Secure Ileal Pouch–Anal Anastomosis for Histologic Indeterminate Colitis
Abstract
1. Introduction
2. Methods
Ethics
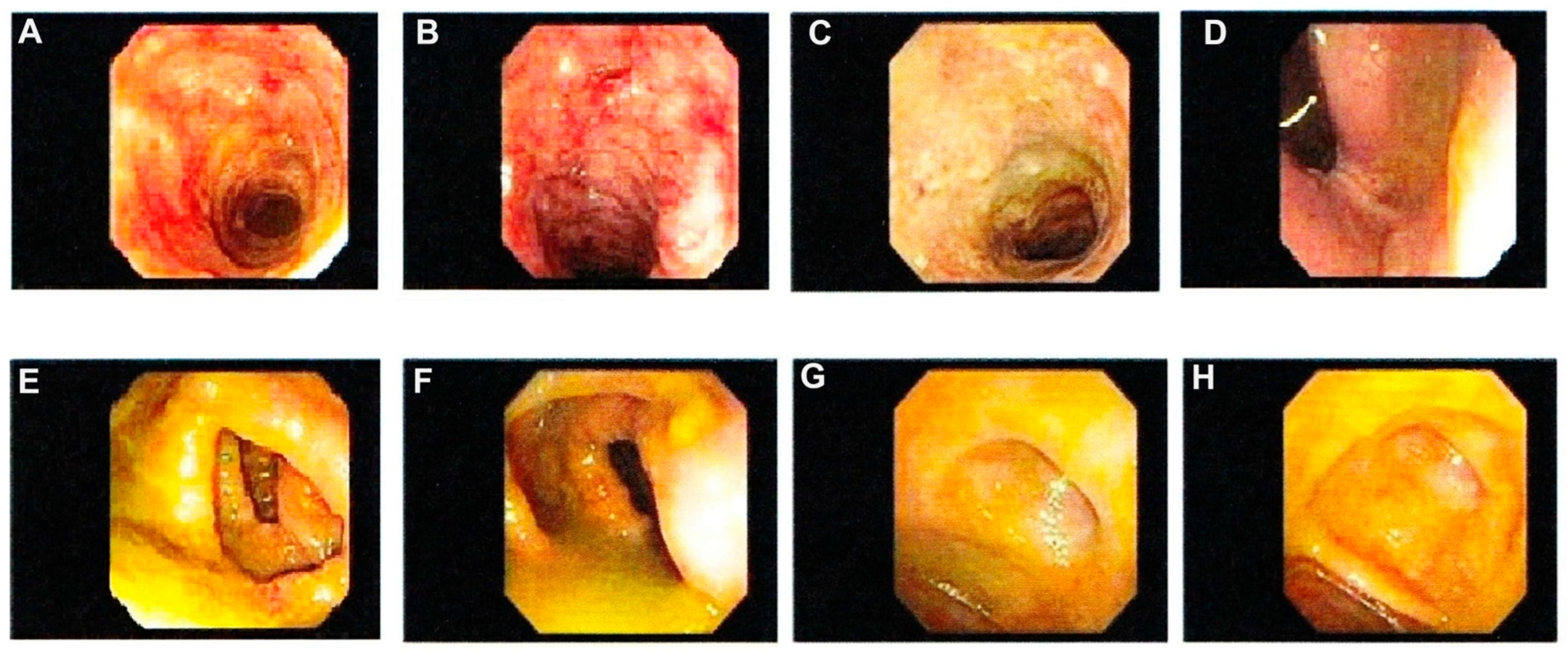
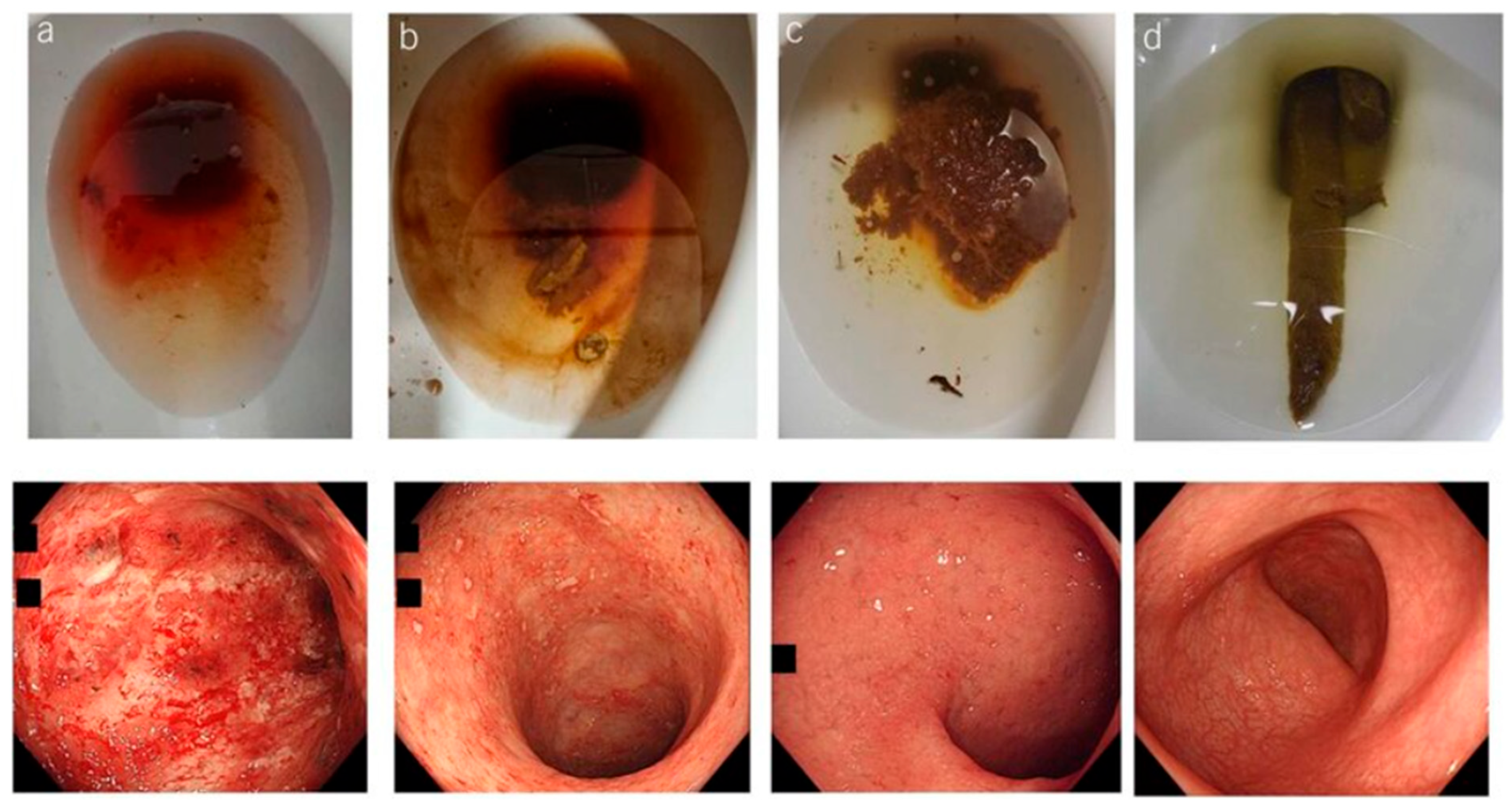
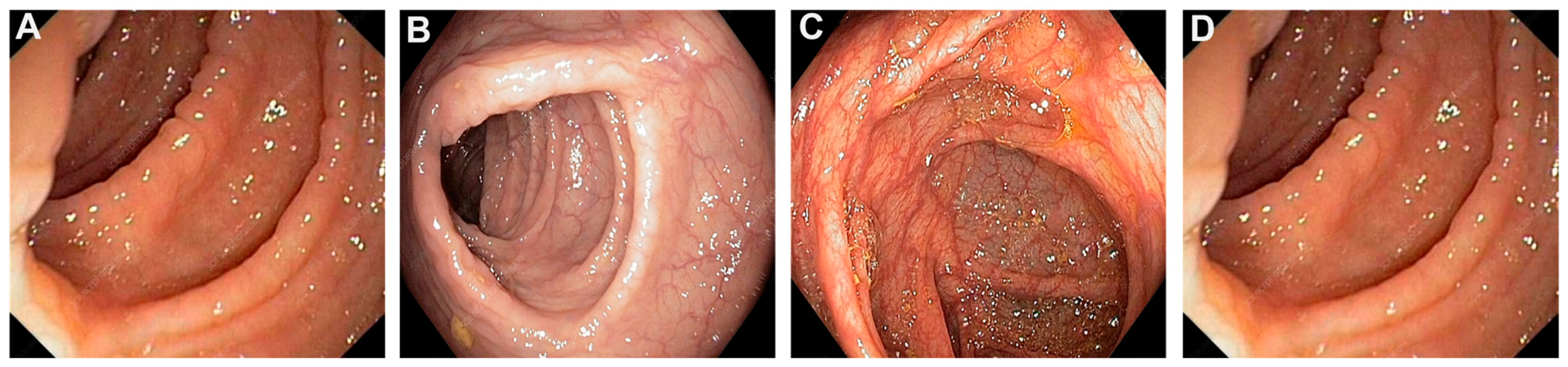
3. Indeterminate Colitis
4. Criteria for Making a Diagnosis
5. Diagnostic Dilemmas for IBD
6. Reclassification of Indeterminate Colitis to Definitive Ulcerative Colitis or Crohn’s Colitis
6.1. DEFA5 Is a Promising Diagnostic Biomarker
6.2. Advances in Assay Development for IBD Diagnostics
6.3. Anti-DEFA5 Monoclonal Antibodies
7. Transcriptome Studies
8. Proteomic Profiling
9. Blood-Based Biomarkers
10. Discussion
11. Conclusions
12. Conference Presentations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.D.; Korolkova, O.Y.; Sakwe, A.M.; Geiger, T.M.; James, S.D.; Muldoon, R.L.; Herline, A.J.; Goodwin, J.S.; Izban, M.G.; Washington, M.K.; et al. Human alpha defensin 5 is a candidate biomarker to delineate inflammatory bowel disease. PLoS ONE 2017, 12, e0179710. [Google Scholar] [CrossRef]
- Tontini, G.E.; Vecchi, M.; Pastorelli, L.; Neurath, M.F.; Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: State of the art and future perspectives. World J. Gastroenterol. 2015, 21, 21–46. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Weismiller, S.; Clarke, K. Indeterminate Colitis—Update on Treatment Options. J. Inflamm. Res. 2021, 14, 6383–6395. [Google Scholar] [CrossRef]
- Thurgate, L.E.; Lemberg, D.A.; Day, A.S.; Leach, S.T. An Overview of Inflammatory Bowel Disease Unclassified in Children. Inflamm. Intest. Dis. 2019, 4, 97–103. [Google Scholar] [CrossRef]
- M’koma, A.E. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina 2022, 58, 567. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory Bowel Disease: Clinical Diagnosis and Pharmaceutical Management of Inflammatory Bowel Disease. Med. Res. Arch. 2023, 11, 18103. [Google Scholar] [CrossRef]
- Lightner, A.L.; Mathis, K.L.; Dozois, E.J.; Hahnsloser, D.; Loftus, E.V.; Raffals, L.E.; Pemberton, J.H. Results at Up to 30 Years After Ileal Pouch–Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Pemberton, J.H.; Loftus, E.J. Crohn’s Disease of the Ileoanal Pouch. Inflamm. Bowel Dis. 2016, 22, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Jia, X.; Zaghiyan, K.; Fleshner, P.R. IPAA in Known Preoperative Crohn’s Disease: A Systematic Review. Dis. Colon Rectum 2020, 64, 355–364. [Google Scholar] [CrossRef]
- Lytvynenko, O.O.; Sorokin, B.V.; Shupyk National Healthcare University of Ukraine; Halchak, I.V.; Lishchenko, O.P.; Demianov, V.O. Synchronous Colon Cancer Associated with Idiopathic Thrombocytopenic Purpura. Clinical Case. Probl. Radiat. Med. Radiobiol. 2024, 29, 465–472. [Google Scholar] [CrossRef]
- Ramos, E.J.B.; Marques, H.P.; Palavecino, M.; Pawlik, T.; Adam, R.; Soubrane, O.; Herman, P.; Cotta-Pereira, R.L. Management of Synchronic Large Liver Metastasis in a Non-Occlusive Colon Tumor. Arq. Bras. Cir. Dig. 2024, 37, e1858. [Google Scholar] [CrossRef]
- Smith, J.C.; Schäffer, M.W.; Ballard, B.R.; Smoot, D.T.; Herline, A.J.; Adunyah, S.E.; M’kOma, A.E. Adenocarcinomas after Prophylactic Surgery for Familial Adenomatous Polyposis. J. Cancer Ther. 2013, 4, 260–270. (In English) [Google Scholar] [CrossRef] [PubMed]
- Parks, A.G.; Nicholls, R.J.; Belliveau, P. Proctocolectomy with ileal reservoir and anal anastomosis. Br. J. Surg. 1980, 67, 533–538. [Google Scholar] [CrossRef]
- Parks, A.G.; Nicholls, R.J. Proctocolectomy without ileostomy for ulcerative colitis. Br. Med. J. 1978, 2, 85–88. (In English) [Google Scholar] [CrossRef]
- Rothenberger, D.A.; Vermeulen, F.D.; Christenson, C.E.; Balcos, E.G.; Nemer, F.D.; Goldberg, S.M.; Belliveau, P.; Nivatvongs, S.; Schottler, J.L.; Fang, D.T.; et al. Restorative proctocolectomy with ileal reservoir and ileoanal anas-tomosis. Am. J. Surg. 1983, 145, 82–88. [Google Scholar] [CrossRef]
- Keighley, M.R.; Grobler, S.; Bain, I. An audit of restorative proctocolectomy. Gut 1993, 34, 680–684. [Google Scholar] [CrossRef]
- Keighley, M.R. The final diagnosis in pouch patients for presumed ulcerative colitis may change to Crohn’s disease: Patients should be warned of the consequences. Acta Chir. Iugosl. 2000, 47, 27–31. [Google Scholar] [PubMed]
- Peyrègne, V.; Francois, Y.; Gilly, F.-N.; Descos, J.-L.; Flourie, B.; Vignal, J. Outcome of ileal pouch after secondary diagnosis of Crohn’s disease. Int. J. Color. Dis. 2000, 15, 49–53. [Google Scholar] [CrossRef]
- M’koma, A.E.; Wise, P.E.; Muldoon, R.L.; Schwartz, D.A.; Washington, M.K.; Herline, A.J. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int. J. Color. Dis. 2007, 22, 1143–1163. [Google Scholar] [CrossRef]
- Cohen, Z.; McLeod, R.S.; Stephen, W.; Stern, H.S.; O’connor, B.; Reznick, R. Continuing Evolution of the Pelvic Pouch Procedure. Ann. Surg. 1992, 216, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Ziv, Y.; Fazio, V.W.; Church, J.M.; Lavery, I.C.; King, T.M.; Ambrosetti, P. Stapled ileal pouch anal anastomoses are safer than handsewn anastomoses in patients with ulcerative colitis. Am. J. Surg. 1996, 171, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.S.; Cohen, Z.; Stern, H.S.; McLeod, R.S. Outcome of the pelvic pouch procedure in patients with prior perianal disease. Dis. Colon Rectum 1997, 40, 647–652. [Google Scholar] [CrossRef]
- Brown, C.J.; MacLean, A.R.; Cohen, Z.; MacRae, H.M.; O’Connor, B.I.; McLeod, R.S. Crohn’s Disease and Indeterminate Colitis and the Ileal Pouch-Anal Anastomosis: Outcomes and Patterns of Failure. Dis. Colon Rectum 2005, 48, 1542–1549. (In English) [Google Scholar] [CrossRef]
- Koltun, W.A.; Schoetz, D.J.; Roberts, P.L.; Murray, J.J.; Coller, J.A.; Veidenheimer, M.C. Indeterminate colitis predisposes to perineal complications after ileal pouch-anal anastomosis. Dis. Colon Rectum 1991, 34, 857–860. [Google Scholar] [CrossRef]
- Yu, C.S.; Pemberton, J.H.; Larson, D. Ileal pouch-anal anastomosis in patients with indeterminate colitis: Long-term results. Dis. Colon Rectum 2000, 43, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.P.; Remzi, F.H.; Gramlich, T.; Dadvand, B.; Fazio, V.W. Equivalent Function, Quality of Life and Pouch Survival Rates After Ileal Pouch-Anal Anastomosis for Indeterminate and Ulcerative Colitis. Ann. Surg. 2002, 236, 43–48. [Google Scholar] [CrossRef]
- McLeod, R.S. Is Ileoanal the Proper Operation for Indeterminate Colitis. Inflamm. Bowel Dis. 2002, 8, 368–369. [Google Scholar] [CrossRef]
- Jimmo, B.; Hyman, N.H. Is Ileal pouch-anal anastomosis really the procedure of choice for patients with ulcerative colitis? Dis. Colon Rectum 1998, 41, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Meucci, G. What is the incidence, prevalence, and natural history of indeterminate colitis? Inflamm. Bowel Dis. 2008, 14, S159–S160. [Google Scholar] [CrossRef]
- Burakoff, R. Indeterminate colitis: Clinical spectrum of disease. J. Clin. Gastroenterol. 2004, 38, S41–S43. (In English) [Google Scholar] [CrossRef]
- Guindi, M.; Riddell, R.H. Indeterminate colitis. J. Clin. Pathol. 2004, 57, 1233–1244. [Google Scholar] [CrossRef]
- Geboes, K.; De Hertogh, G. Indeterminate colitis. Inflamm. Bowel Dis. 2003, 9, 324–331. [Google Scholar] [CrossRef]
- Geboes, K.; Van Eyken, P. Inflammatory bowel disease unclassified and indeterminate colitis: The role of the pathologist. J. Clin. Pathol. 2008, 62, 201–205. [Google Scholar] [CrossRef]
- M’Koma, A.E. Diagnosis of inflammatory bowel disease: Potential role of molecular biometrics. World J. Gastrointest. Surg. 2014, 6, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Price, A.B. Overlap in the spectrum of non-specific inflammatory bowel disease—‘Colitis indeterminate’. J. Clin. Pathol. 1978, 31, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Theodossi, A.; Spiegelhalter, D.J.; Jass, J.; Firth, J.; Dixon, M.; Leader, M.; Levison, D.A.; Lindley, R.; Filipe, I.; Price, A. Observer variation and discriminatory value of biopsy features in inflammatory bowel disease. Gut 1994, 35, 961–968. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Porter, C.Q.; Galanko, J.A.; Rifas-Shiman, S.L.; Ollendorf, D.A.; Sandler, R.S.; Finkelstein, J.A. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 62–68. [Google Scholar] [CrossRef]
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Colitis Foundation of America; Bousvaros, A.; Antonioli, D.A.; Colletti, R.B.; Dubinsky, M.C.; Glickman, J.N.; Gold, B.D.; Griffiths, A.M.; Jevon, G.P.; Higuchi, L.M.; et al. Differentiating Ulcerative Colitis from Crohn Disease in Children and Young Adults: Report of a Working Group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Loginov, A.S.; Parfenov, A.I.; Sivash, E.S.; Tsvetkov, V.F.; Zinov’ev, O.I. Crohn’s disease. The problem of early diagnosis. Ter. Arkh. 1992, 64, 82–85. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1440317 (accessed on 20 January 2020).
- Tremaine, W.J. Review article: Indeterminate colitis—Definition, diagnosis and management. Aliment. Pharmacol. Ther. 2006, 25, 13–17. [Google Scholar] [CrossRef]
- Tremaine, W.J. Is Indeterminate Colitis Determinable? Curr. Gastroenterol. Rep. 2012, 14, 162–165. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Rabau, M.Y.; Haboubi, N.Y. Indeterminate colitis. Tech. Coloproctology 2007, 11, 91–96. [Google Scholar] [CrossRef]
- Rudolph, W.G.; Uthoff, S.M.; McAuliffe, T.L.; Goode, E.T.; Petras, R.E.; Galandiuk, S. Indeterminate colitis: The real story. Dis. Colon Rectum 2002, 45, 1528–1534. [Google Scholar] [CrossRef]
- Wagner-Bartak, N.A.; Levine, M.S.; Rubesin, S.E.; Laufer, I.; Rombeau, J.L.; Lichtenstein, G.R. Crohn’s Disease in the Ileal Pouch After Total Colectomy for Ulcerative Colitis: Findings on Pouch Enemas in Six Patients. Am. J. Roentgenol. 2005, 184, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Ballard, B.R.; M’koma, A.E. Gastrointestinal endoscopy biopsy derived proteomic patterns predict indeterminate colitis into ulcerative colitis and Crohn’s colitis. World J. Gastrointest. Endosc. 2015, 7, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef]
- Laass, M.W.; Roggenbuck, D.; Conrad, K. Diagnosis and classification of Crohn’s disease. Autoimmun. Rev. 2014, 13, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Jevon, G.P.; Madhur, R. Endoscopic and histologic findings in pediatric inflammatory bowel disease. Gastroenterol. Hepatol. 2010, 6, 174–180. [Google Scholar]
- Nosti, P.A.; Stahl, T.J.; Sokol, A.I. Surgical repair of rectovaginal fistulas in patients with Crohn’s disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 166–170. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Rogler, G.; Hahnloser, D.; Thomsen, O.Ø. Diagnosis and management of fistulizing Crohn’s disease. Nat. Clin. Pr. Gastroenterol. Hepatol. 2009, 6, 92–106. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Bartolo, D.; Mortensen, N. Restorative Proctocolectomy; Blackwell Scientific Publications: Oxford, UK, 1993; pp. 11–12. [Google Scholar]
- Wells, A.D.; McMillan, I.; Price, A.B.; Ritchie, J.K.; Nicholls, R.J. Natural history of indeterminate colitis. Br. J. Surg. 1991, 78, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Breaux, W.A.; Bragg, M.A.; M’Koma, A.E. Ubiquitous Colonic Ileal Metaplasia Consistent with the Diagnosis of Crohn’s Colitis among Indeterminate Colitis Cohorts. Med. Res. Arch. 2023, 11, 4188. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Pathology of idiopathic inflammatory bowel disease. In Inflammatory Bowel Disease; StatPearls: Treasure Island, FL, USA, 1988; pp. 329–350. [Google Scholar]
- Atkinson, K.G.; Owen, D.A.; Wankling, G. Restorative proctocolectomy and indeterminate colitis. Am. J. Surg. 1994, 167, 516–518. [Google Scholar] [CrossRef]
- Ashton, J.J.; Coelho, T.; Ennis, S.; Vadgama, B.; Batra, A.; Afzal, N.A.; Beattie, R.M. Endoscopic Versus Histological Disease Extent at Presentation of Paediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 246–251. [Google Scholar] [CrossRef]
- Shen, B. Crohn’s disease of the ileal pouch: Reality, diagnosis, and management. Inflamm. Bowel Dis. 2009, 15, 284–294. (In English) [Google Scholar] [CrossRef]
- Feakins, R.M. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology 2013, 64, 317–335. [Google Scholar] [CrossRef]
- Fornaro, R.; Caratto, M.; Barbruni, G.; Fornaro, F.; Salerno, A.; Giovinazzo, D.; Sticchi, C.; Caratto, E. Surgical and medical treatment in patients with acute severe ulcerative colitis. J. Dig. Dis. 2015, 16, 558–567. [Google Scholar] [CrossRef]
- Sugita, A.; Koganei, K.; Tatsumi, K.; Futatsuki, R.; Kuroki, H.; Yamada, K.; Arai, K.; Fukushima, T. Recent advances in medical and surgical treatment of ulcerative colitis. Nihon Geka Gakkai Zasshi 2015, 116, 99–103. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26050509 (accessed on 29 March 2023).
- Sagar, P.M.; Dozois, R.R.; Wolff, B.G. Long-term results of ileal pouch-anal anastomosis in patients with Crohn’s disease. Dis. Colon Rectum 1996, 39, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.; Overstraeten, A.d.B.v.; Melmed, G.Y.; Mustain, W.C.; Scow, J.S.; Otterson, M.F.; Ogilvie, J.W.; Bordeianou, L.; Brar, M.S.; Wells, K.O.; et al. Improvement in Functional Outcomes Following Ileal Pouch-Anal Anastomosis: Results from the United States Ileal Pouch-Anal Anastomosis Study. Dis. Colon Rectum 2025, 68, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Remzi, F.H.; Brzezinski, A.; Lopez, R.; Bennett, A.E.; Lavery, I.C.; Queener, E.; Fazio, V.W. Risk factors for pouch failure in patients with different phenotypes of Crohn’s disease of the pouch. Inflamm. Bowel Dis. 2008, 14, 942–948. [Google Scholar] [CrossRef]
- Shen, B.; Remzi, F.H.; Lavery, I.C.; Lashner, B.A.; Fazio, V.W. A Proposed Classification of Ileal Pouch Disorders and Associated Complications After Restorative Proctocolectomy. Clin. Gastroenterol. Hepatol. 2008, 6, 145–158. [Google Scholar] [CrossRef]
- Handelsman, J.C.; Gottlieb, L.M.; Hamilton, S.R. Crohn’s disease as a contraindication to Kock pouch (continent ileostomy). Dis. Colon Rectum 1993, 36, 840–843. [Google Scholar] [CrossRef]
- Le, Q.; Melmed, G.; Dubinsky, M.; McGovern, D.; Vasiliauskas, E.A.; Murrell, Z.; Ippoliti, A.; Shih, D.; Kaur, M.; Targan, S.; et al. Surgical Outcome of Ileal Pouch—Anal Anastomosis When Used Intentionally for Well-Defined Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 30–36. [Google Scholar] [CrossRef]
- Shen, B.; Patel, S.; Lian, L. Natural history of Crohn’s disease in patients who underwent intentional restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment. Pharmacol. Ther. 2010, 31, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, D.; Gibert-Gerez, J.; Escrig-Sos, J.; Alcalde-Sánchez, M.; Salvador-Sanchis, J.L. Ileal pouch-anal anastomosis for Crohn’s disease. Current status. Cirugia Espanola 2009, 85, 69–75. [Google Scholar] [CrossRef] [PubMed]
- de Oca, J.; Sánchez-Santos, R.; Ragué, J.M.; Biondo, S.; Parés, D.; Osorio, A.; del Rio, C.; Jaurrieta, E. Long-Term Results of Ileal Pouch–Anal Anastomosis in Crohn’s Disease. Inflamm. Bowel Dis. 2003, 9, 171–175. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E.; Seeley, E.H.; Washington, M.K.; Schwartz, D.A.; Muldoon, R.L.; Herline, A.J.; Wise, P.E.; Caprioli, R.M. Proteomic profiling of mucosal and submucosal colonic tissues yields protein signatures that differentiate the inflammatory colitides. Inflamm. Bowel Dis. 2011, 17, 875–883. [Google Scholar] [CrossRef]
- M’Koma, A.E.; Wise, P.E.; Schwartz, D.A.; Washington, M.K.; Muldoon, R.L.; El-Rifai, W.M.; Herline, A.J. Gene Expression of Colonic Submucosa Differs Between the Inflammatory Colitides. In Proceedings of the Annual Congress—The American Society of Colon and Rectal Surgeons, Minneapolis, MN, USA, 15–19 May 2010; p. 17. [Google Scholar]
- M’Koma, A.E.; Seeley, E.H.; Wise, P.E.; Washingtoin, M.K.; Schwartz, D.A.; Caprioli, R.M.; Muldon, R.L.; Herline, A.J. Proteomic Patterns of Colonic Submucosa Discriminates Inflammatory Colitides. In Proceedings of the Annual Congress—The American Society of Colon and Rectal Surgeons, Hollywood, FL, USA, 30 September–3 October 2009; p. 166. [Google Scholar]
- M’Koma, A.E.; Seeley, E.H.; Wise, P.E.; Washington, M.K.; Schwartz, D.A.; Herline, A.J.; Muldoon, R.L.; Caprioli, R.M. M1096 Proteomic Analysis of Colonic Submucosa Differentiates Crohn’s and Ulcerative Colitis. Gastroenterology 2009, 136, A-349. [Google Scholar] [CrossRef]
- M’Koma, A.; Wise, P.E.; Seeley, E.H.; Washington, M.K.; Schwartz, D.A.; Muldoon, R.L.; Herline, A.J.; Caprioli, R.M. Human Alpha Defensins are Differentially Expressed Between the Inflammatory Colitides. Gastroenterology 2010, 138, S-525. [Google Scholar] [CrossRef]
- Seeley, E.H.; Washington, M.K.; Caprioli, R.M.; M’Koma, A.E. Proteomic patterns of colonic mucosal tissues delineate Crohn’s colitis and ulcerative colitis. Proteom. Clin. Appl. 2013, 7, 541–549. [Google Scholar] [CrossRef]
- Korolkova, O.Y.; Myers, J.N.; Pellom, S.T.; Wang, L.; M’Koma, A.E. Characterization of Serum Cytokine Profile in Predominantly Colonic Inflammatory Bowel Disease to Delineate Ulcerative and Crohn’s Colitides. Clin. Med. Insights Gastroenterol. 2015, 8, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Murrah, V.A.; Gilchrist, E.P.; Moyer, M.P. Attenuation of the natural course of herpes simplex virus infection in human oral epithelial cell cultures by smokeless tobacco extracts suggests the possibility of a synergistic mechanism for carcinogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 81, 63–69. (In English) [Google Scholar] [CrossRef]
- Ikeuchi, H.; Nakano, H.; Uchino, M.; Nakamura, M.; Noda, M.; Yanagi, H.; Yamamura, T. Safety of One-Stage Restorative Proctocolectomy for Ulcerative Colitis. Dis. Colon Rectum 2005, 48, 1550–1555. [Google Scholar] [CrossRef]
- Ikeuchi, H. Surgery for ulcerative colitis. Nihon Geka Gakkai Zasshi 2015, 116, 109–113. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26050511 (accessed on 29 March 2023).
- Thangaiyan, R.; Sakwe, A.M.; Hawkins, A.T.; Washington, M.K.; Ballard, B.R.; Izban, M.G.; Chirwa, S.S.; Hildreth, J.E.K.; Shanker, A.; Blum, D.L.; et al. Functional characterization of novel anti-DEFA5 monoclonal antibody clones 1A8 and 4F5 in inflammatory bowel disease colitis tissues. Inflamm. Res. 2025, 74, 30. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Fiocchi, C.; Chakravarti, S. Ulcerative colitis and Crohn’s disease: Distinctive gene expression profiles and novel susceptibility candidate genes. Hum. Mol. Genet. 2001, 10, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Rockett, J.C.; Burczynski, M.E.; Fornace, A.J.; Herrmann, P.C.; Krawetz, S.A.; Dix, D.J. Surrogate tissue analysis: Monitoring toxicant exposure and health status of inaccessible tissues through the analysis of accessible tissues and cells. Toxicol. Appl. Pharmacol. 2004, 194, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Elsenbruch, S.; Koelzer, J.; Rueffer, A.; Michalsen, A.; Dobos, G.J. Noninvasive Markers in the Assessment of Intestinal Inflammation in Inflammatory Bowel Diseases: Performance of Fecal Lactoferrin, Calprotectin, and PMN-Elastase, CRP, and Clinical Indices. Am. J. Gastroenterol. 2008, 103, 162–169. (In English) [Google Scholar] [CrossRef]
- Shinzaki, S.; Iijima, H.; Nakagawa, T.; Egawa, S.; Nakajima, S.; Ishii, S.; Irie, T.; Kakiuchi, Y.; Nishida, T.; Yasumaru, M.; et al. IgG Oligosaccharide Alterations Are a Novel Diagnostic Marker for Disease Activity and the Clinical Course of Inflammatory Bowel Disease. Am. J. Gastroenterol. 2008, 103, 1173–1181. (In English) [Google Scholar] [CrossRef]
- Kader, H.A.; Tchernev, V.T.; Satyaraj, E.; Lejnine, S.; Kotler, G.; Kingsmore, S.F.; Patel, D.D. Protein Microarray Analysis of Disease Activity in Pediatric Inflammatory Bowel Disease Demonstrates Elevated Serum PLGF, IL-7, TGF-beta1, and IL-12p40 Levels in Crohn’s Disease and Ulcerative Colitis Patients in Remission versus Active Disease. Am. J. Gastroenterol. 2005, 100, 414–423. [Google Scholar] [CrossRef]
- Burczynski, M.E.; Peterson, R.L.; Twine, N.C.; Zuberek, K.A.; Brodeur, B.J.; Casciotti, L.; Maganti, V.; Reddy, P.S.; Strahs, A.; Immermann, F.; et al. Molecular Classification of Crohn’s Disease and Ulcerative Colitis Patients Using Transcriptional Profiles in Peripheral Blood Mononuclear Cells. J. Mol. Diagn. 2006, 8, 51–61. [Google Scholar] [CrossRef]
- Anand, V.; Russell, A.S.; Tsuyuki, R.; Fedorak, R. Perinuclear Antineutrophil Cytoplasmic Autoantibodies and Anti-Saccharomyces Cerevisiae Antibodies as Serological Markers Are Not Specific in the Identification of Crohn’s Disease and Ulcerative Colitis. Can. J. Gastroenterol. Hepatol. 2008, 22, 33–36. [Google Scholar] [CrossRef]
- Sandborn, W.J. Serologic markers in inflammatory bowel disease: State of the art. Rev. Gastroenterol. Disord. 2004, 4, 167–174. [Google Scholar] [PubMed]
- Fukushima, K.; Yonezawa, H.; Fiocchi, C. Inflammatory Bowel Disease-Associated Gene Expression in Intestinal Epithelial Cells by Differential cDNA Screening and mRNA Display. Inflamm. Bowel Dis. 2003, 9, 290–301. [Google Scholar] [CrossRef]
- Shkoda, A.; Werner, T.; Daniel, H.; Gunckel, M.; Rogler, G.; Haller, D. Differential Protein Expression Profile in the Intestinal Epithelium from Patients with Inflammatory Bowel Disease. J. Proteome Res. 2007, 6, 1114–1125. [Google Scholar] [CrossRef]
- Felley-Bosco, E.; André, M. Proteomics and chronic inflammatory bowel diseases. Pathol. Res. Pr. 2004, 200, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X. Serologic Markers in Inflammatory Bowel Disease. Clin. Chem. 2006, 52, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Mumolo, M.G.; Ceccarelli, L.; Bellini, M.; Romano, M.R.; Sterpi, C.; Ricchiuti, A.; Marchi, S.; Bottai, M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005, 54, 364–368. [Google Scholar] [CrossRef]
- Lockhart-Mummery, H.E.; Morson, B.C. Crohn’s Disease (Regional Enteritis) of the Large Intestine and its Distinction from Ulcerative Colitis. Gut 1960, 1, 87–105. [Google Scholar] [CrossRef]
- Lockhart-Mummery, H.E.; Morson, B.C. Crohn’s Disease of the Large Intestine. Gut 1964, 5, 493–509. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14244023 (accessed on 20 January 2020). [CrossRef]
- Clamp, S.E.; Myren, J.; Bouchier, I.A.; Watkinson, G.; de Dombal, F.T. Diagnosis of inflammatory bowel disease: An international multicentre scoring system. Br. Med. J. 1982, 284, 91–95. [Google Scholar] [CrossRef]
- Lee, K.; Medline, A.; Shockey, S. Indeterminate colitis in the spectrum of inflammatory bowel-disease. Arch. Pathol. Lab. Med. 1979, 103, 173–176. [Google Scholar] [PubMed]
- Lewin, K.S.J. Granulomatous colitis and atypical ulcerative colitis. Gastyroenterology 1966, 50, 211–223. [Google Scholar] [CrossRef]
- Berre, N.L.H.D.; Kerbaol, M.; Caulet, S.; Bretagne, J.F.; Chaperon, J.; Gosselin, M.; Ramée, M.P. Histological discrimination of idiopathic inflammatory bowel disease from other types of colitis. J. Clin. Pathol. 1995, 48, 749–753. [Google Scholar] [CrossRef]
- Kangas, E.; Matikainen, M.; Mattila, J. Is “indeterminate colitis” Crohn’s disease in the long-term follow-up? Int. Surg. 1994, 79, 120–123. [Google Scholar] [PubMed]
- Hamilton, S.R. The differential diagnosis of idiopathic inflammatory disease by colorectal biopsy. Int. J. Color. Dis. 1987, 2, 113–117. [Google Scholar] [CrossRef]
- Pezim, M.E.; Pemberton, J.H.; Beart, R.W., Jr.; Wolff, B.G.; Dozois, R.R.; Nivatvongs, S.; Devine, R.; Ilstrup, D.M. Outcome of “indeterminant” colitis following ileal pouch-anal anastomosis. Dis. Colon Rectum 1989, 32, 653–658. [Google Scholar] [CrossRef]
- Deutsch, A.A.; McLeod, R.S.; Cullen, J.; Cohen, Z. Results of the pelvic-pouch procedure in patients with Crohn’s disease. Dis. Colon Rectum 1991, 34, 475–477. [Google Scholar] [CrossRef]
- Grobler, S.P.; Hosie, K.B.; Affie, E.; Thompson, H.; Keighley, M.R. Outcome of restorative proctocolectomy when the diagnosis is suggestive of Crohn’s disease. Gut 1993, 34, 1384–1388. [Google Scholar] [CrossRef]
- Mylonakis, E.; Allan, R.N.; Keighley, M.R.B. How does pouch construction for a final diagnosis of Crohn’s disease compare with ileoproctostomy for established Crohn’s proctocolitis? Dis. Colon Rectum 2001, 44, 1137–1142. [Google Scholar] [CrossRef]
- Achkar, J.-P.; Shen, B. Medical management of postoperative complications of inflammatory bowel disease: Pouchitis and crohn’s disease recurrence. Curr. Gastroenterol. Rep. 2001, 3, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Telakis, E.T. Indeterminate colitis—Definition, diagnosis, characteristics and management. Ann. Gastroenterol. 2008, 3, 173–179. [Google Scholar]
- Malaty, H.M.; Ferry, G.D.; Abraham, B.; Mehta, S.; Garnett, E. The natural course of inflammatory bowel disease-indeterminate from childhood to adulthood: Within a 25 year period. Clin. Exp. Gastroenterol. 2013, 6, 115–121. [Google Scholar] [CrossRef]
- Hildebrand, H.; Fredrikzon, B.; Holmquist, L.; Kristiansson, B.; Lindquist, B. Chronic inflammatory bowel disease in children and adolescents in Sweden. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 293–297. [Google Scholar] [CrossRef]
- Hildebrand, H.; Brydolf, M.; Holmquist, L.; Krantz, I.; Kristiansson, B. Incidence and prevalence of inflammatory bowel disease in children in South-Western Sweden. Acta Paediatr. 2008, 83, 640–645. [Google Scholar] [CrossRef]
- Lindberg, E.; Lindquist, B.; Holmquist, L.; Hildebrand, H. Inflammatory bowel disease in children and adolescents in Sweden, 1984–1995. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 259–264. [Google Scholar] [CrossRef]
- Malaty, H.M.; Fan, X.; Opekun, A.R.; Thibodeaux, C.; Ferry, G.D. Rising Incidence of Inflammatory Bowel Disease Among Children: A 12-year Study. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, S.; Judd, R.H.; Hoffmann, R.G.; Heikenen, J.; Telega, G.; Khan, F.; Weisdorf-Schindele, S.; Pablo, W.S.; Perrault, J.; Park, R.; et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in wisconsin: A statewide population-based study. J. Pediatr. 2003, 143, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. (In English) [Google Scholar] [CrossRef]
- Turunen, P.; Kolho, K.L.; Auvinen, A.; Iltanen, S.; Huhtala, H.; Ashorn, M. Incidence of inflammatory bowel disease in Finnish chil-dren, 1987–2003. Inflamm. Bowel Dis. 2006, 12, 677–683. (In English) [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.P.; Mehta, S.; El-Serag, H.B. Natural History of Pediatric-onset Inflammatory Bowel Disease: A systematic review. J. Clin. Gastroenterol. 2012, 46, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Ekbom, A. Indeterminate IBD: The magnitude of the problem. Inflamm. Bowel Dis. 2000, 6, S14–S15. [Google Scholar]
- Wells, P.; Halliwell, M.; Skidmore, R.; Woodcock, J.; Webb, A. Tumour detection by ultrasonic Doppler blood-flow signals. Ultrasonics 1977, 15, 231–232. [Google Scholar] [CrossRef]
- Dayton, M.T.; Larsen, K.R.; Christiansen, D.D. Similar Functional Results and Complications After Ileal Pouch–Anal Anastomosis in Patients with Indeterminate vs Ulcerative Colitis. Arch. Surg. 2002, 137, 690–695. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Abadom, V.; Dilworth, H.P.; Thompson, R.; Oliva-Hemker, M.; Cuffari, C. Indeterminate colitis: A significant subgroup of pediatric IBD. Inflamm. Bowel Dis. 2006, 12, 258–262. [Google Scholar] [CrossRef]
- Meucci, G.; Bortoli, A.; Riccioli, F.A.; Girelli, C.M.; Radaelli, F.; Rivolta, R.; Tatarella, M. Frequency and clinical evolution of indeterminate colitis: A retrospective multi-centre study in northern Italy. GSMII (Gruppo di Studio per le Malattie Infiammatorie Intestinali). Eur. J. Gastroenterol. Hepatol. 1999, 11, 909–913. [Google Scholar] [CrossRef]
- Tatsumi, K.; Sugita, A.; Koganei, K.; Futatsuki, R.; Kuroki, H.; Yamada, K.; Nakao, S.; Sako, M.; Kimura, H.; Arai, K.; et al. Long-term outcomes of ileal pouch-anal canal anastomosis in children with ulcerative colitis. Nihon Shokakibyo Gakkai Zasshi (Jpn. J. Gastro-Enterol.) 2013, 110, 2081–2088. [Google Scholar]
- Pellino, G.; Sciaudone, G.; Candilio, G.; De Fatico, G.S.; Landino, I.; Canonico, S.; Selvaggi, F. Restorative proctocolectomy with ileal pouch-anal anastomosis is safe and effective in selected very elderly patients suffering from ulcerative colitis. Int. J. Surg. 2014, 12, S56–S59. [Google Scholar] [CrossRef]
- Ceriati, E.; De Peppo, F.; Rivosecchi, M. Role of surgery in pediatric ulcerative colitis. Pediatr. Surg. Int. 2013, 29, 1231–1241. [Google Scholar] [CrossRef]
- Bikhchandani, J.; Polites, S.F.; Wagie, A.E.M.; Habermann, E.B.; Cima, R.R. National Trends of 3- Versus 2-Stage Restorative Proctocolectomy for Chronic Ulcerative Colitis. Dis. Colon Rectum 2015, 58, 199–204. [Google Scholar] [CrossRef]
- Pellino, G.; Sciaudone, G.; Miele, E.; Candilio, G.; De Fatico, G.S.; Riegler, G.; Staiano, A.; Canonico, S.; Selvaggi, F. Functional Outcomes and Quality of Life after Restorative Proctocolectomy in Paediatric Patients: A Case-Control Study. Gastroenterol. Res. Pr. 2014, 2014, 340341. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Stocchi, L.; Kiran, R.P.; Shen, B.; Remzi, F.H. Do Clinical Characteristics of de Novo Pouch Crohn’s Disease After Restorative Proctocolectomy Affect Ileal Pouch Retention? Dis. Colon Rectum 2014, 57, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Panis, Y. Is there a place for ileal pouch-anal anastomosis in patients with Crohn’s colitis? Neth. J. Med. 1998, 53, S47–S51. [Google Scholar] [CrossRef]
- Panis, Y.; Poupard, B.; Hautefeuille, P.; Valleur, P.; Nemeth, J.; Lavergne, A. Ileal pouch/anal anastomosis for Crohn’s disease. Lancet 1996, 347, 854–857. [Google Scholar] [CrossRef]
- Koktysz, R.; Kozłowski, W.; Trawiński, J.; Wojtuń, S.; Gil, J. Histoclinic of “indeterminate colitis”. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2007, 22, 446–448. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’Koma, A.E. Secure Ileal Pouch–Anal Anastomosis for Histologic Indeterminate Colitis. J. Clin. Med. 2025, 14, 8390. https://doi.org/10.3390/jcm14238390
M’Koma AE. Secure Ileal Pouch–Anal Anastomosis for Histologic Indeterminate Colitis. Journal of Clinical Medicine. 2025; 14(23):8390. https://doi.org/10.3390/jcm14238390
Chicago/Turabian StyleM’Koma, Amosy E. 2025. "Secure Ileal Pouch–Anal Anastomosis for Histologic Indeterminate Colitis" Journal of Clinical Medicine 14, no. 23: 8390. https://doi.org/10.3390/jcm14238390
APA StyleM’Koma, A. E. (2025). Secure Ileal Pouch–Anal Anastomosis for Histologic Indeterminate Colitis. Journal of Clinical Medicine, 14(23), 8390. https://doi.org/10.3390/jcm14238390





