How to Minimize Hyper-Continence After Intracorporeal Robotic Neobladder Configuration in Women? The Three-Layer Posterior Reconstruction During Florence Robotic IntraCorporeal Neobladder (FloRIN)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Dataset
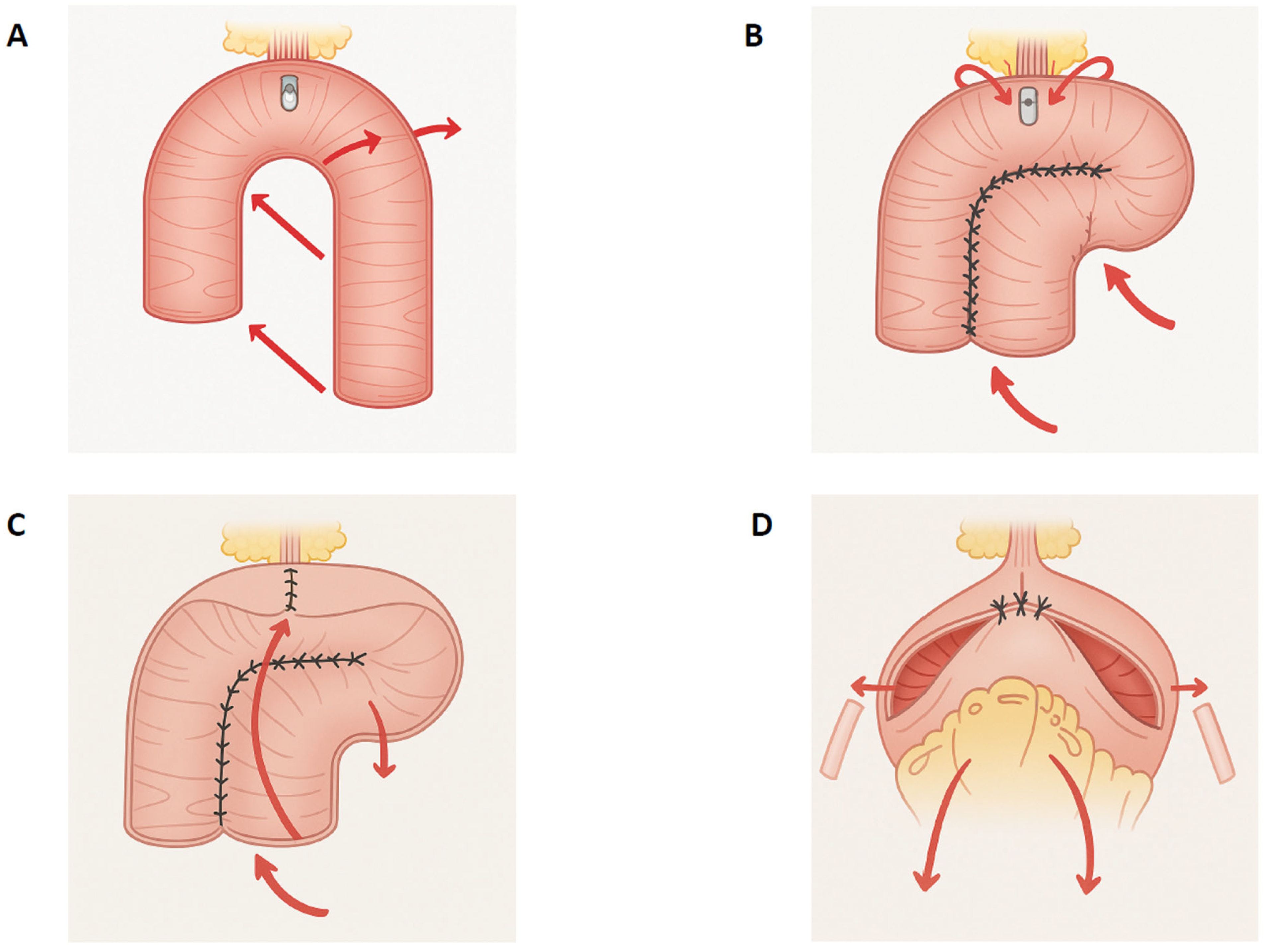
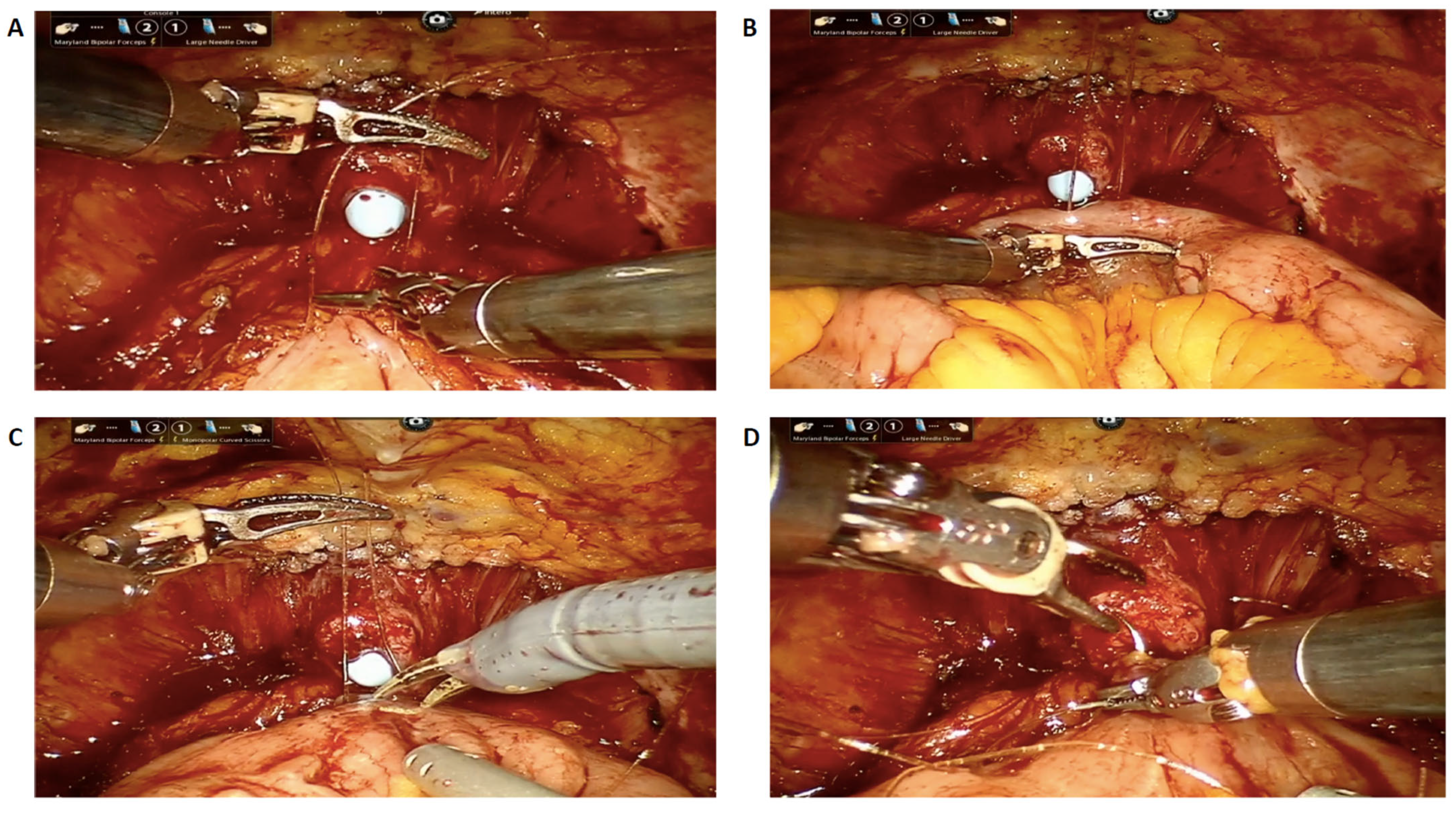
2.2. Surgical Technique
2.3. Urodynamic Study
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Fallara, G.; Di Maida, F.; Bravi, C.A.; De Groote, R.; Piramide, F.; Turri, F.; Andras, I.; Moschovas, M.; Larcher, A.; Breda, A.; et al. A systematic review and meta-analysis of robot-assisted vs. open radical cystectomy: Where do we stand and future perspective. Minerva Urol. Nephrol. 2023, 75, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Piramide, F.; Turri, F.; Amparore, D.; Fallara, G.; De Groote, R.; Knipper, S.; Wuernschimmel, C.; Bravi, C.A.; Lambert, E.; Di Maida, F.; et al. Atlas of Intracorporeal Orthotopic Neobladder Techniques After Robot-assisted Radical Cystectomy and Systematic Review of Clinical Outcomes. Eur. Urol. 2024, 85, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Khetrapal, P.; Conroy, S.; Kelly, J.D.; Catto, J.W.F. Comparing open-radical cystectomy and robot-assisted radical cystectomy: Current status and analysis of the evidence. Curr. Opin. Urol. 2020, 30, 400–406. [Google Scholar] [CrossRef]
- Hussein, A.A.; Elsayed, A.S.; Aldhaam, N.A.; Jing, Z.; Peabody, J.O.; Wijburg, C.J.; Wagner, A.; Canda, A.E.; Khan, M.S.; Scherr, D.; et al. A Comparative Propensity-Score Matched Analysis of Perioperative Outcomes of Intracorporeal versus Extracorporeal Urinary Diversion after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. BJU Int. 2020, 126, 265–272. [Google Scholar] [CrossRef]
- Rich, J.M.; Tillu, N.; Geduldig, J.; Ben-David, R.; Lavallee, E.; Wang, Y.A.; Attalla, K.; Dey, L.; Aron, M.; Ballon, J.; et al. Contemporary outcomes for robotic radical cystectomy and intracorporeal neobladder urinary diversion. Urol. Oncol. 2025, 43, 392.e13–392.e21. [Google Scholar] [CrossRef]
- Rautiola, J.; Martini, A.; Mertens, L.S.; Skokic, V.; Di Gianfrancesco, L.; Bravi, C.A.; Heinzelbecker, J.; Mendrek, M.; Buse, S.; Ploussard, G.; et al. Outcomes after robot-assisted radical cystectomy with orthotopic neobladder in women. World J. Urol. 2024, 42, 617. [Google Scholar] [CrossRef]
- von Deimling, M.; Laukhtina, E.; Pradere, B.; Pallauf, M.; Klemm, J.; Fisch, M.; Shariat, S.F.; Rink, M. Radical cystectomy and urinary diversion in women: Techniques, outcomes, and challenges-a narrative review. Transl. Androl. Urol. 2022, 11, 1598–1610. [Google Scholar] [CrossRef]
- Zahran, M.H.; El-Hefnawy, A.S.; Zidan, E.M.; El-Bilsha, M.A.; Taha, D.-E.; Ali-El-Dein, B. Health-related quality of life after radical cystectomy and neobladder reconstruction in women: Impact of voiding and continence status. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2014, 21, 887–892. [Google Scholar] [CrossRef]
- Nseyo, U.; Ginsberg, D. Functional Outcomes of Orthotopic Neobladder in Women. Curr. Urol. Rep. 2024, 25, 277–285. [Google Scholar] [CrossRef]
- Gakis, G.; Stenzl, A. Considerations for orthotopic diversions in women. Curr. Opin. Urol. 2015, 25, 550–554. [Google Scholar] [CrossRef]
- Laukhtina, E.; von Deimling, M.; Pradere, B.; Yanagisawa, T.; Rajwa, P.; Kawada, T.; Quhal, F.; Pallauf, M.; Bianchi, A.; Majdoub, M.; et al. Urinary function in female patients after traditional, organ-sparing and nerve-sparing radical cystectomy for bladder cancer: A systematic review and pooled analysis. BJU Int. 2024, 133, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Rocco, B.; Luciani, L.G.; Collins, J.; Sanchez-Salas, R.; Adding, C.; Mattevi, D.; Hosseini, A.; Wiklund, P. Posterior reconstruction during robotic-assisted radical cystectomy with intracorporeal orthotopic ileal neobladder: Description and outcomes of a simple step. J. Robot. Surg. 2021, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rocco, B.; Assumma, S.; Calcagnile, T.; Sangalli, M.; Turri, F.; Micali, S.; Gaia, G.; Bozzini, G.; Sighinolfi, M.C. Reproducibility of a modified posterior reconstruction during robotic intracorporeal neobladder reconfiguration. Int. Braz. J. Urol. 2022, 49, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Mineo Bianchi, F.; Romagnoli, D.; D’Agostino, D.; Salvaggio, A.; Giampaoli, M.; Corsi, P.; Bianchi, L.; Borghesi, M.; Schiavina, R.; Brunocilla, E.; et al. Posterior muscle-fascial reconstruction and knotless urethro-neo bladder anastomosis during robot-assisted radical cystectomy: Description of the technique and its impact on urinary continence. Arch. Ital. Di Urol. Androl. Organo Uff. Soc. Ital. Di Ecogr. Urol. E Nefrol. 2019, 91, 5–10. [Google Scholar] [CrossRef]
- Minervini, A.; Di Maida, F.; Tasso, G.; Mari, A.; Bossa, R.; Sforza, S.; Grosso, A.A.; Tellini, R.; Vittori, G.; Siena, G.; et al. Robot assisted radical cystectomy with Florence robotic intracorporeal neobladder (FloRIN): Analysis of survival and functional outcomes after first 100 consecutive patients upon accomplishment of phase 3 IDEAL framework. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 2651–2657. [Google Scholar] [CrossRef]
- Lambertini, L.; Di Maida, F.; Cadenar, A.; Nardoni, S.; Grosso, A.A.; Valastro, F.; Spinelli, P.; Fantechi, R.; Tuccio, A.; Vittori, G.; et al. Stentless florence robotic intracorporeal neobladder (FloRIN), a feasibility prospective randomized clinical trial. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2024, 50, 107259. [Google Scholar] [CrossRef]
- Di Maida, F.; Grosso, A.A.; Tasso, G.; Gemma, L.; Lambertini, L.; Nardoni, S.; Mari, A.; Tuccio, A.; Vittori, G.; Masieri, L.; et al. Robot assisted radical cystectomy with Florence Robotic Intracorporeal Neobladder (FloRIN): Functional and urodynamic features compared with a contemporary series of open Vescica Ileale Padovana (VIP). Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2022, 48, 1854–1861. [Google Scholar] [CrossRef]
- Anceschi, U.; DI Maida, F.; Flammia, R.S.; Bigazzi, B.; Grosso, A.A.; Fede Spicchiale, C.; Mari, A.; Brassetti, A.; Tuderti, G.; Ferriero, M.C.; et al. Robotic intracorporeal Padua ileal neobladder vs. Florin pouch: Comparison of mid-term urodynamic and functional profiles. Minerva Urol. Nephrol. 2022, 74, 825–827. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Wein, A.; Wagg, A. (Eds.) Incontinence, 6th ed.; ICI-ICS, International Continence Society: Bristol, UK, 2017. [Google Scholar]
- Satkunasivam, R.; Santomauro, M.; Chopra, S.; Plotner, E.; Cai, J.; Miranda, G.; Salibian, S.; Aron, M.; Ginsberg, D.; Daneshmand, S.; et al. Robotic Intracorporeal Orthotopic Neobladder: Urodynamic Outcomes, Urinary Function, and Health-related Quality of Life. Eur. Urol. 2016, 69, 247–253. [Google Scholar] [CrossRef]
- Rautiola, J.; Björklund, J.; Ben-David, R.; Skokic, V.; Cacciamani, G.; Desai, M.; Dey, L.; Mehrazin, R.; Miranda, G.; Sfakianos, J.; et al. Pelvic organ-sparing robot-assisted radical cystectomy in women with bladder cancer. BJU Int. 2025, 136, 747–754. [Google Scholar] [CrossRef]
- Pappot, N.; Maibom, S.L.; Vejlgaard, M.; Joensen, U.N. Investigating the Potential to Offer Reproductive Organ Preserving Radical Cystectomy to More Female Bladder Cancer Patients. Clin. Genitourin. Cancer 2025, 23, 102303. [Google Scholar] [CrossRef] [PubMed]
- Yeguez, A.C.; Talwar, R.; Smith, A.L. Optimizing Care for Women Through Gynecologic Organ Considerations During Cystectomy: A Pre-Operative Checklist of Important Considerations. Urology 2025, 197, 194–199. [Google Scholar] [CrossRef]
- Chung, Y.; Lee, S.; Jeong, B.C.; Ku, J.H.; Kwon, T.G.; Kim, T.-H.; Lee, J.Y.; Hong, S.H.; Han, W.K.; Ham, W.S.; et al. Risk factors for urinary retention after robot-assisted radical cystectomy with orthotopic neobladder diversion: A multicenter study. J. Robot. Surg. 2024, 19, 1. [Google Scholar] [CrossRef]
- Zahran, M.H.; Eldemerdash, Y.; Taha, D.-E.; Sheir, K.; Shaaban, A.A.; Ali-El-Dein, B. Chronic urinary retention after radical cystectomy and orthotopic neobladder in women: Risk factors and relation to time. Urol. Oncol. 2017, 35, 671.e11–671.e16. [Google Scholar] [CrossRef]
- Chen, Z.; He, P.; Zhou, X.; Li, P.; Li, Q.; Zheng, J.; Li, X.; Zhou, Z. Preliminary Functional Outcome Following Robotic Intracorporeal Orthotopic Ileal Neobladder Suspension with Round Ligaments in Women with Bladder Cancer. Eur. Urol. 2022, 82, 295–302. [Google Scholar] [CrossRef]
- Pyrgidis, N.; Schulz, G.B.; Ebner, B.; Jokisch, F.; Eismann, L.; Karatas, D.; Fouladgar, S.T.; Hermans, J.; Keller, P.; Stief, C.; et al. Radical Cystectomy with Ileal Orthotopic Neobladder after 70 Years Leads to Worse Health-Related Quality of Life. J. Clin. Med. 2024, 13, 6102. [Google Scholar] [CrossRef]
| Age, median (IQR) | 72 (67–75) | |
| BMI, median (IQR) | 24 (22–27) | |
| Smoking status | Never smokers, n (%) | 7 (21.8) |
| Former smokers, n (%) | 20 (62.6) | |
| Current smokers, n (%) | 5 (15.6) | |
| Neo-adjuvant chemotherapy, n (%) | 23 (71.9) | |
| CCI age-adjusted, median (IQR) | 3 (3–4) | |
| Previous abdominal surgery, n (%) | 18 (56.3) | |
| Hydronephrosis observed at CT scan, n (%) | 0 (0) | |
| cT ≤ 2, n (%) | 28 (87.5) | |
| cT ≥ 3, n (%) | 4 (12.5) | |
| cN0, n (%) | 29 (90.6) | |
| cN+, n (%) | 3 (9.4) | |
| Preoperative urinary incontinence, n (%) | 3 (9.4) | |
| Preoperative Pelvic Organ Prolapse ≥ III, n (%) | 2 (6.3) | |
| Intraoperative complications, n (%) | 0 (0) |
| Intraoperative blood transfusions, n (%) | 0 (0) |
| Conversion to open surgery, n (%) | 0 (0) |
| Console time (minutes), median (IQR) | 331 (308–347) |
| Estimated Blood Loss (mL), median (IQR) | 330 (260–390) |
| Time to canalization (days), median (IQR) | 5 (4–7) |
| Stentless procedure, n (%) | 5 (15.6) |
| Hemoglobin level decrease (g/dL), median (IQR) | 2.7 (1.8–3.5) |
| Δ creatinine between baseline—3rd POD, median (IQR) | 0.5 (0.2–1.2) |
| Δ creatinine between discharge and 3rd month assessment, median (IQR) | 0.12 (0.03–0.41) |
| Δ creatinine between discharge and 6th month assessment, median (IQR) | 0.11 (0.04–0.43) |
| Early major (Clavien Dindo ≥ 3) complications (≤30 days), n (%) | 3 (9.4) |
| Nephrostomy placement | 2 (6.5) |
| Ileo-ileal anastomosis revision | 1 (3.1) |
| Delayed major (Clavien Dindo ≥ 3) complications (>30 days), n (%) | 4 (12.5) |
| Nephrostomy placement | 3 (9.4) |
| Pneumatic dilatation of the uretero-ileal anastomosis | 1 (3.1) |
| Presence of hydronephrosis at 3rd month follow-up assessment, n (%) | 2 (6.3) |
| Readmission rate 30-day, n (%) | 2 (6.3) |
| Median follow-up time, months (IQR) | 36 (30–42) |
| Hyper-continence status, n (%) | 3 (9.4) | |
| Median self-catheterization number, median (IQR) | 3 (2–5) | |
| Urodynamic evaluation | First desire (mL), median (IQR) | 190 (170–240) |
| Qmax mL/s, median (IQR) | 17 (14–25) | |
| Qave mL/s, median (IQR) | 11 (7–18) | |
| Compliance mL/cmH2O, median (IQR) | 22 (18–25) | |
| Abdominal leak points, n (%) | 9 (28.1) | |
| Abdominal leak points minimum volume (mL), median (IQR) | 175 (145–230) | |
| Abdominal leak points minimum pressure (cmH2O), median (IQR) | 47 (38–55) | |
| Phasic neobladder contractions, n (%) | 8 (25) | |
| Maximal urethral closure pressure (cmH2O), median (IQR) | 44 (32–58) | |
| Post Voidal Residual cc, median (IQR) | 40 (30–55) | |
| Variable | Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | p | 95% CI | OR | p | 95% CI | ||||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||
| BMI (kg/m2) | >25 | 1.46 | 0.02 | 1.05 | 1.57 | 1.24 | 0.03 | 1.12 | 1.39 |
| ≤25 | - | - | - | - | - | - | - | - | |
| Age | >70 years | 1.35 | 0.03 | 1.10 | 1.66 | 1.18 | 0.04 | 1.09 | 1.73 |
| 50–69 years | 1.21 | 0.04 | 1.15 | 1.89 | 1.10 | 0.15 | 0.97 | 1.62 | |
| <50 years | - | - | - | - | - | - | - | - | |
| ASA score | >3 | 1.12 | 0.04 | 1.15 | 1.92 | 1.02 | 0.23 | 0.98 | 1.11 |
| ≤3 | - | - | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Maida, F.; Lambertini, L.; Grosso, A.A.; Salamone, V.; Paganelli, D.; Di Stefano, L.; Conte, F.; Lipparini, F.; Salvi, M.; Oriti, R.; et al. How to Minimize Hyper-Continence After Intracorporeal Robotic Neobladder Configuration in Women? The Three-Layer Posterior Reconstruction During Florence Robotic IntraCorporeal Neobladder (FloRIN). J. Clin. Med. 2025, 14, 8397. https://doi.org/10.3390/jcm14238397
Di Maida F, Lambertini L, Grosso AA, Salamone V, Paganelli D, Di Stefano L, Conte F, Lipparini F, Salvi M, Oriti R, et al. How to Minimize Hyper-Continence After Intracorporeal Robotic Neobladder Configuration in Women? The Three-Layer Posterior Reconstruction During Florence Robotic IntraCorporeal Neobladder (FloRIN). Journal of Clinical Medicine. 2025; 14(23):8397. https://doi.org/10.3390/jcm14238397
Chicago/Turabian StyleDi Maida, Fabrizio, Luca Lambertini, Antonio Andrea Grosso, Vincenzo Salamone, Daniele Paganelli, Laura Di Stefano, Francesca Conte, Filippo Lipparini, Matteo Salvi, Rino Oriti, and et al. 2025. "How to Minimize Hyper-Continence After Intracorporeal Robotic Neobladder Configuration in Women? The Three-Layer Posterior Reconstruction During Florence Robotic IntraCorporeal Neobladder (FloRIN)" Journal of Clinical Medicine 14, no. 23: 8397. https://doi.org/10.3390/jcm14238397
APA StyleDi Maida, F., Lambertini, L., Grosso, A. A., Salamone, V., Paganelli, D., Di Stefano, L., Conte, F., Lipparini, F., Salvi, M., Oriti, R., Mari, A., & Minervini, A. (2025). How to Minimize Hyper-Continence After Intracorporeal Robotic Neobladder Configuration in Women? The Three-Layer Posterior Reconstruction During Florence Robotic IntraCorporeal Neobladder (FloRIN). Journal of Clinical Medicine, 14(23), 8397. https://doi.org/10.3390/jcm14238397







