The Marshall Complex in the Human Heart: Embryology, Microanatomy, Autonomic Features and Clinical Implications for Atrial Fibrillation—A State-of-the-Art Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection of Publications and Data Extraction
2.4. Study Selection Summary
2.5. Ethical Considerations
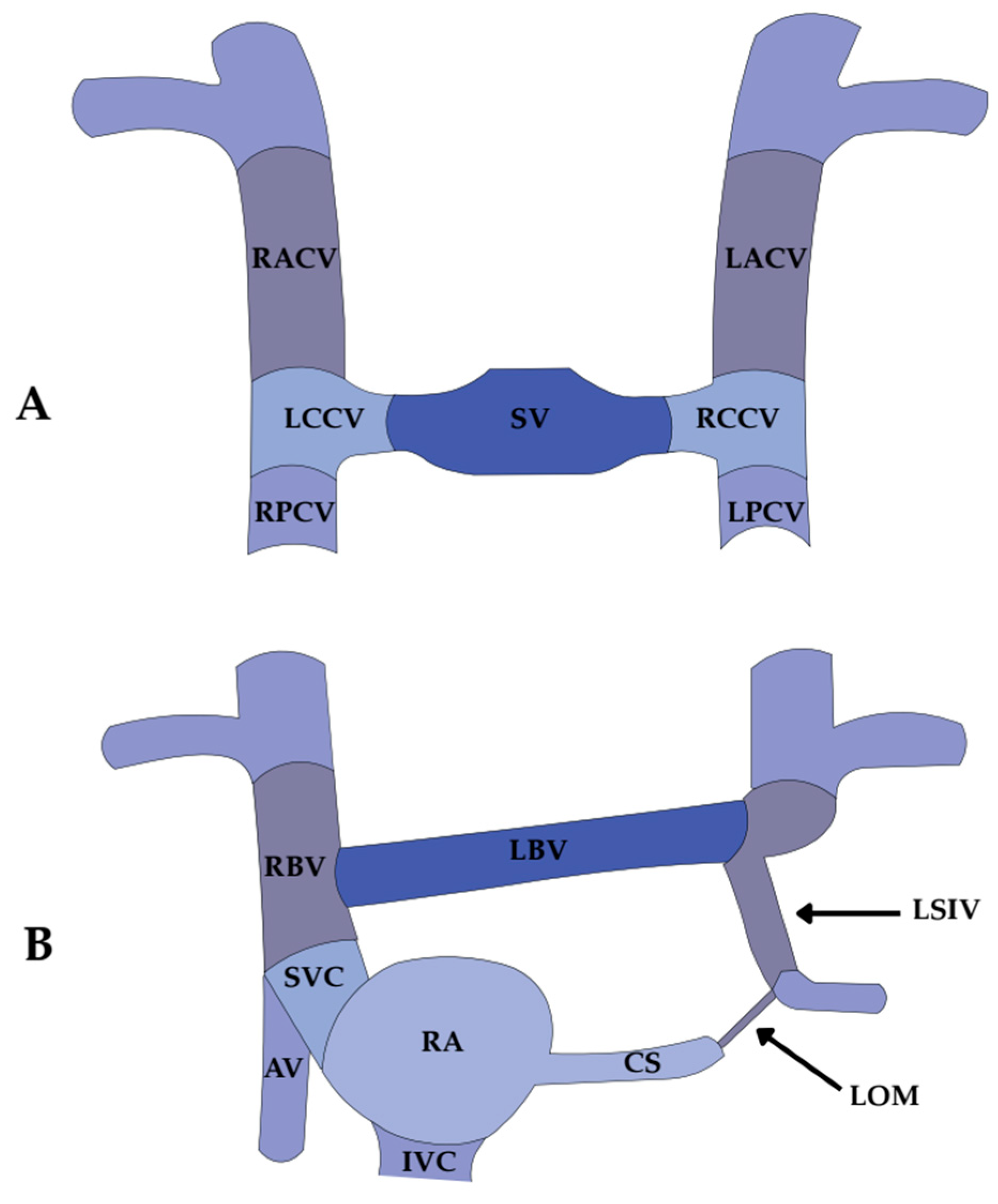
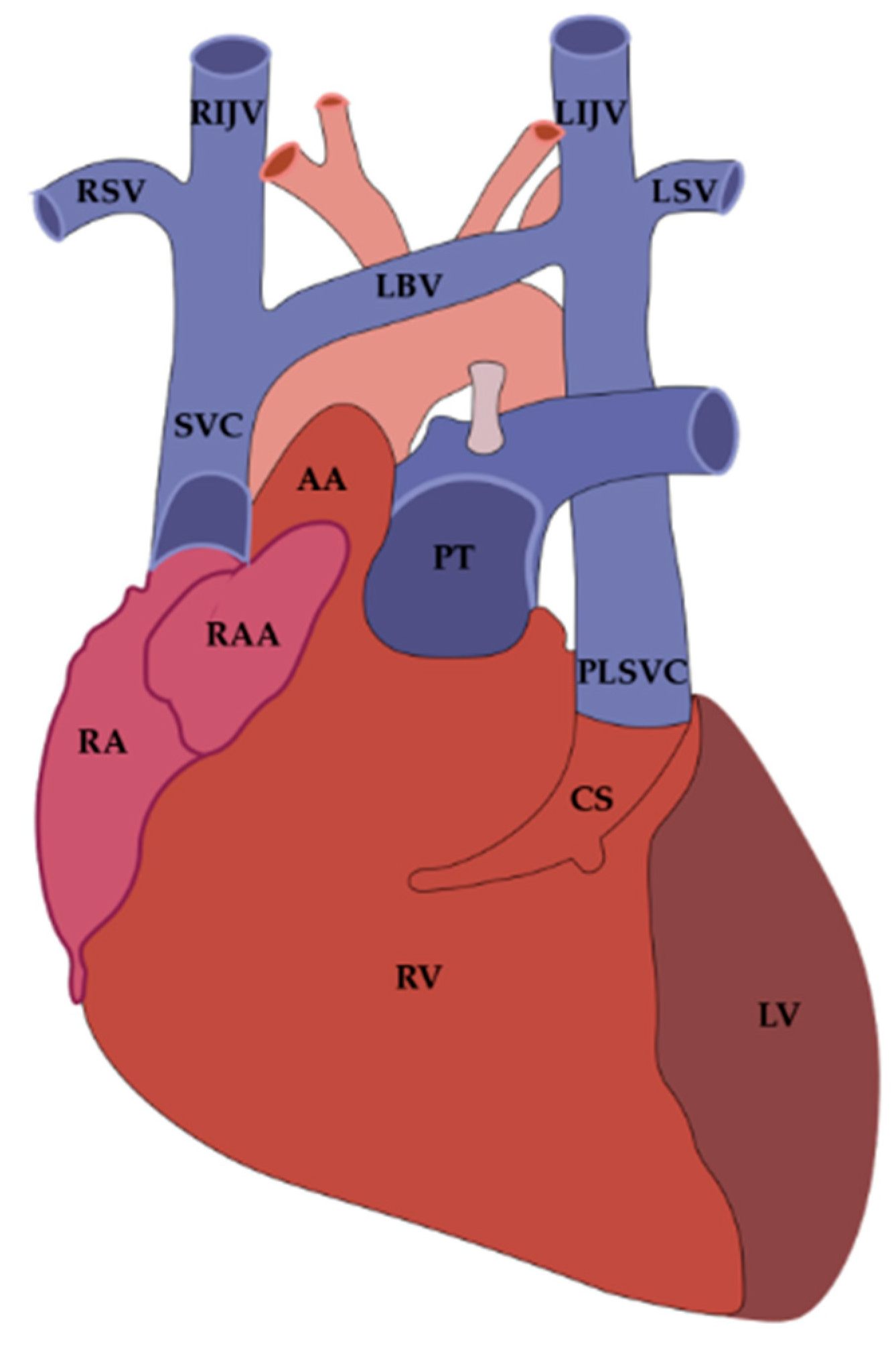
3. Embryological Origin of the Vein and Ligament of Marshall
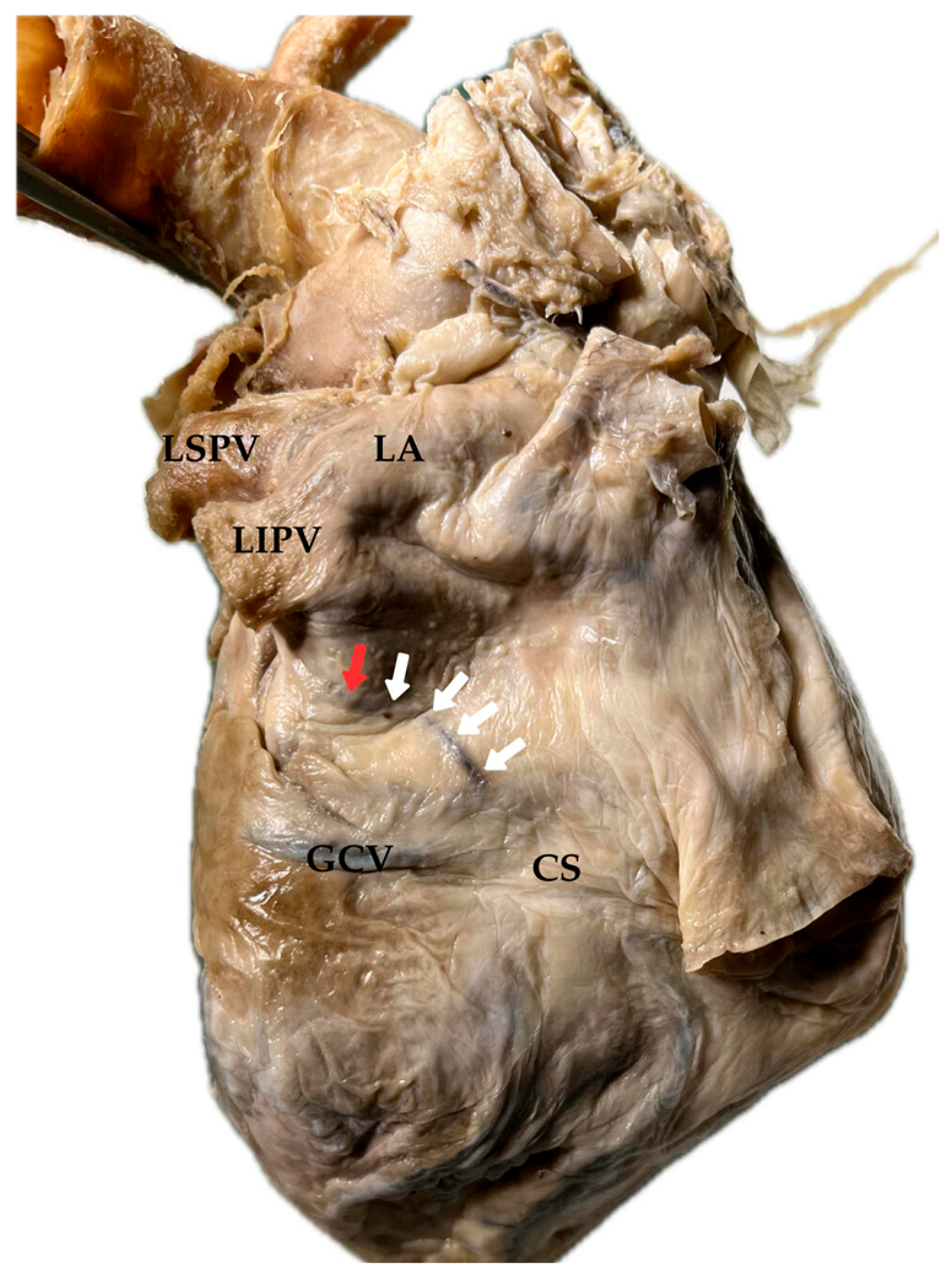
4. Anatomical Course and Morphological Variability of the Vein and Ligament of Marshall
4.1. Presence and Prevalence of the Vein and Ligament of Marshall
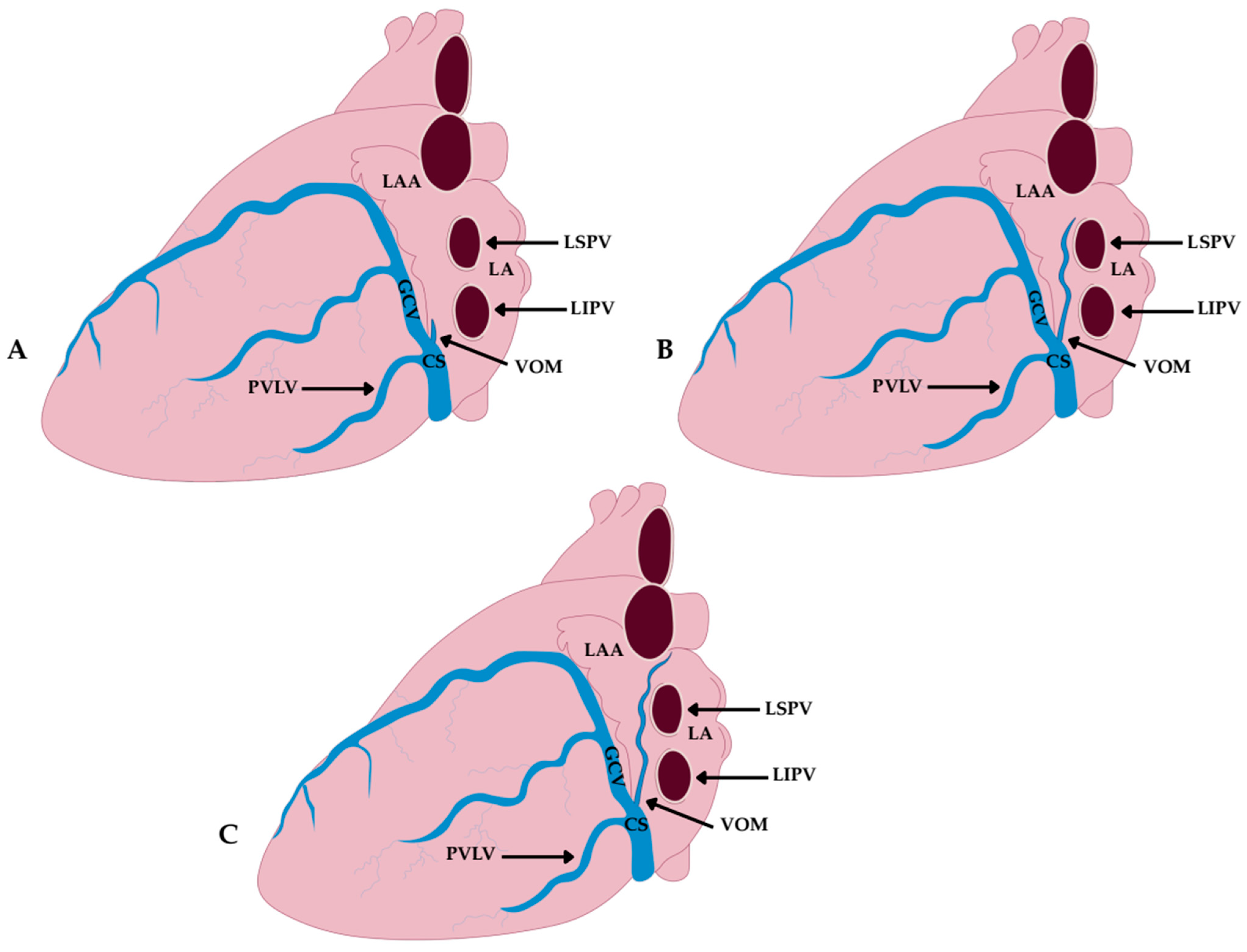
4.2. Morphological Variations in the Vein and Ligament of Marshall
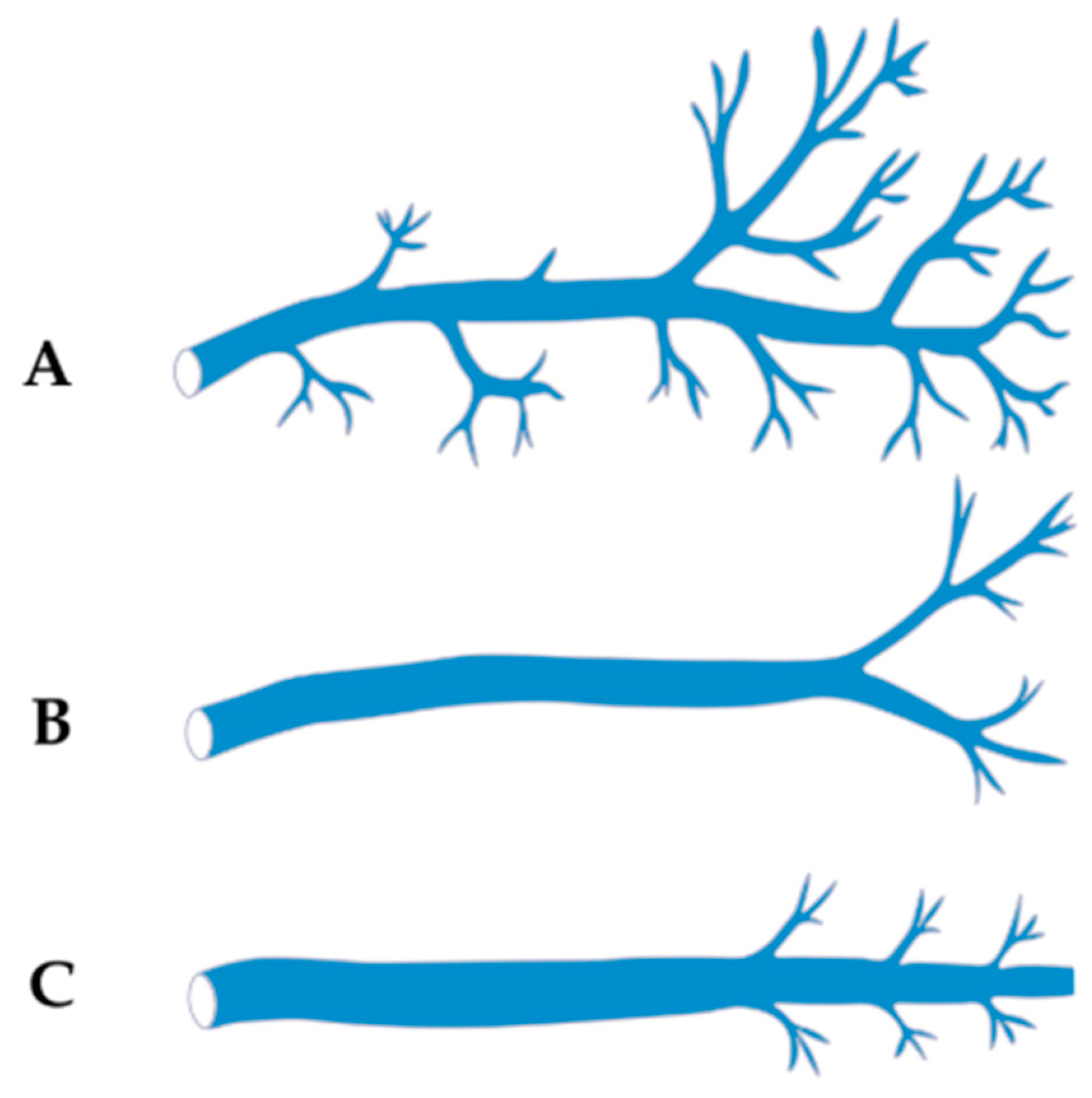
4.2.1. Classification Based on Branching Pattern/Tributaries (Cendrowska-Pinkosz & Urbanowicz)
- Dendritic Type—characterized by numerous fine tributaries converging into a single trunk draining into the CS. The length of the common trunk ranged from 0.5 to 1.8 mm, and this type was found in approximately 17.5% of cases.
- Forked Type—defined by the presence of two main tributaries merging into a single vein at a distance of 0.5–1.3 mm below the point of connection, without additional lateral branches. This was the most common variant, observed in about 48.5% of cases.
- Simple Type—lacking both initial tributaries and distal side branches, identified in approximately 34% of cases.
4.2.2. Classification Based on Length and Anatomical Extent (Delgove et al.)
- Short Type—terminating within the CS, without further extension toward the LA; observed in 16% of cases.
- Intermediate Type—extending up to the posterior wall of the LA; found in 57% of cases.
- Long Type—running all the way to the roof of the LA; present in 27% of cases.
4.3. Size and Dimensions of the Vein and Ligament of Marshall
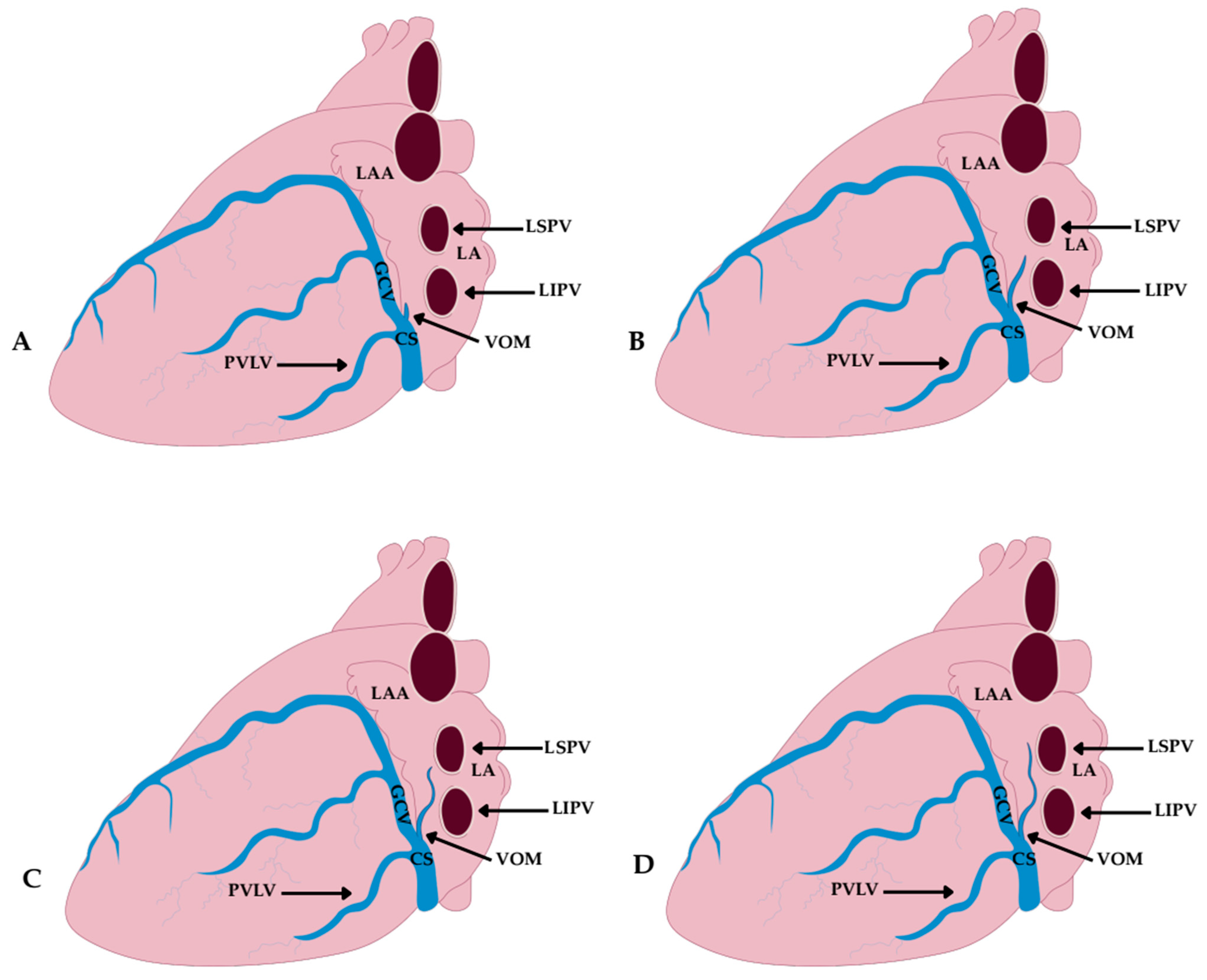
4.4. Topography and Ostium of the Vein and Ligament of Marshall
- Type I—terminating below the left inferior pulmonary vein; observed in 21.9% of cases.
- Type II—reaching the level of the left inferior pulmonary vein; observed in 47.7% of cases.
- Type III—ascending to the intervein area; observed in 17.2% of cases.
- Type IV—extending up to the left superior pulmonary vein; observed in 13.3% of cases.
- Group A—ostium at the level of the posterior vein of the left ventricle and the GCV opening; observed in 24% of cases.
- Group B—ostium at the level of the posterior vein of the left ventricle; 11% of cases.
- Group C—ostium at the level of the GCV; 7% of cases.
- Group D—independent ostium, separate from other tributaries of the CS; the most common variant, seen in 58% of cases.
5. Histological and Functional Structure of the Vein and Ligament of Marshall
5.1. Muscular Components of the Vein and Ligament of Marshall
- Proximal segment—connected to the muscular sleeve of the CS, serving as a potential conduction pathway between the CS and the MB.
- Middle segment—extending toward the LA ridge and forming connections with the left PV.
- Distal segment—running superiorly above the PV and in some cases reaching the free wall of the LA.
5.2. Nerve Components of the Vein and Ligament of Marshall
5.3. Nerve Ganglia of the Vein and Ligament of Marshall
5.4. Vascular Components of the Vein and Ligament of Marshall
5.5. Fibro-Fatty Tissue of the Ligament of Marshall
6. The Importance of the Vein and Ligament of Marshall in Atrial Fibrillation
7. The Role of the Vein and Ligament of Marshall in Other Atrial Arrhythmias
7.1. Perimitral Atrial Flutter (PMFL)
7.2. Focal Atrial Tachycardia (FAT)
7.3. Post-AF Ablation Atrial Tachycardia
8. Ablation Strategies Targeting the Vein and Ligament of Marshall in the Treatment of Atrial Arrhythmias
8.1. EIVOM
8.2. Endocardial RF Ablation
8.3. Epicardial (Pericardial) Ablation
8.4. Laser Ablation
8.5. Cryoablation
8.6. Hybrid Techniques and Imaging Support
9. Possible Complications of Ablation Procedures
10. Important Clinical Implications of Ablation Procedures
11. Imaging of the Vein and Ligament of Marshall
11.1. CT
11.2. CSA
11.3. ICE and TEE
11.4. EAM
11.5. Intraoperative Electrophysiological Localization
12. Age and Sex-Related Differences in the Vein and Ligament of Marshall
12.1. Age-Related Aspects
12.2. Sex-Related Aspects
12.3. Clinical Implications
13. Limitation of Current Knowledge
14. Future Directions
15. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOM | Vein of Marshall |
| LOM | Ligament of Marshall |
| LSVC | Left Superior Vena Cava |
| PLSVC | Persistent Left Superior Vena Cava |
| LA | Left Atrium |
| GCV | Great Cardiac Vein |
| CS | Coronary Sinus |
| CT | Computed Tomography |
| CSA | Coronary Sinus Angiography |
| MB | Marshall Bundles |
| AF | Atrial Fibrillation |
| EIVOM | Ethanol Infusion into the Vein of Marshall |
| LSPV | Left Superior Pulmonary Vein |
| LIPV | Left Inferior Pulmonary Vein |
| PV | Pulmonary Vein |
| RACV | Right Anterior Cardinal Vein |
| LACV | Left Anterior Cardinal Vein |
| RPCV | Reft Posterior Cardinal Vein |
| LPCV | Left Posterior Cardinal Vein |
| SV | Sinus Venosus |
| LBV | Left Brachiocephalic Vein |
| SVC | Superior Vena Cava |
| RA | Right Atrium |
| IVC | Inferior Vena Cava |
| AV | Azygos Vein |
| RBV | Right Brachiocephalic Vein |
| RAA | Right Atrial Appendage |
| RV | Right Ventricle |
| LV | Left Ventricle |
| AA | Ascending Aorta |
| PT | Pulmonary Trunk |
| RSV | Right Subclavian Vein |
| LSV | Left Subclavian Vein |
| RIJV | Right Internal Jugular Vein |
| LIJV | Left Internal Jugular Vein |
| LAA | Left Atrial Appendage |
| LPV | Left Pulmonary Vein |
| OVLA | Oblique Vein and Ligament Area |
| AT | Atrial Tachyarrhythmias |
| Cx-43 | Connexin-43 |
| Cx-40 | Connexin-40 |
| ANP | Atrial Natriuretic Peptide |
| ECG | Electrocardiogram |
| ERP | Effective Refractory Period |
| PMFL | Perimitral Atrial Flutter |
| FAT | Focal Atrial Tachycardia |
| PVI | Pulmonary Vein Isolation |
| RF | Radiofrequency |
| PFA | Pulsed Field Ablation |
| ICE | Intracardiac Echocardiography |
| TEE | Transesophageal Echocardiography |
| ESC | European Society of Cardiology |
| CMR | Cardiac Magnetic Resonance |
| CTA | Computed Tomography Angiography |
| EAM | Electroanatomical Mapping |
References
- Yagel, S.; Kivilevitch, Z.; Cohen, S.M.; Valsky, D.V.; Messing, B.; Shen, O.; Achiron, R. The fetal venous system, Part I: Normal embryology, anatomy, hemodynamics, ultrasound evaluation and Doppler investigation. Ultrasound Obstet. Gynecol. 2010, 35, 741–750. [Google Scholar] [CrossRef]
- Mozes, G.E.; Gloviczki, P. Venous embryology and anatomy. In The Vein Book; Bergan, J.J., Ed.; Academic Press: San Diego, CA, USA, 2007; pp. 15–25. [Google Scholar] [CrossRef]
- Marshall, J. On the development of the great anterior veins in man and mammalia: Including an account of certain remnants of foetal structure found in the adult, a comparative view of these great veins in the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philos. Trans. R. Soc. Lond. 1850, 140, 133–169. [Google Scholar]
- Shah, S.S.; Teague, S.D.; Lu, J.C.; Dorfman, A.L.; Kazerooni, E.A.; Agarwal, P.P. Imaging of the coronary sinus: Normal anatomy and congenital abnormalities. Radiographics 2012, 32, 991–1008. [Google Scholar] [CrossRef]
- Azizova, A.; Onder, O.; Arslan, S.; Ardali, S.; Hazırolan, T. Persistent left superior vena cava: Clinical importance and differential diagnoses. Insights Imaging 2020, 11, 110. [Google Scholar] [CrossRef]
- Perles, Z.; Nir, A.; Gavri, S.; Golender, J.; TaShma, A.; Ergaz, Z.; Rein, A.J. Prevalence of persistent superior vena cava and association with congenital heart anomalies. Am. J. Cardiol. 2013, 112, 1214–1218. [Google Scholar] [CrossRef]
- von Lüdinghausen, M.; Ohmachi, N.; Besch, S.; Mettenleiter, R. Atrial veins of the human heart. Clin. Anat. 1995, 8, 169–189. [Google Scholar] [CrossRef]
- Cendrowska-Pinkosz, M.; Urbanowicz, Z. Analysis of the course and the ostium of the oblique vein of the left atrium. Folia Morphol. 2000, 59, 163–166. [Google Scholar]
- Kim, D.T.; Lai, A.C.; Hwang, C.; Fan, L.-T.; Karagueuzian, H.S.; Chen, P.-S.; Fishbein, M.C. The ligament of Marshall: A structural analysis in human hearts with implications for atrial arrhythmias. J. Am. Coll. Cardiol. 2000, 36, 1324–1327. [Google Scholar] [CrossRef]
- Hwang, C.; Chen, P.S. Ligament of Marshall: Why it is important for atrial fibrillation ablation. Heart Rhythm. 2009, 6, S35–S40. [Google Scholar] [CrossRef]
- Rodríguez-Mañero, M.; Schurmann, P.; Valderrábano, M. Ligament and vein of Marshall: A therapeutic opportunity in atrial fibrillation. Heart Rhythm. 2016, 13, 593–601. [Google Scholar] [CrossRef]
- Desimone, C.V.; Noheria, A.; Lachman, N.; Edwards, W.D.; Gami, A.S.; Maleszewski, J.J.; Friedman, P.A.; Munger, T.M.; Hammill, S.C.; Packer, D.L.; et al. Myocardium of the superior vena cava, coronary sinus, vein of Marshall, and the pulmonary vein ostia: Gross anatomic studies in 620 hearts. J. Cardiovasc. Electrophysiol. 2012, 23, 1304–1309. [Google Scholar] [CrossRef]
- Żabówka, A.; Jakiel, M.; Bolechała, F.; Jakiel, R.; Jasińska, K.A.; Hołda, M.K. Topography of the oblique vein of the left atrium (vein of Marshall). Kardiol. Pol. 2020, 78, 688–693. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, H.; Yu, F.; Mi, L.; Hua, W.; Zhang, S.; Yao, Y.; Tang, M. Angiographic characteristics of the vein of Marshall in patients with and without atrial fibrillation. J. Clin. Med. 2022, 11, 5384. [Google Scholar] [CrossRef]
- Takagi, T.; Derval, N.; Pambrun, T.; Nakatani, Y.; André, C.; Ramirez, F.D.; Nakashima, T.; Krisai, P.; Kamakura, T.; Pineau, X.; et al. Optimized computed tomography acquisition protocol for ethanol infusion into the vein of Marshall. JACC Clin. Electrophysiol. 2022, 8, 168–178. [Google Scholar] [CrossRef]
- Młynarski, R.; Młynarska, A.; Gołba, K.S.; Sosnowski, M. Visualisation of the oblique vein of the left atrium (vein of Marshall) using cardiac computed tomography: Is the game worth the candle? Kardiol. Pol. 2018, 76, 1344–1349. [Google Scholar] [CrossRef]
- Delgove, A.; Walton, R.; Pallares-Lupon, N.; Ozenne, V.; Constantin, M.; Arnaud, M.; Le Quilliec, E.; Haïssaguerre, M.; Hocini, M.; Jaïs, P.; et al. Anatomy of the oblique vein of the left atrium: Contribution of microCT analysis of human hearts. Surg. Radiol. Anat. 2025, 47, 182. [Google Scholar] [CrossRef]
- Ulphani, J.S.; Arora, R.; Cain, J.H.; Villuendas, R.; Shen, S.; Gordon, D.; Inderyas, F.; Harvey, L.A.; Morris, A.; Goldberger, J.J.; et al. The ligament of Marshall as a parasympathetic conduit. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1629–H1635. [Google Scholar] [CrossRef]
- Makino, M.; Inoue, S.; Matsuyama, T.; Ogawa, G.; Sakai, T.; Kobayashi, Y.; Katagiri, T.; Ota, H. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J. Cardiovasc. Electrophysiol. 2006, 17, 594–599. [Google Scholar] [CrossRef]
- Takigawa, M.; Martin, C.; Jaïs, P. Mechanisms of vein of Marshall-related tachyarrhythmias and the impact of ethanol infusion. Rev. Cardiovasc. Med. 2024, 25, 112. [Google Scholar] [CrossRef]
- Cho, K.H.; Hayashi, S.; Jin, Z.W.; Kim, J.H.; Murakami, G.; Rodríguez-Vázquez, J.F. The so-called absorption process of the pulmonary vein into the left atrium of the heart: A histological study using human embryos and fetuses. Surg. Radiol. Anat. 2023, 45, 469–478. [Google Scholar] [CrossRef]
- Valderrábano, M. Vein of Marshall ethanol infusion in the treatment of atrial fibrillation: From concept to clinical practice. Heart Rhythm. 2021, 18, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, M. Ligament of Marshall arrhythmogenesis and vein of Marshall ethanol: A problem with a solution. Heart Rhythm. 2018, 15, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, M.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: The VENUS randomized clinical trial. JAMA 2020, 324, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Báez-Escudero, J.L.; Morales, P.F.; Dave, A.S.; Kim, Y.H.; Okishige, K.; Valderrábano, M. Ethanol infusion in the vein of Marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012, 9, 1207–1215. [Google Scholar] [CrossRef]
- Derval, N.; Duchateau, J.; Denis, A.; Ramirez, F.D.; Mahida, S.; André, C.; Krisai, P.; Nakatani, Y.; Kitamura, T.; Takigawa, M.; et al. Marshall bundle elimination, pulmonary vein isolation, and line completion for anatomical ablation of persistent atrial fibrillation (Marshall-PLAN): Prospective, single-center study. Heart Rhythm. 2021, 18, 529–537. [Google Scholar] [CrossRef]
- Anderson, R.H.; Brown, N.A.; Moorman, A.F.M. Development and structures of the venous pole of the heart. Dev. Dyn. 2006, 235, 2–9. [Google Scholar] [CrossRef]
- Gray, H.; Standring, S. (Eds.) Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: London, UK, 2021. [Google Scholar]
- Goyal, S.K.; Punnam, S.R.; Verma, G.; Ruberg, F.L. Persistent left superior vena cava: A case report and review of literature. Cardiovasc. Ultrasound. 2008, 6, 50. [Google Scholar] [CrossRef]
- Irwin, R.B.; Greaves, M.; Schmitt, M. Left superior vena cava: Revisited. Eur. Heart J. Cardiovasc. Imaging. 2012, 13, 284–291. [Google Scholar] [CrossRef]
- Tyrak, K.W.; Hołda, J.; Hołda, M.K.; Koziej, M.; Piątek, K.; Klimek-Piotrowska, W. Persistent left superior vena cava. Cardiovasc. J. Afr. 2017, 28, e1–e4. [Google Scholar] [CrossRef]
- Sonavane, S.K.; Milner, D.M.; Singh, S.P.; Abdel Aal, A.K.; Shahir, K.S.; Chaturvedi, A. Comprehensive imaging review of the superior vena cava. Radiographics. 2015, 35, 1873–1892. [Google Scholar] [CrossRef]
- Demos, T.C.; Posniak, H.V.; Pierce, K.L.; Olson, M.C.; Muscato, M. Venous anomalies of the thorax. Am. J. Roentgenol. 2004, 182, 1139–1150. [Google Scholar] [CrossRef]
- Depes, D.; Chłopaś, K.; Gil, K.; Ratajska, A. The autonomic nerves around the vein of Marshall: A postmortem study with clinical implications. APMIS 2024, 132, 165–175. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, S.; Choi, J.-I.; Lee, K.-N.; Baek, Y.-S.; Uhm, J.-S.; Shim, J.; Kim, J.S.; Park, S.W.; Hwang, C.; et al. Impact of persistent left superior vena cava on radiofrequency catheter ablation in patients with atrial fibrillation. Europace 2019, 21, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Ortale, J.R.; Gabriel, E.A.; Iost, C.; Márquez, C.Q. The anatomy of the coronary sinus and its tributaries. Surg. Radiol. Anat. 2001, 23, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.; Scanavacca, M.I.; Correia, A.T.; Sosa, E.A.; Aiello, V.D. Anatomic relations of the Marshall vein: Importance for catheterization of the coronary sinus in ablation procedures. Europace 2007, 9, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Linhares, R.R.; Silva, C.E.S.; Monaco, C.G.; Ferreira, L.D.C.; Gil, M.A.; Ortiz, J.; Anderson, R.H.; Aiello, V.D. Echocardiographic identification of the oblique vein of the left atrium: Its relationship to the persistent left superior caval vein. Cardiol. Young 2010, 20, 269–274. [Google Scholar] [CrossRef]
- Żabówka, A.; Hołda, J.; Strona, M.; Małek, Ł.; Koziej, M.; Hołda, M.K. Morphology of the Vieussens valve and its imaging in cardiac multislice computed tomography. J. Cardiovasc. Electrophysiol. 2019, 30, 1325–1329. [Google Scholar] [CrossRef]
- Yu, X.; He, W.; Xie, J.; He, B.; Luo, D.; Wang, X.; Jiang, H.; Lu, Z. Selective ablation of ligament of Marshall inhibits ventricular arrhythmias during acute myocardial infarction: Possible mechanisms. J. Cardiovasc. Electrophysiol. 2019, 30, 374–382. [Google Scholar] [CrossRef]
- Chandler, N.; Aslanidi, O.; Buckley, D.; Inada, S.; Birchall, S.; Atkinson, A.; Kirk, D.; Monfredi, O.; Molenaar, P.; Anderson, R.; et al. Computer three-dimensional anatomical reconstruction of the human sinus node and a novel paranodal area. Anat. Rec. 2011, 294, 970–979. [Google Scholar] [CrossRef]
- Langmuur, S.J.J.; Taverne, Y.J.H.J.; van Schie, M.S.; Bogers, A.J.J.C.; de Groot, N.M.S. Optimization of intra-operative electrophysiological localization of the ligament of Marshall. Front. Cardiovasc. Med. 2022, 9, 1030064. [Google Scholar] [CrossRef]
- Pambrun, T.; Derval, N.; Duchateau, J.; Denis, A.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; Nakashima, T.; Nakatani, Y.; et al. Epicardial course of the musculature related to the great cardiac vein: Anatomical considerations and clinical implications for mitral isthmus block after vein of Marshall ethanol infusion. Heart Rhythm. 2021, 18, 1951–1958. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, V.; Miyagawa, S.; Szalay, Z.; Risteli, J.; Kostin, S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J. Cell. Mol. Med. 2008, 12, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Okumura, Y.; Watanabe, I.; Nakai, T.; Ohkubo, K.; Kofune, T.; Kofune, M.; Mano, H.; Sonoda, K.; Hirayama, A. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ. J. 2011, 75, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, D.; Gurses, K.M.; Yalcin, M.U.; Yalcin, M.; Canpolat, U.; Tukek, T.; Aytemir, K.; Oto, A. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. J. Cardiovasc. Comput. Tomogr. 2015, 9, 295–302. [Google Scholar] [CrossRef]
- Chugh, S.S.; Roth, G.A.; Gillum, R.F.; Mensah, G.A. Global burden of atrial fibrillation in developed and developing nations. Glob. Heart 2014, 9, 113–119. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wolf, P.A.; Benjamin, E.J.; Levy, D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am. J. Cardiol. 1998, 82, 2N–9N. [Google Scholar] [CrossRef]
- Hayashi, T.; Fukamizu, S.; Mitsuhashi, T.; Kitamura, T.; Aoyama, Y.; Hojo, R.; Sugawara, Y.; Sakurada, H.; Hiraoka, M.; Fujita, H.; et al. Peri-Mitral Atrial Tachycardia Using the Marshall Bundle. JACC Clin. Electrophysiol. 2016, 2, 22–32. [Google Scholar] [CrossRef]
- Briceño, D.F.; Valderrábano, M. Recurrent perimitral flutter due to vein of Marshall epicardial connections bypassing the mitral isthmus: Response to ethanol infusion. Circ. Arrhythm. Electrophysiol. 2014, 7, 986–987. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018, 20, e1–e160. [Google Scholar] [CrossRef]
- Katritsis, D.; Giazitzoglou, E.; Korovesis, S.; Paxinos, G.; Anagnostopoulos, C.E.; Camm, A.J. Epicardial foci of atrial arrhythmias apparently originating in the left pulmonary veins. J. Cardiovasc. Electrophysiol. 2002, 13, 319–323. [Google Scholar] [CrossRef]
- Valderrábano, M.; Liu, X.; Sasaridis, C.; Sidhu, J.; Little, S.; Khoury, D.S. Ethanol infusion in the vein of Marshall: Adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009, 6, 1552–1558. [Google Scholar] [CrossRef]
- Valderrábano, M.; Morales, P.F.; Rodríguez-Mañero, M.; Lloves, C.; Schurmann, P.A.; Dave, A.S. The human left atrial venous circulation as a vascular route for atrial pharmacological therapies: Effects of ethanol infusion. JACC Clin. Electrophysiol. 2017, 3, 1020–1032. [Google Scholar] [CrossRef]
- Zhang, H.-D.; Ding, L.; Yu, F.-Y.; Mi, L.-J.; Zhang, K.; Weng, S.-X.; Jiang, Z.-H.; Tang, M. Angiographic assessment of vein of Marshall in atrial fibrillation: Implications for identification and cannulation. Heliyon 2023, 9, e21266. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Lupercio, F.; Goodman-Meza, D.; Ruiz, J.C.; Briceno, D.F.; Fisher, J.D.; Gross, J.; Ferrick, K.; Kim, S.; Di Biase, L.; et al. Electroanatomic mapping systems (CARTO/EnSite NavX) vs conventional mapping for ablation procedures in a training program. J. Interv. Card. Electrophysiol. 2016, 45, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bazoukis, G.; Elkholy, K.; Stavrakis, S.; Heist, E.K.; Armoundas, A.A. Efficacy of commonly used 3D mapping systems in acute success rates of catheter ablation procedures. Heart Int. 2024, 18, 9–25. [Google Scholar] [CrossRef] [PubMed]
- La Fazia, V.M.; Mohanty, S.; Gianni, C.; Zito, E.; Pierucci, N.; Stifano, G.; Torlapati, P.G.; Della Rocca, D.G.; Bode, W.D.; Burkhardt, J.D.; et al. Feasibility and Safety of Pulsed Field Ablation for Coronary Sinus and Left Atrial Appendage Isolation and Mitral Isthmus Ablation: Acute and Chronic Findings. Circ. Arrhythm. Electrophysiol. 2025, 18, e014026. [Google Scholar] [CrossRef]
- Poa, L.; Puig, M.; Zubiate, P.; Ranzenbach, E.; Shari-Knutson, M.; Poa, C.; Poa, H. Laser ablation of atrial fibrillation: Mid-term clinical experience. J. Atr. Fibrillation 2009, 2, 198. [Google Scholar] [CrossRef]
- Saremi, F.; Muresian, H.; Sánchez-Quintana, D. Coronary veins: Comprehensive CT-anatomic classification and review of variants and clinical implications. Radiographics 2012, 32, E1–E32. [Google Scholar] [CrossRef]
- Genc, B.; Solak, A.; Sahin, N.; Gur, S.; Kalaycioglu, S.; Ozturk, V. Assessment of the coronary venous system by using cardiac CT. Diagn. Interv. Radiol. 2013, 19, 286–293. [Google Scholar] [CrossRef]
- Aranyó, J.; Juncà, G.; Sarrias, A.; Bazan, V.; Cea, D.; Villuendas, R.; Gálvez-Montón, C.; Fernandez-Nofrerias, E.; Bayes-Genís, A.; Delgado, V.; et al. Left atrial structure and function following ethanol infusion into the vein of Marshall (MR-SHALL Study). J. Cardiovasc. Electrophysiol. 2025, 36, 157–167. [Google Scholar] [CrossRef]
- Chen, Y.A.; Nguyen, E.T.; Dennie, C.; Wald, R.M.; Crean, A.M.; Yoo, S.-J.; Jimenez-Juan, L. Computed tomography and magnetic resonance imaging of the coronary sinus: Anatomic variants and congenital anomalies. Insights Imaging. 2014, 5, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, X.; Chen, D.; Li, X.; Yang, L.; Liu, Z.; Chen, Y.; Zhang, J. Female sex is an independent risk factor for recurrence after ethanol Marshall bundle elimination in atrial fibrillation ablation. Front. Cardiovasc. Med. 2025, 12, 1556222. [Google Scholar] [CrossRef]
- Duchnowski, P.; Śmigielski, W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Pol. Heart J. 2024, 82, 423–426. [Google Scholar] [CrossRef]
| Study Type | Author (Year) | Method | Cases (N) | Prevalence of VOM |
|---|---|---|---|---|
| Cadaveric | Cendrowska-Pinkosz & Urbanowicz (2000) [8] | Gross Dissection | 100 | 97% |
| Cadaveric | DeSimone et al. (2012) [12] | Gross Dissection | 589 | 98% |
| Cadaveric | de Oliveira et al. (2007) [37] | Gross Dissection | 23 | 87% |
| Cadaveric | Żabówka et al. (2020) [13] | Gross Dissection | 200 | 71% |
| Cadaveric | Delgove et al. (2025) [17] | Micro-CT (ex vivo) | 18 | 18% |
| Clinical Imaging | Młynarski et al. (2018) [16] | CT (conventional) | 300 | 20,33% |
| Clinical Imaging | Takagi et al. (2022) [15] | CT (conventional) | 132 | 35% |
| Clinical Imaging | Takagi et al. (2022) [15] | CT (VOM-CT protocol) | 126 | 63% |
| Clinical Imaging | Ding et al. (2022) [14] | CSA | 290 | 73% |
| Study Type | Author (Year) | Method | Cases (N) | VOM Length (mm) | VOM Diameter (mm) | Distance from CS Ostium (mm) | Remarks |
|---|---|---|---|---|---|---|---|
| Cadaveric | Żabówka et al. (2020) [13] | Gross Dissection | 142 | 30.8 ± 13.6 (9–72); classical PV pattern: 27.6 ± 10.4; variant pattern: 48.3 ± 5.6 | – | – | p < 0.001 for length differences |
| Cadaveric | Ortale et al. (2001) [36] | Gross Dissection | 16 | – | 1.0 ± 0.4 (0.4–1.8) | – | – |
| Cadaveric | de Oliveira et al. (2007) [37] | Gross Dissection | 20 | – | 1.23 ± 0.38 (ostial) | 30.9 ± 10.2 | – |
| Cadaveric | DeSimone et al. (2012) [12] | Gross Dissection | 579 | – | – | 24 ± 4 | Patent segment length: 9.3 ± 6.6 |
| Cadaveric | Delgove et al. (2025) [17] | Micro-CT (ex vivo) | 15 | 36.5 ± 19.4 (12.3–72.2) | – | – | Based on micro-CT segmentation and arborization types |
| Clinical Imaging | Młynarski et al. (2018) [16] | CT (conventional) | 61 | 9.24 ± 7.58 (visible segment) | 1.72 ± 0.69 (segmental) | – | – |
| Clinical Imaging | Takagi et al. (2022) [15] | CT (VOM-CT protocol) | 79 | – | 1.6 ± 0.3 (1.1–2.6; segmental) | 36 ± 7 (22–52) | Dedicated VOM-CT protocol |
| Clinical Imaging | Ding et al. (2022) [14] | CSA | 257 | – | AF: 1.9 ± 0.9 (ostial); non–AF: 1.7 ± 0.7 | – | p < 0.05 for diameter differences |
| Strategy | Target/Area | Key Mechanism | When to Consider (Clinical Role) | Efficacy Assessment |
|---|---|---|---|---|
| EIVOM [22,24,25,26,56,57] | VOM/LOM, epicardial connections, mitral isthmus region | Chemoablation: conduction block, autonomic denervation, branch occlusion | Persistent AF, difficult/unstable mitral isthmus block, failed RF ablation, anatomical strategy | Successful VOM cannulation, “staining” of the vein, bidirectional isthmus block, absence of Marshall potentials |
| Endocardial RF Ablation [11,14,23,37,42,49,53,54,55,58,59,60] | Mitral isthmus region, atrial roof line, CS/VOM-related potentials | Endocardial RF lesion sets: conduction block, substrate modification; mapping from CS/VOM improves precision and assessment of block continuity | First-line ablation strategy for AF; PMFL prevention/treatment; patients with VOM/LOM-related epicardial connections (often supplemented by EIVOM when block is incomplete) | Achievement of durable bidirectional mitral isthmus block, validated by electroanatomic mapping and CS/VOM potentials; clinical efficacy limited by epicardial conduction bridges |
| Pulsed Field Ablation [61] | Mitral isthmus, CS region | Non-thermal irreversible electroporation | Persistent AF; mitral isthmus ablation when thermal energy fails; can complement EIVOM for epicardial gaps | Durable mitral isthmus lesions demonstrated; promising synergy with EIVOM |
| Epicardial ablation [42,43,54,55] | Epicardial connections along LOM/VOM and GCV | Targeted epicardial RF | When endocardial ablation is insufficient; necessary to close epicardial bypass circuits | Durable bidirectional isthmus block; caution due to tamponade risk |
| Cryoablation [46,47,49] | Vulnerable areas/sites with high perforation risk | Cryothermal necrosis (freezing) | Alternative to RF depending on anatomy and operator experience | Continuous lines without conduction; longer applications required |
| Laser ablation [62] | Localized damage | Photothermal tissue injury | Preliminary reports only; not a standard for VOM/LOM | – |
| Hybrid approach (EIVOM + PVI + RF) [22,24,25,26] | Elimination of muscular and autonomic components + completion of lines | Combination of chemoablation (EIVOM) with RF/PVI lines | Persistent/refractory AF, recurrences | Freedom from AF/AT, durable isthmus block, no conduction through VOM/LOM |
| Method | What It Shows | Clinical Role | Level of Evidence |
|---|---|---|---|
| CT (conventional protocol) [9,15,16,37,63,64] | Partial visualization of the VOM, occasional indirect depiction of the LOM | Anatomic studies, reference for other methods | Ex vivo, experimental |
| CT (VOM-CT protocol) [15,63,64] | More accurate visualization of the VOM | Procedural planning, limited sensitivity | Clinical, limited |
| CMR (high-resolution) [65,66] | Visualization of LA wall, venous territories, structural remodeling; occasional depiction of VOM course when optimized sequences used | Structural assessment before/after EIVOM; substrate characterization in AF | Clinical, emerging evidence |
| CTA (optimized /high-resolution) for venous system [16,65] | Better spatial resolution and contrast opacification; reliable identification of small venous structures including VOM; improved detection of anatomic variants | Pre-procedural VOM assessment; enhanced anatomical mapping | Clinical, supportive |
| micro-CT [17] | Precise morphology of VOM/LOM, branching patterns, course | Improved ablation planning, assessment of VOM accessibility | Clinical, moderate-quality evidence |
| CSA [14,24,25,37,58] | VOM ostium, morphology, cannulation | Intra-procedural standard prior to EIVOM | Clinical, high |
| ICE and TEE [9,15,24,37,38] | Indirect visualization of the VOM (contrast), catheter positioning | Intra-procedural monitoring and complication control | Clinical, supportive |
| EAM (CARTO, EnSite) [43,59,60] | Functional identification of VOM/LOM (electrogram signals) | Functional identification of VOM/LOM (electrogram signals) | Clinical, adjunctive |
| Intraoperative Electrophysiological Localization [42] | Functional identification of the LOM based on local electrograms | Surgical adjunct, refractory AF cases | Experimental/clinical case reports |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutka, W.B.; Bochenek, A.; Lepich, T.; Malinowski, M.; Bajor, G. The Marshall Complex in the Human Heart: Embryology, Microanatomy, Autonomic Features and Clinical Implications for Atrial Fibrillation—A State-of-the-Art Narrative Review. J. Clin. Med. 2025, 14, 8422. https://doi.org/10.3390/jcm14238422
Dutka WB, Bochenek A, Lepich T, Malinowski M, Bajor G. The Marshall Complex in the Human Heart: Embryology, Microanatomy, Autonomic Features and Clinical Implications for Atrial Fibrillation—A State-of-the-Art Narrative Review. Journal of Clinical Medicine. 2025; 14(23):8422. https://doi.org/10.3390/jcm14238422
Chicago/Turabian StyleDutka, Wojciech Bartosz, Adam Bochenek, Tomasz Lepich, Marcin Malinowski, and Grzegorz Bajor. 2025. "The Marshall Complex in the Human Heart: Embryology, Microanatomy, Autonomic Features and Clinical Implications for Atrial Fibrillation—A State-of-the-Art Narrative Review" Journal of Clinical Medicine 14, no. 23: 8422. https://doi.org/10.3390/jcm14238422
APA StyleDutka, W. B., Bochenek, A., Lepich, T., Malinowski, M., & Bajor, G. (2025). The Marshall Complex in the Human Heart: Embryology, Microanatomy, Autonomic Features and Clinical Implications for Atrial Fibrillation—A State-of-the-Art Narrative Review. Journal of Clinical Medicine, 14(23), 8422. https://doi.org/10.3390/jcm14238422






