Provisional Reduction Plating Versus External Fixation in the Staged Management of Gustilo–Anderson Type II–III Open Tibial Shaft Fractures
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. Surgical Technique and Rehabilitation
2.2.1. Group A: Provisional Miniplate and External Fixation (n = 32, Figure 1)
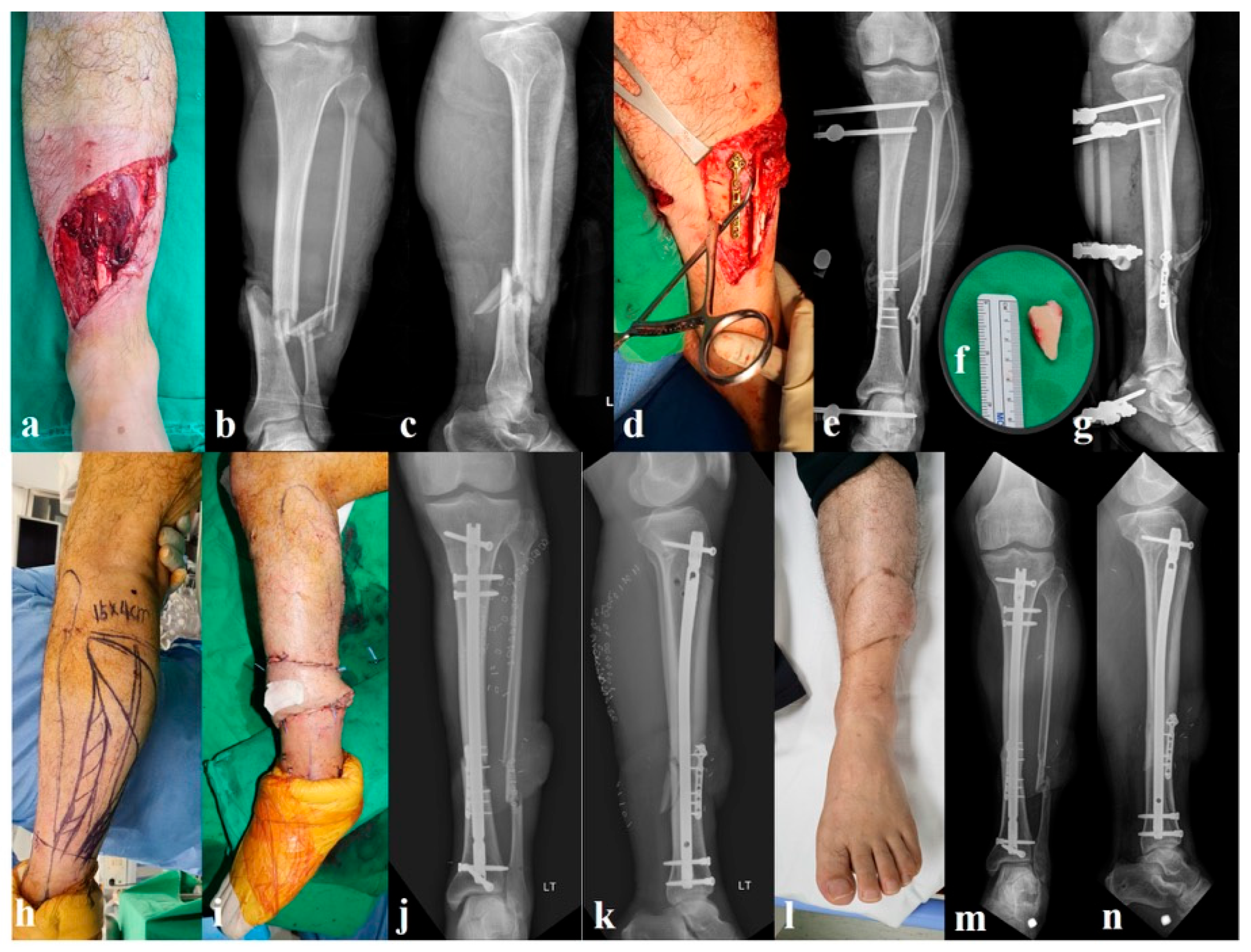
2.2.2. Group B: External Fixation Alone (n = 27, Figure 2)

2.3. Outcomes and Data Collection
2.4. Statistical Analyses
3. Results
3.1. Demographics and Injury Characteristics
3.2. Infection Outcomes
3.3. Fracture Union and Healing
3.4. Radiographic Alignment and Malunion
3.5. Operative Metrics
3.6. Functional Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMN | Intramedullary nailing |
| LEFS | Lower Extremity Functional Scale |
| AO/OTA | Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association |
| FRI | Fracture-related infection |
References
- Mundi, R.; Chaudhry, H.; Niroopan, G.; Petrisor, B.; Bhandari, M. Open tibial fractures: Updated guidelines for management. JBJS Rev. 2015, 3, e1. [Google Scholar] [CrossRef]
- Bhandari, M.; Guyatt, G.H.; Swiontkowski, M.F.; Schemitsch, E.H. Treatment of open fractures of the shaft of the tibia. J. Bone Jt. Surg. Br. 2001, 83, 62–68. [Google Scholar] [CrossRef]
- Melvin, J.S.; Dombroski, D.G.; Torbert, J.T.; Kovach, S.J.; Esterhai, J.L.; Mehta, S. Open tibial shaft fractures: I. Evaluation and initial wound management. J. Am. Acad. Orthop. Surg. 2010, 18, 10–19. [Google Scholar] [CrossRef]
- Hull, P. The management of open tibial fractures. Eur. J. Orthop. Surg. Traumatol. 2008, 18, 441–447. [Google Scholar] [CrossRef]
- Yoon, Y.-C.; Kim, Y.; Song, H.K.; Yoon, Y.H. Efficacy of staged surgery in the treatment of open tibial fractures with severe soft tissue injury and bone defect. Yonsei Med. J. 2022, 63, 915–926. [Google Scholar] [CrossRef]
- Yoon, Y.-C.; Oh, C.-W.; Lee, D.-W.; Sim, J.-A.; Oh, J.-K. Miniplate osteosynthesis in fracture surgeries: Case series with review of concepts. Injury 2020, 51, 878–886. [Google Scholar] [CrossRef]
- Ludwig, M.; Hymes, R.A.; Schulman, J.; Pitta, M.; Ramsey, L. Intramedullary nailing of open tibial fractures: Provisional plate fixation. Orthopedics 2016, 39, e931–e936. [Google Scholar] [CrossRef]
- Dunbar, R.P.; Nork, S.E.; Barei, D.P.; Mills, W.J. Provisional plating of Type III open tibia fractures prior to intramedullary nailing. J. Orthop. Trauma 2005, 19, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Dheenadhayalan, J.; Nagashree, V.; Devendra, A.; Velmurugesan, P.S.; Rajasekaran, S. Management of open fractures: A narrative review. J. Clin. Orthop. Trauma 2023, 44, 102246. [Google Scholar] [CrossRef] [PubMed]
- Revak, T.; Mahle, P.; Nicolaou, D.; Watson, J.T. Permanent reduction plate and intramedullary nailing of open tibia fractures: Do we need to take them out? Injury 2021, 52, 2439–2443. [Google Scholar] [CrossRef]
- Papakostidis, C.; Kanakaris, N.K.; Pretel, J.; Faour, O.; Morell, D.J.; Giannoudis, P.V. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury 2011, 42, 1408–1415. [Google Scholar] [CrossRef]
- Garnavos, C.; Kanakaris, N.K.; Lasanianos, N.G.; Tzortzi, P.; West, R.M. New classification system for long-bone fractures supplementing the AO/OTA classification. Orthopedics 2012, 35, e709–e719. [Google Scholar] [CrossRef]
- Nicholson, J.A.; Yapp, L.Z.; Keating, J.F.; Simpson, A.H.R.W. Monitoring of fracture healing. Update on current and future imaging modalities to predict union. Injury 2021, 52 (Suppl. S2), S29–S34. [Google Scholar] [CrossRef]
- Wittauer, M.; Burch, M.-A.; McNally, M.; Vandendriessche, T.; Clauss, M.; Della Rocca, G.J.; Giannoudis, P.V.; Metsemakers, W.-J.; Morgenstern, M. Definition of long-bone nonunion: A scoping review of prospective clinical trials to evaluate current practice. Injury 2021, 52, 3200–3205. [Google Scholar] [CrossRef]
- Zhao, K.; Lv, H.; Zhang, C.; Wang, Z.; Hou, Z.; Chen, W.; Zhang, Q.; Zhang, Y. Application of the multiplanar fracture redactor in the treatment of tibial shaft fractures with intramedullary nails. Sci. Rep. 2021, 11, 8428. [Google Scholar] [CrossRef]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys. Ther. 1999, 79, 371–383. [Google Scholar] [PubMed]
- Strage, K.E.; Parry, J.A.; Mauffrey, C. Standardizing statistics and data reporting in orthopaedic research. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Tejwani, N. Open tibial shaft fracture fixation strategies: Intramedullary nailing, external fixation, and plating. OTA Int. 2024, 7 (Suppl. S4), e316. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Itoman, M.; Uchino, M.; Fukushima, K.; Nitta, H.; Kojima, Y. Immediate versus delayed intramedullary nailing for open fractures of the tibial shaft: A multivariate analysis of factors affecting deep infection and fracture healing. Indian J. Orthop. 2008, 42, 410–419. [Google Scholar] [CrossRef]
- Bach, A.W.; Hansen, S.T. Plates versus external fixation in severe open tibial shaft fractures. Clin. Orthop. Relat. Res. 1989, 241, 89–94. [Google Scholar] [CrossRef]
- Haller, J.M.; Githens, M.; Scolaro, J.; Firoozabadi, R. Does provisional plating of closed tibia fractures have higher complication rates? J. Orthop. Trauma 2017, 31, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Wang, G.; Zhang, L.; Zhang, L.; Chen, S.; Du, H.; Zhao, Y.; Tang, P. Intramedullary nailing versus plating for distal tibia fractures without articular involvement: A meta-analysis. J. Orthop. Surg. Res. 2015, 10, 95. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group A (n = 32) | Group B (n = 27) | p-Value | |
|---|---|---|---|---|
| Age (years) | 37.5 ± 12.4 | 39.1 ± 11.7 | 0.61 | |
| Male | 22 (68.8) | 19 (70.4) | 0.89 | |
| Previous medical history | HTN | 7 | 6 | 0.64 |
| DM | 4 | 5 | ||
| Others | 6 | 3 | ||
| Smoking history | 13 | 11 | 1.00 | |
| Injury mechanism | MVC | 14 | 11 | 0.88 |
| Motorcycle | 8 | 6 | ||
| Fall | 6 | 5 | ||
| Crush | 3 | 4 | ||
| Others | 1 | 1 | ||
| Gustilo–Anderson classification | II | 12 | 12 | 0.96 |
| IIIA | 10 | 8 | ||
| IIIB | 7 | 5 | ||
| IIIC | 2 | 2 | ||
| AO/OTA classification | 42-A | 18 (56.3) | 16 (59.3) | 0.89 |
| 42-B | 10 (31.3) | 7 (25.9) | ||
| 42-C | 4 (12.5) | 4 (14.8) | ||
| Polytrauma (ISS ≥ 16) | 9 (28) | 10 (37) | 0.58 | |
| ASA score | I | 5 | 4 | 0.92 |
| II | 15 | 14 | ||
| III | 10 | 8 | ||
| IV | 2 | 1 | ||
| Variable | Group A (n = 32) | Group B (n = 27) | p-Value | |
|---|---|---|---|---|
| Time from injury to first I&D (hours) | 10 [2–21] | 11 [2–18] | 0.48 | |
| External fixator duration before IMN (days) 1 | 10 ± 5 | 12 ± 7 | 0.22 | |
| Operative time at IMN (minutes) | 71.2 ± 13.6 | 84.6 ± 19.8 | <0.05 | |
| C-arm screening time (seconds) | 95 ± 40 | 132 ± 55 | <0.05 | |
| Soft-tissue coverage within 7 days 1 | 25 (78.1) | 20 (74.1) | 0.77 | |
| Coverage method 1 | Local/rotational | 8 | 6 | — |
| Free flap | 4 | 4 | ||
| IV antibiotic duration (days) 1 | 11 ± 4 | 10 ± 4 | 0.34 | |
| Variable | Group A (n = 32) | Group B (n = 27) | p-Value |
|---|---|---|---|
| Time to union (weeks) | 23.5 ± 4.8 | 26.7 ± 5.6 | 0.08 |
| Coronal angulation at union (°) | 1.8 ± 2.5 | 4.2 ± 3.5 | 0.07 |
| Sagittal angulation at union (°) | 2.1 ± 2.7 | 4.8 ± 4.1 | 0.06 |
| LEFS at final follow-up | 76.1 ± 6.7 | 71.4 ± 5.2 | 0.09 |
| Time to protected WB (weeks) 1 | 4.0 ± 2.5 | 4.8 ± 1.8 | 0.11 |
| Deep infection (FRI 2018 definition) 1 | 6 (18.8) | 4 (14.8) | 0.74 |
| Superficial infection | 1 (3.1) | 2 (7.4) | 0.59 |
| Nonunion | 6 (18.8) | 5 (18.5) | 1.00 |
| Malunion (>5° or >2 cm) | 1 (3.1) | 5 (18.5) | 0.08 |
| Pin-site infection while in external fixation 1 | 3 (9.4) | 2 (7.4) | 1.00 |
| Reoperation (any cause) 1 | 12 (37.5) | 9 (33.3) | 0.79 |
| Exchange nailing for nonunion | 2 | 2 | — |
| Bone graft for nonunion | 4 | 3 | |
| DAIR (for FRI) 1 | 4 | 3 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.-C.; Yoon, S.H.; Kim, M.J.; Oh, H.-K. Provisional Reduction Plating Versus External Fixation in the Staged Management of Gustilo–Anderson Type II–III Open Tibial Shaft Fractures. J. Clin. Med. 2025, 14, 8421. https://doi.org/10.3390/jcm14238421
Yoon Y-C, Yoon SH, Kim MJ, Oh H-K. Provisional Reduction Plating Versus External Fixation in the Staged Management of Gustilo–Anderson Type II–III Open Tibial Shaft Fractures. Journal of Clinical Medicine. 2025; 14(23):8421. https://doi.org/10.3390/jcm14238421
Chicago/Turabian StyleYoon, Yong-Cheol, Seok Hwan Yoon, Min Jun Kim, and Hyoung-Keun Oh. 2025. "Provisional Reduction Plating Versus External Fixation in the Staged Management of Gustilo–Anderson Type II–III Open Tibial Shaft Fractures" Journal of Clinical Medicine 14, no. 23: 8421. https://doi.org/10.3390/jcm14238421
APA StyleYoon, Y.-C., Yoon, S. H., Kim, M. J., & Oh, H.-K. (2025). Provisional Reduction Plating Versus External Fixation in the Staged Management of Gustilo–Anderson Type II–III Open Tibial Shaft Fractures. Journal of Clinical Medicine, 14(23), 8421. https://doi.org/10.3390/jcm14238421






