuli-Vieira, E.; Statkievicz, C.; Pereira Santos, D.L.; Augusto Neto, R.T.; Pinto Cde, F.S.; Bennardo, F.; Mourão, C.F. Histomorphometric and Micro-CT Evaluation of Cerabone and Bio-Oss in Maxillary Sinus Lifting: A Randomized Clinical Trial. Med. (Kaunas Lith.) 2024, 60, 1834. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Z.P.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int. J. Dent. 2012, 2012, 737262. [Google Scholar] [CrossRef] [PubMed]
- Rios, H.F.; Avila, G.; Galindo, P.; Bratu, E.; Wang, H.-L. The influence of remaining alveolar bone upon lateral window sinus augmentation implant survival. Implant Dent. 2009, 18, 402–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in maxillofacial surgery: Membranes and grafts. Int. J. Biomed. Sci. IJBS 2011, 7, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Rapani, A.; Lombardi, T.; Bernardello, F.; Nicolin, V.; Berton, F. Does new bone formation vary in different sites within the same maxillary sinus after lateral augmentation? A prospective histomorphometric study. Clin. Oral Implant. Res. 2022, 33, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, B.; Jaunich, M.; Müller, W.-D.; Beuer, F.; Zafiropoulos, G.-G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Troedhan, A.; Schlichting, I.; Kurrek, A.; Wainwright, M. Primary implant stability in augmented sinuslift-sites after completed bone regeneration: A randomized controlled clinical study comparing four subantrally inserted biomaterials. Sci. Rep. 2014, 4, 5877. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Katranji, A. ABC sinus augmentation classification. Int. J. Periodontics Restor. Dent. 2008, 28, 383–389. [Google Scholar]
| ABB | BCP | p Value | |
|---|---|---|---|
| Age (mean (min-max)) | 45 (36–62) | 47 (38–63) | 0.464 |
| Gender (n (%)) | 0.142 | ||
| Female | 12 (60.0%) | 8 (36.4%) | |
| Male | 8 (40.0%) | 14 (63.6%) | |
| Bone Height Before (mm) | 2.99 (0.57) | 2.93 (0.52) | 0.700 |
| Bone Height After (mm) | 8.98 (0.77) | 9.05 (1.01) | 0.666 |
| ABB | BCP | p Value 1,2 | |
|---|---|---|---|
| Length of the biopsy (mm) | 5.26 (1.96) | 5.12 (2.05) | 0.805 |
| Total area of the biopsy (mm2) | 6.71 (2.93) | 7.06 (3.25) | 0.863 |
| Relative area of mineralized tissue in pristine bone (%) | 53.81 (17.09) | 54.57 (15.83) | 0.912 |
| Relative area of non-mineralized tissue in pristine bone (%) | 46.19 (17.09) | 45.43 (15.83) | 0.912 |
| Relative area of mineralized tissue in grafted bone (%) | 32.24 (13.71) | 30.19 (13.69) | 0.727 |
| Relative area of non-mineralized tissue in grafted bone (%) | 24.82 (13.31) | 40.90 (19.69) | 0.002 |
| Relative area of remnant biomaterial in grafted bone (%) | 42.94 (14.88) | 28.91 (19.42) | 0.002 |
| Osteoid Lines in pristine bone (n) | 0.80 (1.16) | 1.20 (1.28) | 0.160 |
| Osteoid Lines in grafted bone (n) | 2.52 (2.67) | 2.93 (2.25) | 0.326 |
| Osteocytes in grafted bone (n/mm2) | 284.95 (226.85) | 539.85 (195.54) | <0.001 |
| Osteoblasts in grafted bone (n/mm2) | 64.52 (75.77) | 125.24 (86.54) | 0.002 |
| Osteoclasts in grafted bone (n/mm2) | 76.34 (120.25) | 47.44 (71.13) | 0.424 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flichy-Fernández, A.J.; Padial-Molina, M.; Martin-Morales, N.; Alegre-Domingo, T.; Peñarrocha-Diago, M.; O’Valle, F.; Galindo-Moreno, P. Anorganic Bovine Bone vs. Biphasic Calcium Phosphate in a Large Series of Maxillary Sinus Floor Elevations—A Non-Randomized Clinico-Morphological Study. J. Clin. Med. 2025, 14, 8464. https://doi.org/10.3390/jcm14238464
Flichy-Fernández AJ, Padial-Molina M, Martin-Morales N, Alegre-Domingo T, Peñarrocha-Diago M, O’Valle F, Galindo-Moreno P. Anorganic Bovine Bone vs. Biphasic Calcium Phosphate in a Large Series of Maxillary Sinus Floor Elevations—A Non-Randomized Clinico-Morphological Study. Journal of Clinical Medicine. 2025; 14(23):8464. https://doi.org/10.3390/jcm14238464
Chicago/Turabian StyleFlichy-Fernández, Antonio J., Miguel Padial-Molina, Natividad Martin-Morales, Teresa Alegre-Domingo, Miguel Peñarrocha-Diago, Francisco O’Valle, and Pablo Galindo-Moreno. 2025. "Anorganic Bovine Bone vs. Biphasic Calcium Phosphate in a Large Series of Maxillary Sinus Floor Elevations—A Non-Randomized Clinico-Morphological Study" Journal of Clinical Medicine 14, no. 23: 8464. https://doi.org/10.3390/jcm14238464
APA StyleFlichy-Fernández, A. J., Padial-Molina, M., Martin-Morales, N., Alegre-Domingo, T., Peñarrocha-Diago, M., O’Valle, F., & Galindo-Moreno, P. (2025). Anorganic Bovine Bone vs. Biphasic Calcium Phosphate in a Large Series of Maxillary Sinus Floor Elevations—A Non-Randomized Clinico-Morphological Study. Journal of Clinical Medicine, 14(23), 8464. https://doi.org/10.3390/jcm14238464








