Risk of Hypophosphatemia Following Postpartum Anemia Treatment with IV Ferric Carboxymaltose, IV Ferric Derisomaltose, and Oral Ferrous Sulfate
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization and Blinding
2.4. Study Outcomes
- Serum phosphate level at six weeks postpartum.
- The proportion of participants with severe hypophosphatemia, defined as <0.34 mmol/L (equivalent to <1.05 mg/dL), at six weeks follow-up.
- The change in serum phosphate level from study inclusion to six weeks postpartum.
2.5. Sample Size
2.6. Statistical Analysis
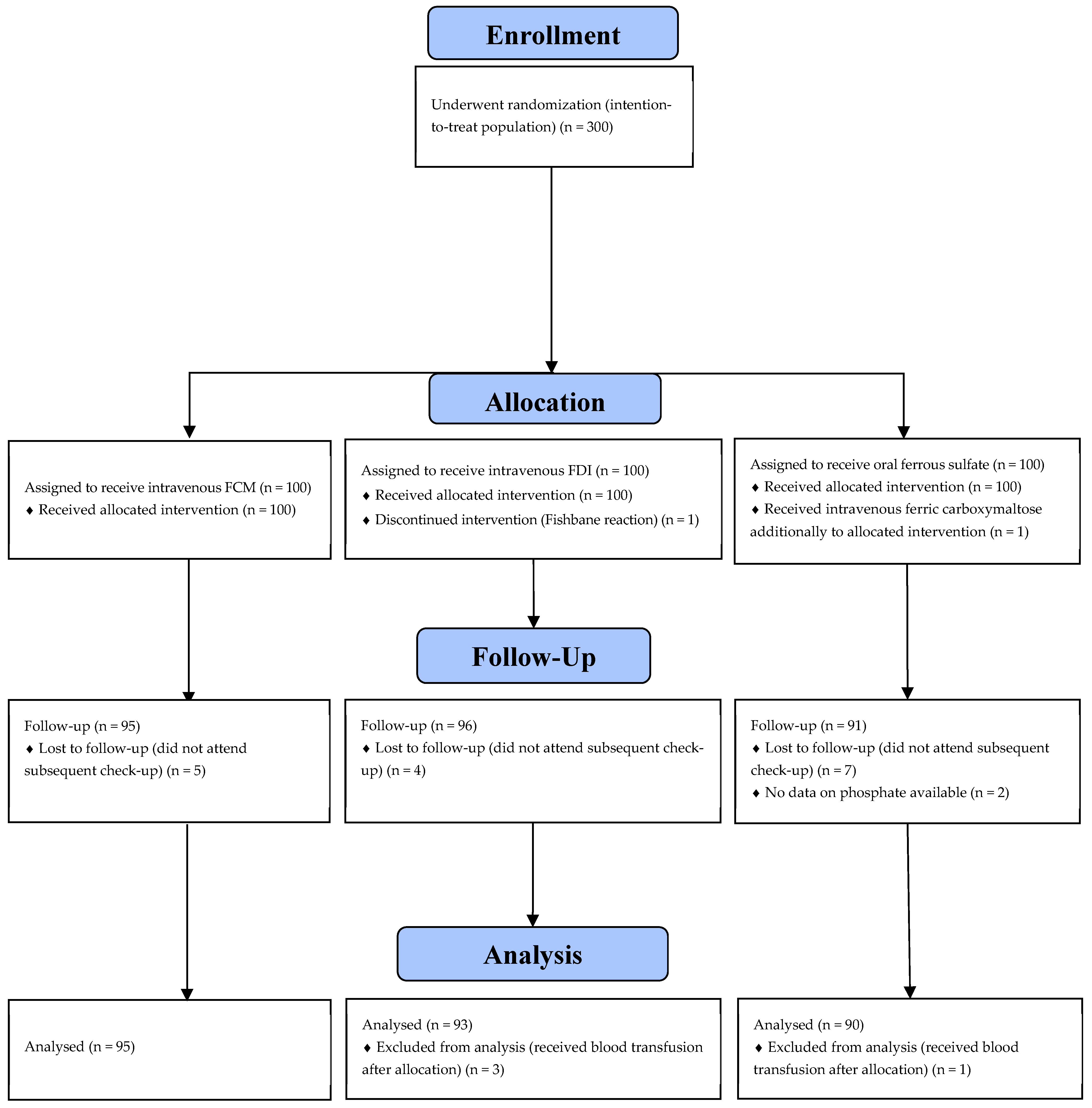
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IDA | iron deficiency anemia |
| IV | intravenous |
| FCM | ferric carboxymaltose |
| FDI | ferric derisomaltose |
References
- Milman, N. Postpartum anemia I: Definition, prevalence, causes, and consequences. Ann. Hematol. 2011, 90, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Cogswell, M.E.; McDonald, T. Have we forgotten the significance of postpartum iron deficiency? Am. J. Obstet. Gynecol. 2005, 193, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Mintsopoulos, V.; Tannenbaum, E.; Malinowski, A.K.; Shehata, N.; Walker, M. Identification and treatment of iron-deficiency anemia in pregnancy and postpartum: A systematic review and quality appraisal of guidelines using AGREE II. Int. J. Gynaecol. Obstet. 2024, 164, 460–475. [Google Scholar] [CrossRef]
- Zoller, H.; Wagner, S.; Schaefer, B. What is wrong in doing good? Br. J. Haematol. 2023, 202, 1089–1090. [Google Scholar] [CrossRef]
- Milman, N. Postpartum anemia II: Prevention and treatment. Ann. Hematol. 2012, 91, 143–154. [Google Scholar] [CrossRef]
- Ruiz de Viñaspre-Hernández, R.; Gea-Caballero, V.; Juárez-Vela, R.; Iruzubieta-Barragán, F.J. The definition, screening, and treatment of postpartum anemia: A systematic review of guidelines. Birth 2021, 48, 14–25. [Google Scholar] [CrossRef]
- Bhandari, S.; Pereira, D.I.A.; Chappell, H.F.; Drakesmith, H. Intravenous Irons: From Basic Science to Clinical Practice. Pharmaceuticals 2018, 11, 82. [Google Scholar] [CrossRef]
- Schaefer, B.; Meindl, E.; Wagner, S.; Tilg, H.; Zoller, H. Intravenous iron supplementation therapy. Mol. Asp. Med. 2020, 75, 100862. [Google Scholar] [CrossRef]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853.e2. [Google Scholar] [CrossRef]
- Auerbach, M.; Macdougall, I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodial. Int. 2017, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Breymann, C.; Gliga, F.; Bejenariu, C.; Strizhova, N. Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. Int. J. Gynaecol. Obstet. 2008, 101, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Seid, M.H.; Butcher, A.D.; Chatwani, A. Ferric Carboxymaltose as Treatment in Women with Iron-Deficiency Anemia. Anemia 2017, 2017, 9642027. [Google Scholar] [CrossRef] [PubMed]
- Seid, M.H.; Derman, R.J.; Baker, J.B.; Banach, W.; Goldberg, C.; Rogers, R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: A randomized controlled clinical trial. Am. J. Obstet. Gynecol. 2008, 199, 435.e1–435.e7. [Google Scholar] [CrossRef]
- Moore, R.A.; Gaskell, H.; Rose, P.; Allan, J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011, 11, 4. [Google Scholar] [CrossRef]
- Van Wyck, D.B.; Martens, M.G.; Seid, M.H.; Baker, J.B.; Mangione, A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: A randomized controlled trial. Obstet. Gynecol. 2007, 110, 267–278. [Google Scholar] [CrossRef]
- Becuzzi, N.; Zimmermann, R.; Krafft, A. Long-term efficacy of postpartum intravenous iron therapy. Biomed Res. Int. 2014, 2014, 815437. [Google Scholar] [CrossRef]
- Boots, J.M.M.; Quax, R.A.M. High-Dose Intravenous Iron with Either Ferric Carboxymaltose or Ferric Derisomaltose: A Benefit-Risk Assessment. Drug Saf. 2022, 45, 1019–1036. [Google Scholar] [CrossRef]
- Schaefer, B.; Tobiasch, M.; Wagner, S.; Glodny, B.; Tilg, H.; Wolf, M.; Zoller, H. Hypophosphatemia after intravenous iron therapy: Comprehensive review of clinical findings and recommendations for management. Bone 2022, 154, 116202. [Google Scholar] [CrossRef]
- Vilaca, T.; Velmurugan, N.; Smith, C.; Abrahamsen, B.; Eastell, R. Osteomalacia as a Complication of Intravenous Iron Infusion: A Systematic Review of Case Reports. J. Bone Miner. Res. 2022, 37, 1188–1199. [Google Scholar] [CrossRef]
- Fukumoto, S. FGF23-related hypophosphatemic rickets/osteomalacia: Diagnosis and new treatment. J. Mol. Endocrinol. 2021, 66, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Chertow, G.M.; Macdougall, I.C.; Kaper, R.; Krop, J.; Strauss, W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 2018, 3, e124486. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Rubin, J.; Achebe, M.; Econs, M.J.; Peacock, M.; Imel, E.A.; Thomsen, L.L.; Carpenter, T.O.; Weber, T.; Brandenburg, V.; et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA 2020, 323, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Koch, T.A.; Bregman, D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013, 28, 1793–1803. [Google Scholar] [CrossRef]
- Ganzoni, A.M. Intravenous iron-dextran: Therapeutic and experimental possibilities. Schweiz. Med. Wochenschr. 1970, 100, 301–303. (In German) [Google Scholar]
- Zoller, H.; Schaefer, B.; Glodny, B. Iron-induced hypophosphatemia: An emerging complication. Curr. Opin. Nephrol. Hypertens. 2017, 26, 266–275. [Google Scholar] [CrossRef]
- Chu, Z.; Cushway, T.; Wong, M.; Lim, K.X.; Peh, W.M.; Ng, C.T.; Lim, W.Y.; Ong, S.G.K.; Tey, T.T.; Foo, F.J.; et al. Incidence and predictors of hypophosphataemia after ferric carboxymaltose use-A 3-year experience from a single institution in Singapore. Br. J. Haematol. 2023, 202, 1199–1204. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Mwangi, M.N.; Moya, E.; Ataide, R.; Mzembe, G.; Harding, R.; Zinenani, T.; Larson, L.M.; Demir, A.Y.; Nkhono, W.; et al. Ferric carboxymaltose versus standard-of-care oral iron to treat second-trimester anaemia in Malawian pregnant women: A randomised controlled trial. Lancet 2023, 401, 1595–1609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasricha, S.R.; Moya, E.; Ataíde, R.; Mzembe, G.; Harding, R.; Mwangi, M.N.; Zinenani, T.; Prang, K.H.; Kaunda, J.; Mtambo, O.P.L.; et al. Ferric carboxymaltose for anemia in late pregnancy: A randomized controlled trial. Nat. Med. 2025, 31, 197–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Detlie, T.E.; Lindstrøm, J.C.; Jahnsen, M.E.; Finnes, E.; Zoller, H.; Moum, B.; Jahnsen, J. Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment. Pharmacol. Ther. 2019, 50, 397–406. [Google Scholar] [CrossRef]
- Prats, M.; Font, R.; García, C.; Cabré, C.; Jariod, M.; Vea, A.M. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: Post-hoc analysis of a prospective study. BMC Nephrol. 2013, 14, 167. [Google Scholar] [CrossRef]
- Kovacs, C.S. Calcium and bone metabolism during pregnancy and lactation. J. Mammary Gland Biol. Neoplasia 2005, 10, 105–118. [Google Scholar] [CrossRef]
- Glaspy, J.A.; Lim-Watson, M.Z.; Libre, M.A.; Karkare, S.S.; Hadker, N.; Bajic-Lucas, A.; Strauss, W.E.; Dahl, N.V. Hypophosphatemia Associated with Intravenous Iron Therapies for Iron Deficiency Anemia: A Systematic Literature Review. Ther. Clin. Risk Manag. 2020, 16, 245–259. [Google Scholar] [CrossRef]
| Statistics/Category | Treatment Group | p Value | ||
|---|---|---|---|---|
| IV FCM (n = 95) | IV FDI (n = 93) | Oral Ferrous Sulfate (n = 90) | ||
| Maternal age, years | 31 (27–35) | 30 (27–34) | 31 (27–36) | 0.40 |
| Prepregnancy BMI, kg/m2 | 24 (21–27) | 24 (22–27) | 23 (21–27) | 0.63 |
| BMI at delivery, kg/m2 | 28 (26–32) | 30 (27–32) | 29 (26–32) | 0.54 |
| Multiple gestation | 6 (6) | 6 (6) | 3 (3) | 0.57 |
| Nulliparity | 64 (67) | 67 (72) | 50 (56) | 0.06 |
| Gestational age at birth, completed weeks | 39 (38–40) | 39 (38–40) | 39 (38–40) | 0.66 |
| Transfusion of RBC before intervention | 5 (5) | 6 (6) | 7 (8) | 0.79 |
| Cesarean section | 28 (29) | 22 (24) | 30 (33) | 0.35 |
| Calculated iron deficit 1, g | 1422 (1280–1560) | 1437 (1345–1582) | / | 0.26 |
| Hemoglobin, g/L | 92 (87–97) | 91 (87–95) | 95 (91–98) | 0.002 |
| Ferritin, µ/L | 28 (13–48) | 21 (12–34) | 22 (12–40) | 0.17 |
| Transferrin saturation, % | 9 (7–14) | 10 (7–15) | 10 (7–16) | 0.35 |
| Plasma iron µmol/L | 7 (4–10) | 8 (5–12) | 7 (5–11) | 0.11 |
| TIBC, µmol/L | 71 (64–78) | 73 (67–80) | 71 (65–79) | 0.31 |
| CRP, mg/L | 57 (35–95) | 53 (33–78) | 46 (33–71) | 0.30 |
| Baseline phosphate level, mmol/L or mg/dL | 1.15 (1.01–1.33) 3.57 (3.13–4.12) | 1.16 (1.04–1.30) 3.60 (3.22–4.03) | 1.22 (1.08–1.35) 3.78 (3.35–4.19) | 0.13 |
| Hypophosphatemia at inclusion (<0.83 mmol/L) (equivalent to <2.57 mg/dL) | 2 (2) | 3 (3) | 3 (4) | 0.67 |
| Severe hypophosphatemia at inclusion (<0.34 mmol/L) (equivalent to <1.05 mg/dL) | 0 (0) | 0 (0) | 0 (0) | / |
| Statistics/Category | Treatment Group | p Value | ||
|---|---|---|---|---|
| IV FCM (n = 95) | IV FDI (n = 93) | Oral Ferrous Sulfate (n = 90) | ||
| Hypophosphatemia (<0.83 mmol/L) (equivalent to 2.57 mg/dL) six weeks postpartum, % | 3 (3) | 2 (2) | 2 (2) | 0.89 |
| Phosphate level six weeks postpartum, mmol/L or mg/dL | 1.24 (1.10–1.35) 3.84 (3.41–4.19) | 1.23 (1.09–1.35) 3.81 (3.38–4.19) | 1.25 (1.15–1.34) 3.88 (3.57–4.15) | 0.86 |
| Severe hypophosphatemia (<0.34 mmol/L) (equivalent to <1.05 mg/dL) six weeks postpartum, % | 0 (0) | 0 (0) | 1 (1) | 0.35 |
| Change in phosphate level from study inclusion to six weeks postpartum, mmol/L or mg/dL | −0.08 (−0.26–0.17) 0.25 (−0.81–0.53) | −0.09 (−0.26–0.08) −0.28 (−0.81–0.25) | −0.01 (−0.18–0.09) −0.03 (−0.56–0.28) | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavčar, L.B.; Lučovnik, M.; Hrobat, H.; Gornik, L.; Preložnik Zupan, I. Risk of Hypophosphatemia Following Postpartum Anemia Treatment with IV Ferric Carboxymaltose, IV Ferric Derisomaltose, and Oral Ferrous Sulfate. J. Clin. Med. 2025, 14, 8393. https://doi.org/10.3390/jcm14238393
Tavčar LB, Lučovnik M, Hrobat H, Gornik L, Preložnik Zupan I. Risk of Hypophosphatemia Following Postpartum Anemia Treatment with IV Ferric Carboxymaltose, IV Ferric Derisomaltose, and Oral Ferrous Sulfate. Journal of Clinical Medicine. 2025; 14(23):8393. https://doi.org/10.3390/jcm14238393
Chicago/Turabian StyleTavčar, Lea Bombač, Miha Lučovnik, Hana Hrobat, Lea Gornik, and Irena Preložnik Zupan. 2025. "Risk of Hypophosphatemia Following Postpartum Anemia Treatment with IV Ferric Carboxymaltose, IV Ferric Derisomaltose, and Oral Ferrous Sulfate" Journal of Clinical Medicine 14, no. 23: 8393. https://doi.org/10.3390/jcm14238393
APA StyleTavčar, L. B., Lučovnik, M., Hrobat, H., Gornik, L., & Preložnik Zupan, I. (2025). Risk of Hypophosphatemia Following Postpartum Anemia Treatment with IV Ferric Carboxymaltose, IV Ferric Derisomaltose, and Oral Ferrous Sulfate. Journal of Clinical Medicine, 14(23), 8393. https://doi.org/10.3390/jcm14238393






