Cell-Free DNA Levels During the First Hours After Liver Transplantation: A Key Biomarker for Patient Survival and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Patient Surveillance and Treatment
2.3. DNA Extraction from Tissue and Serum Samples
2.4. Quantification of cfDNA by Real-Time Quantitative PCR (qPCR)
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. Characteristics of the Patients
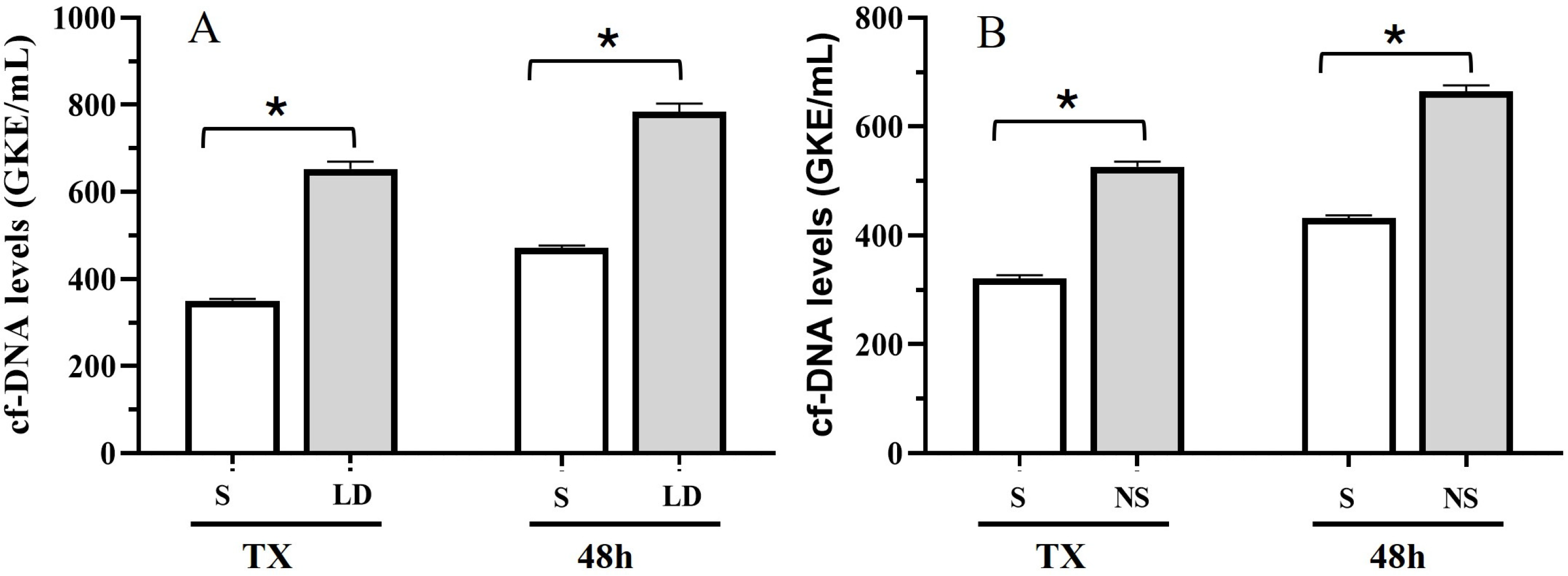
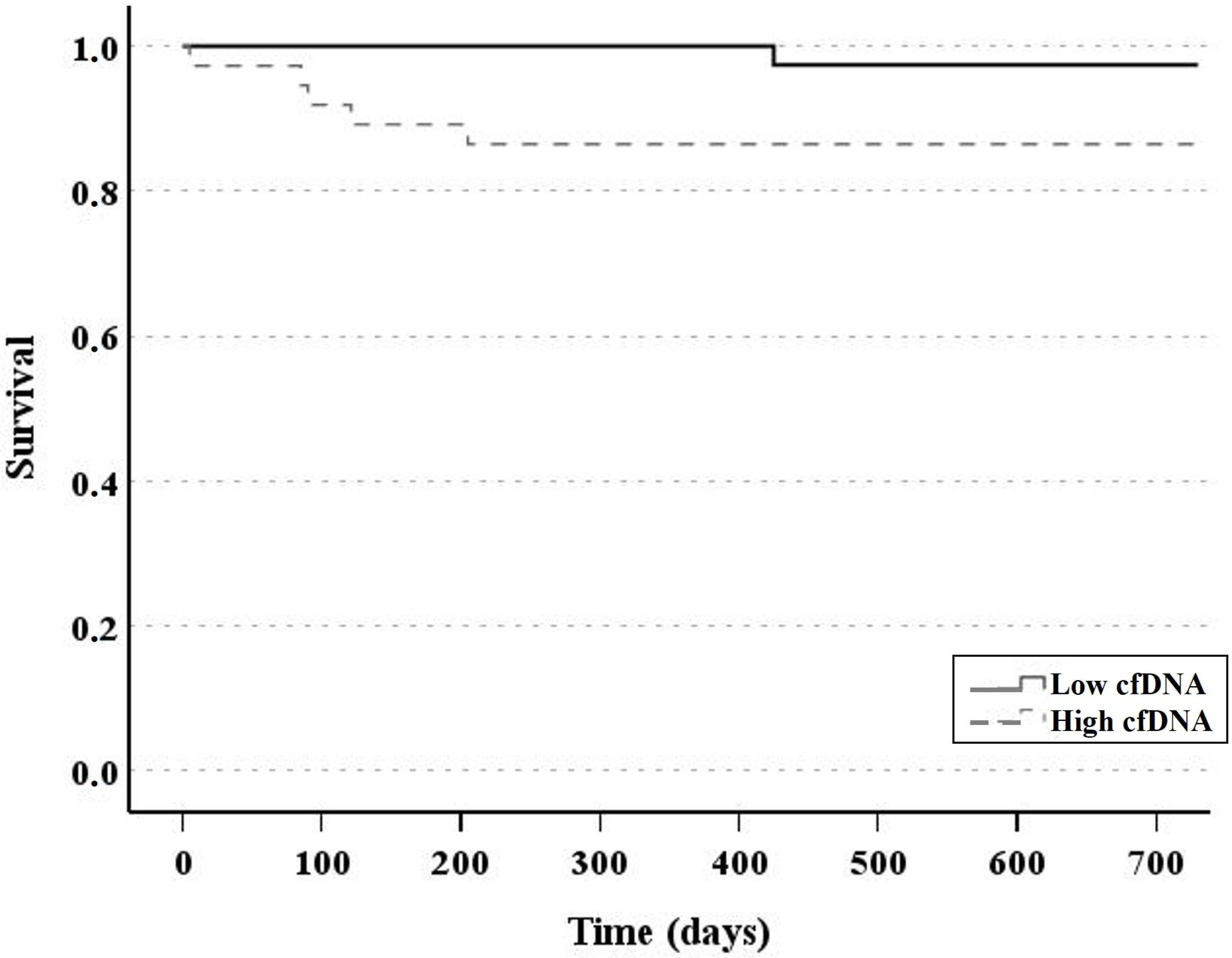
3.2. Patient Outcomes and cfDNA Levels
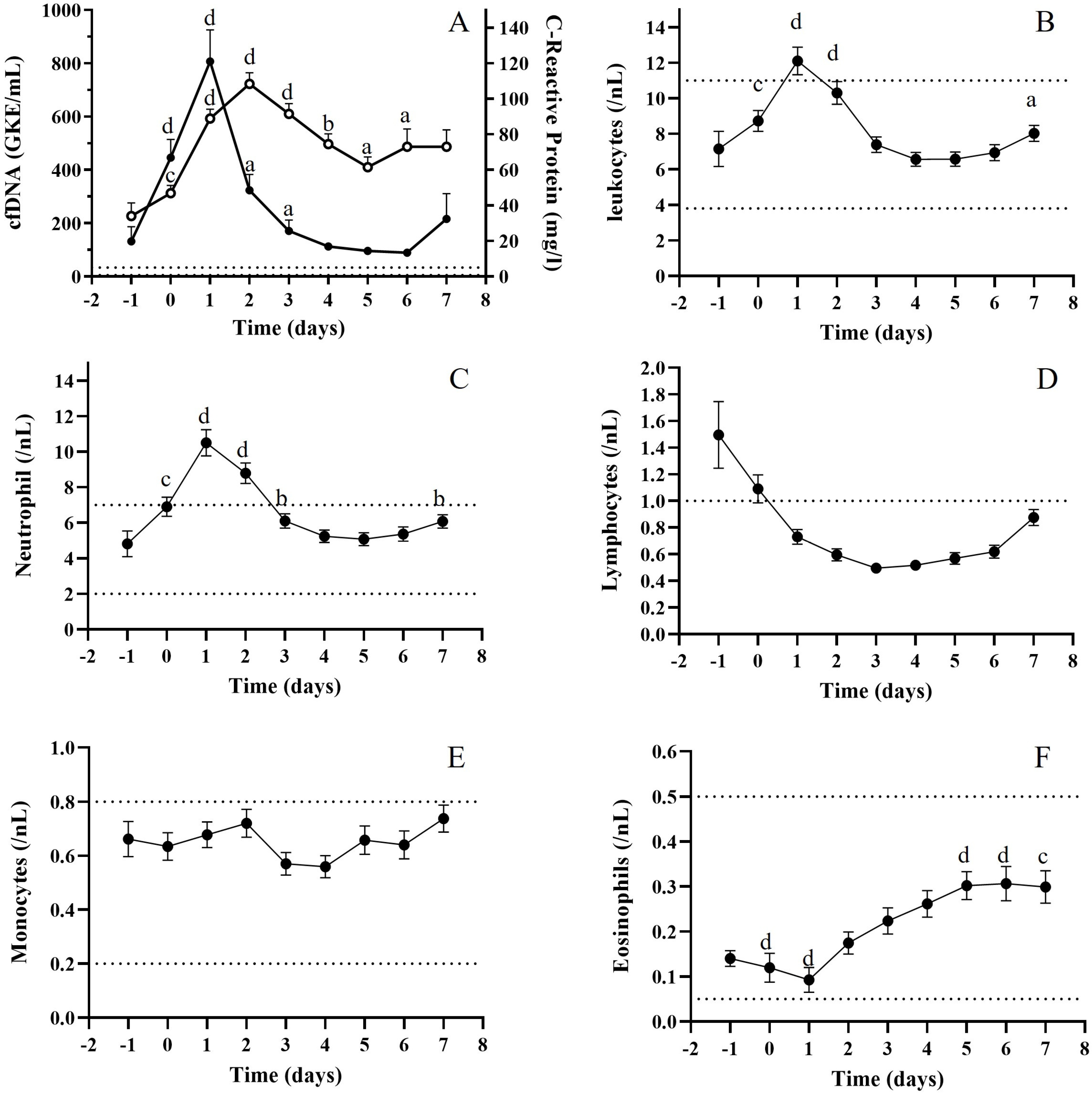
3.3. Relationship Between cfDNA and Biochemical Biomarkers
3.4. Relationship Between cfDNA and Immunological Parameters
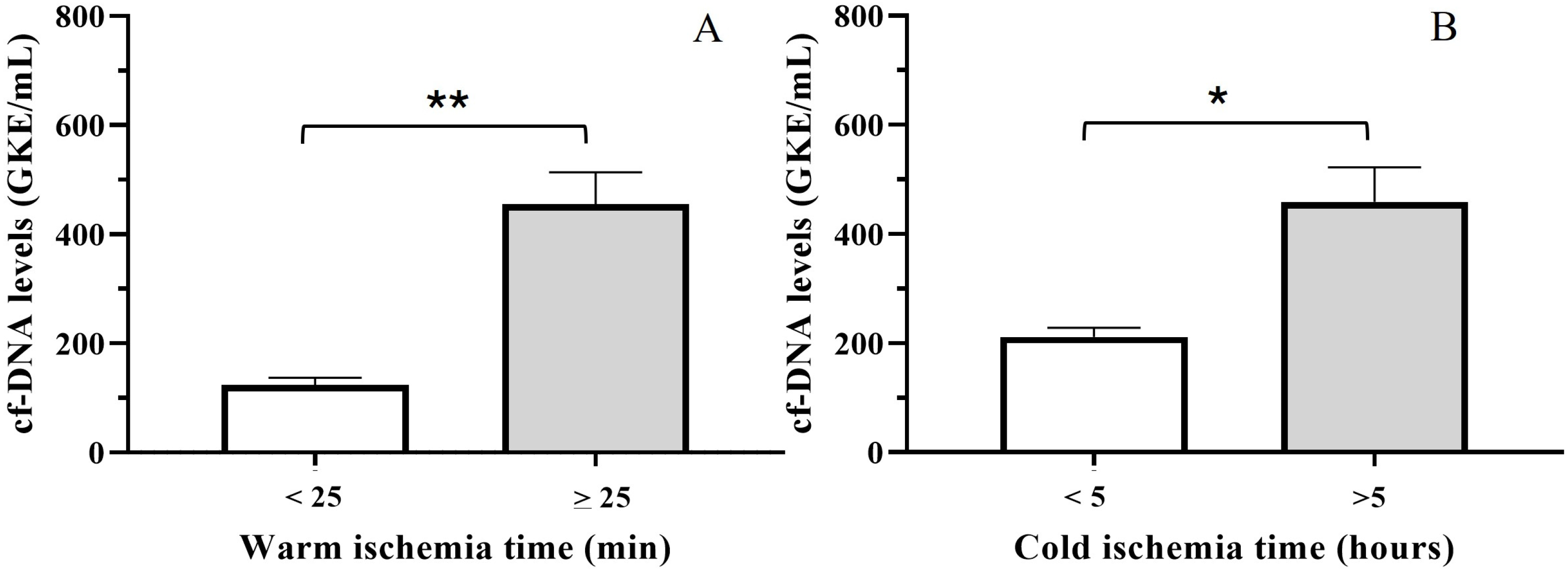
3.5. Relationship Between cfDNA and Ischemia Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serper, M.; Asrani, S.; VanWagner, L.; Reese, P.P.; Kim, M.; Wolf, M.S. Redefining Success After Liver Transplantation: From Mortality Toward Function and Fulfillment. Liver Transpl. 2022, 28, 304–313. [Google Scholar] [CrossRef]
- Buros, C.; Dave, A.A.; Furlan, A. Immediate and Late Complications After Liver Transplantation. Radiol. Clin. N. Am. 2023, 61, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Christenson, R.H.; Beck, J.; Schütz, E.; Sherwood, K.; Price, C.P.; Keown, P.A.; Walson, P.D. Donor-derived cell-free DNA testing in solid organ transplantation: A value proposition. J. Appl. Lab. Med. 2020, 5, 993–1004. [Google Scholar] [CrossRef]
- García-Fernández, N.; Macher, H.C.; Suárez-Artacho, G.; Gómez-Bravo, M.A.; Molinero, P.; Guerrero, J.M.; Porras-López, M.; Rubio, A. Donor-specific cell-free DNA qPCR quantification as a noninvasive accurate biomarker for early rejection detection in liver transplantation. J. Clin. Med. 2022, 12, 36. [Google Scholar] [CrossRef]
- Krenzien, F.; Katou, S.; Papa, A.; Sinn, B.; Benzing, C.; Feldbrügge, L.; Kamali, C.; Brunnbauer, P.; Splith, K.; Lorenz, R.R.; et al. Increased Cell-Free DNA Plasma Concentration Following Liver Transplantation Is Linked to Portal Hepatitis and Inferior Survival. J. Clin. Med. 2020, 9, 1543. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Cannistrà, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Mazzitelli, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Kohli, V.; Selzner, M.; Madden, J.F.; Bentley, R.C.; Clavien, P.A. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 1999, 67, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Drachenberg, C.I.; de la Torre, A.; Bartlett, S.T.; Lim, J.W.; Plotkin, J.S.; Johnsona, L.B. Apoptosis and hepatic allograft reperfusion injury. Clin. Transplant. 1998, 12, 219–223. [Google Scholar] [CrossRef]
- Macher, H.C.; García-Fernández, N.; Adsuar-Gómez, A.; Porras-López, M.; González-Calle, A.; Noval-Padillo, J.; Guerrero, J.M.; Molinero, P.; Borrego-Domínguez, J.M.; Herruzo-Avilés, Á.; et al. Donor-specific circulating cell free DNA as a noninvasive biomarker of graft injury in heart transplantation. Clin. Chim. Acta. 2019, 495, 590–597. [Google Scholar] [CrossRef]
- Silva de Miranda, F.; Garrone Barauna, V.; dos Santos, L.; Costa, G.; Frizera Vassallo, P.; Gastalho Campos, L.C. Properties and Application of Cell-Free DNA as a Clinical Biomarker. Int. J. Mol. Sci. 2021, 22, 9110. [Google Scholar] [CrossRef]
- Schütz, E.; Fischer, A.; Beck, J.; Harden, M.; Koch, M.; Wuensch, T.; Stockmann, M.; Nashan, B.; Kollmar, O.; Matthaei, J.; et al. Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study. PLoS Med. 2017, 14, e1002286. [Google Scholar] [CrossRef]
- Macher, H.C.; Suarez-Artacho, G.; Guerrero, J.M.; Gómez-Bravo, M.A.; Álvarez-Gómez, S.; Bernal-Bellido, C.; Dominguez-Pascual, I.; Rubio, A. Monitoring of transplanted liver health by quantification of organ-specific genomic marker in circulating DNA from receptor. PLoS ONE 2014, 9, e113987. [Google Scholar] [CrossRef] [PubMed]
- Blasi, A.; Patel, V.C.; Adelmeijer, J.; Azarian, S.; Aziz, F.; Fernández, J.; Bernal, W.; Lisman, T. Plasma levels of circulating DNA are associated with outcome, but not with activation of coagulation in decompensated cirrhosis and ACLF. JHEP Rep. 2019, 1, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Aggarwal, S.; Bhardwaj, S.; Ramakrishna, G.; Pandey, C.K. Serial perioperative cell-free DNA levels in donors and recipients undergoing living donor liver transplantation. Acta Anaesthesiol. Scand. 2017, 61, 1084–1094. [Google Scholar] [CrossRef]
- Atamaniuk, J.; Kopecky, C.; Skoupy, S.; Säemann, M.D.; Weichhart, T. Apoptotic cell-free DNA promotes inflammation in haemodialysis patients. Nephrol. Dial. Transplant. 2012, 27, 902–905. [Google Scholar] [CrossRef]
- Dholakia, S.; De Vlaminck, I.; Khush, K.K. Adding Insult on Injury: Immunogenic Role for Donor-derived Cell-free DNA? Transplantation 2020, 104, 2266–2271. [Google Scholar] [CrossRef]
- Han, D.S.C.; Lo, Y.M.D. The Nexus of cfDNA and Nuclease Biology. Trends Genet. 2021, 37, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Marsman, G.; Zeerleder, S.; Luken, B.M. Extracellular histones; cell-free DNA; or nucleosomes: Differences in immunostimulation. Cell Death Dis. 2016, 7, e2518. [Google Scholar] [CrossRef]
- Ye, L.; He, S.; Mao, X.; Zhang, Y.; Cai, Y.; Li, S. Effect of Hepatic Macrophage Polarization and Apoptosis on Liver Ischemia and Reperfusion Injury During Liver Transplantation. Front. Immunol. 2020, 11, 1193. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.Y.; Cheng, Y.L.; Cheng, P.W.; Li, C.-Y.; Li, C.J. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Wang, Y.; He, W.; Li, M.; Hu, X.; Ye, Q. Research advances in liver ischemia reperfusion injury. Chin. J. Hepatobiliary Surg. 2016, 22, 349–351. [Google Scholar]
- Von Meijenfeldt, F.A.; Burlage, L.C.; Bos, S.; Adelmeijer, J.; Porte, R.J.; Lisman, T. Elevated Plasma Levels of Cell-Free DNA During Liver Transplantation Are Associated With Activation of Coagulation. Liver Transplant. 2018, 24, 1716–1725. [Google Scholar] [CrossRef] [PubMed]




| Total (n = 115) | Stable (n = 84) | Hepatic Damage (n = 31) | p | ||
|---|---|---|---|---|---|
| Age (years) | 56 [18–71] | 57 [22–71] | 51 [18–68] | 0.01 | |
| Sex (male) | 62 (53.4%) | 45 (52.9%) | 17 (54.8%) | 0.51 | |
| Donnor age | 62 [14–84] | 63 [17–84] | 58 [14–82] | 0.31 | |
| Warm Ischemia Time (min) | 31 [22–60] | 31 [23–70] | 31 [22–60] | 0.85 | |
| Cold Ischemia Time (min) | 385 [250–575] | 385 [270–551] | 377 [250–575] | 0.25 | |
| Previous ICU | 11 (9.5%) | 5 (5.8%) | 6 (19.4%) | 0.04 | |
| Previous ICU time (days) | 3 [1–58] | 3 [2–58] | 2 [1–34] | 0.29 | |
| ICU post-TX (days) | 6 [3–55] | 6 [3–55] | 7 [3–54] | 0.07 | |
| Hospitalization (days) | 18 [4–120] | 17 [4–120] | 29 [11–109] | 0.0002 | |
| MELD score | 15 [6–37] | 15 [6–37] | 14 [6–30] | 0.72 | |
| Cause of TX | 0.09 | ||||
| AC | 30 (26%) | 25 (29.8%) | 5 (16.1%) | ||
| AC+HCC | 24 (20.7%) | 15 (17.9%) | 9 (29%) | ||
| HCC | 26 (22.6%) | 23 (27.4%) | 3 (9.7%) | ||
| PBC | 5 (4.34%) | 3 (3.6%) | 2 (6.5%) | ||
| FHF | 6 (5.22%) | 4 (4.8%) | 2 (6.5%) | ||
| AMIL | 5 (4.34%) | 4 (4.8%) | 1 (3.2%) | ||
| OTHERS | 19 (13.4%) | 10 (11.9%) | 9 (29%) | ||
| Rejection during first month | 14 (12.1%) | ||||
| Rejection (2 years after TX) | 21 (18.2%) | ||||
| Stable (n = 101) | Rejection (n = 14) | p | |
|---|---|---|---|
| Age (years) | 57 [18–71] | 48 [21–60] | <0.0001 |
| Sex (male) | 54 (53%) | 8 (57.1%) | 0.5 |
| Donnor age (years) | 62 [17–84] | 64 [14–82] | 0.84 |
| Warm Ischemia (min) | 32 [22–60] | 30 [22–36] | 0.24 |
| Cold Ischemia (min) | 385 [270–575] | 380 [250–440] | 0.31 |
| Previous ICU | 8 (7.8%) | 3 (21.4%) | 0.13 |
| Previous ICU (days) | 3 [1–58] | 1 [1–34] | 0.5 |
| ICU post-TX (days) | 6 [3–55] | 6 [4–14] | 0.89 |
| Hospitalization (days) | 17 [4–120] | 31 [11–67] | 0.01 |
| cfDNA | Total | Stable Patients | Patients with Liver Damage | |||
|---|---|---|---|---|---|---|
| CC | p | CC | p | CC | p | |
| ALT | 0.306 ** | <0.0001 | 0.348 ** | <0.0001 | 0.212 ** | <0.005 |
| AST | 0.337 ** | <0.0001 | 0.398 ** | <0.0001 | 0.222 ** | 0.01 |
| Gamma-GT | −0.065 | 0.129 | −0.102 * | <0.05 | −0.007 | 0.93 |
| Bilirubin | 0.009 | 0.8 | 0.004 | 0.938 | −0.113 | 0.153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macher, H.C.; Rubio-Prieto, J.L.; García-Fernández, N.; Molinero, P.; Gómez-Bravo, M.A.; Guerrero, J.M.; Suárez-Artacho, G.; Rubio, A. Cell-Free DNA Levels During the First Hours After Liver Transplantation: A Key Biomarker for Patient Survival and Outcomes. J. Clin. Med. 2025, 14, 8400. https://doi.org/10.3390/jcm14238400
Macher HC, Rubio-Prieto JL, García-Fernández N, Molinero P, Gómez-Bravo MA, Guerrero JM, Suárez-Artacho G, Rubio A. Cell-Free DNA Levels During the First Hours After Liver Transplantation: A Key Biomarker for Patient Survival and Outcomes. Journal of Clinical Medicine. 2025; 14(23):8400. https://doi.org/10.3390/jcm14238400
Chicago/Turabian StyleMacher, Hada C., José L. Rubio-Prieto, Noelia García-Fernández, Patrocinio Molinero, Miguel A. Gómez-Bravo, Juan M. Guerrero, Gonzalo Suárez-Artacho, and Amalia Rubio. 2025. "Cell-Free DNA Levels During the First Hours After Liver Transplantation: A Key Biomarker for Patient Survival and Outcomes" Journal of Clinical Medicine 14, no. 23: 8400. https://doi.org/10.3390/jcm14238400
APA StyleMacher, H. C., Rubio-Prieto, J. L., García-Fernández, N., Molinero, P., Gómez-Bravo, M. A., Guerrero, J. M., Suárez-Artacho, G., & Rubio, A. (2025). Cell-Free DNA Levels During the First Hours After Liver Transplantation: A Key Biomarker for Patient Survival and Outcomes. Journal of Clinical Medicine, 14(23), 8400. https://doi.org/10.3390/jcm14238400





