Efficacy of Virtual Reality Interventions for Motor Function Improvement in Cerebral Palsy Patients: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Reporting Guidelines
2.2. Search Strategy and Information Sources
2.3. Eligibility Criteria
2.4. Study Selection and Data Collection
2.5. Risk of Bias Assessment
2.6. Statistical Analysis and Evidence Synthesis
3. Results
3.1. Study Selection and Characteristics
3.2. Primary and Secondary Outcomes
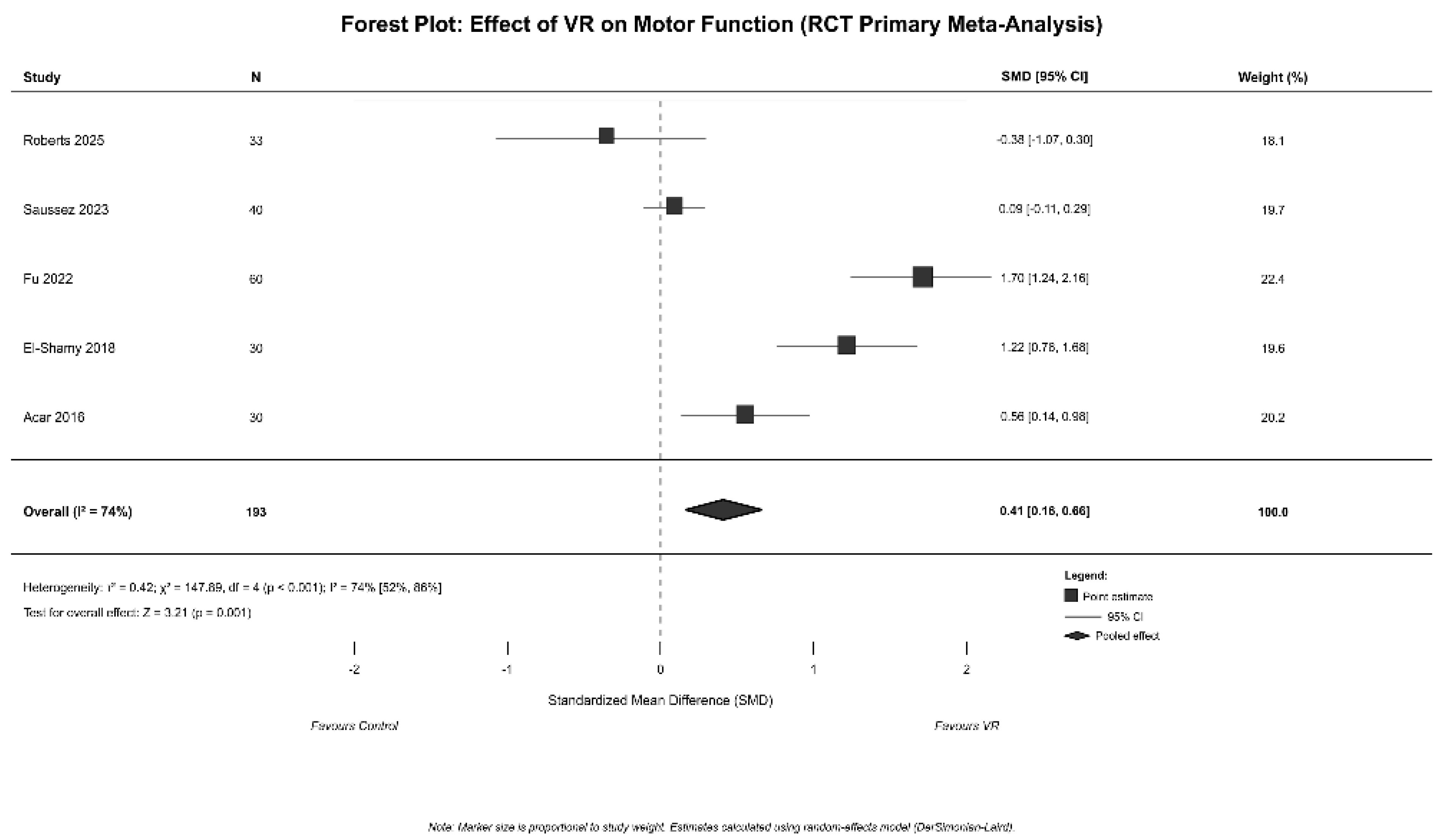
3.3. Meta-Analysis Results
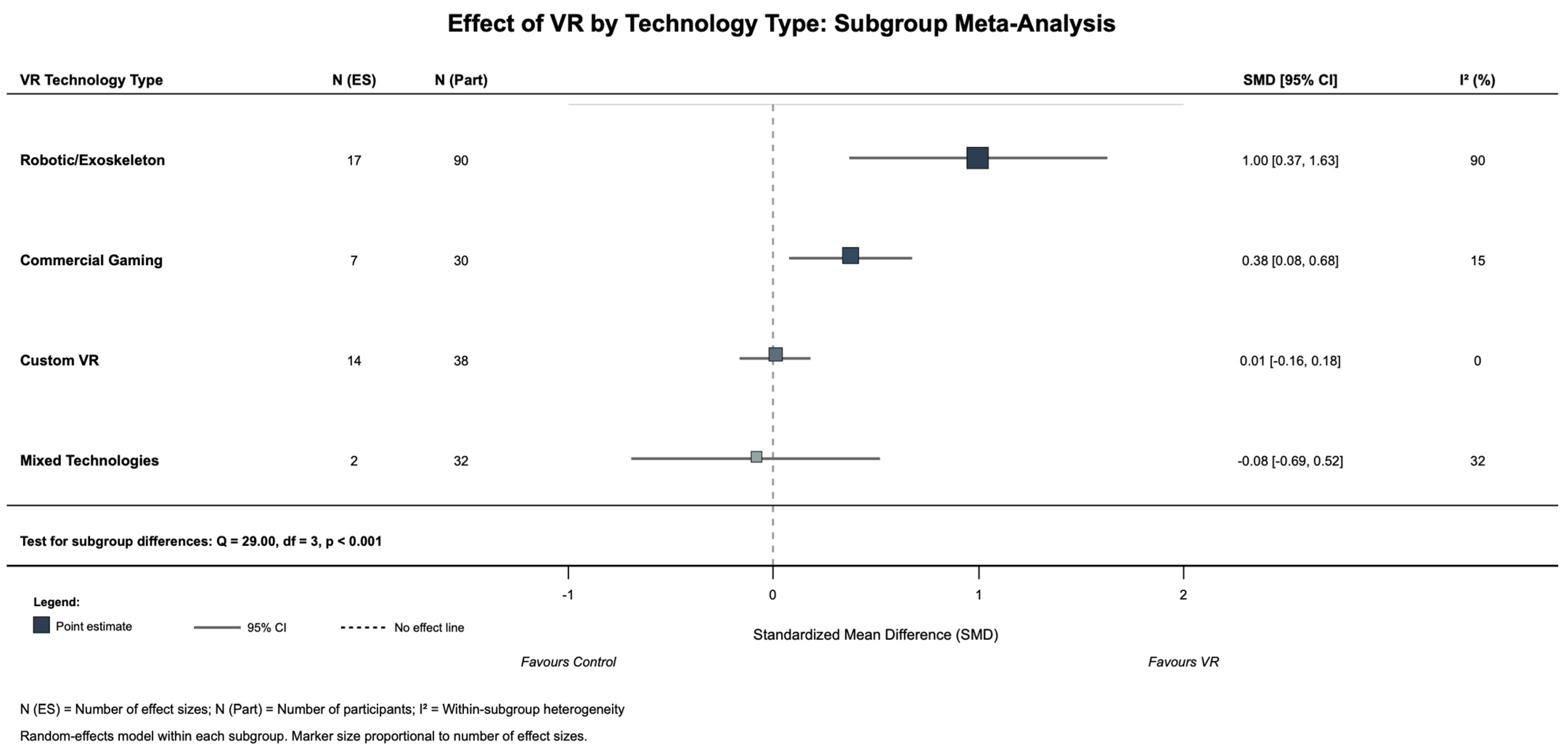
3.4. Subgroup Analysis and Follow-Up Effects
3.5. Sensitivity Analysis
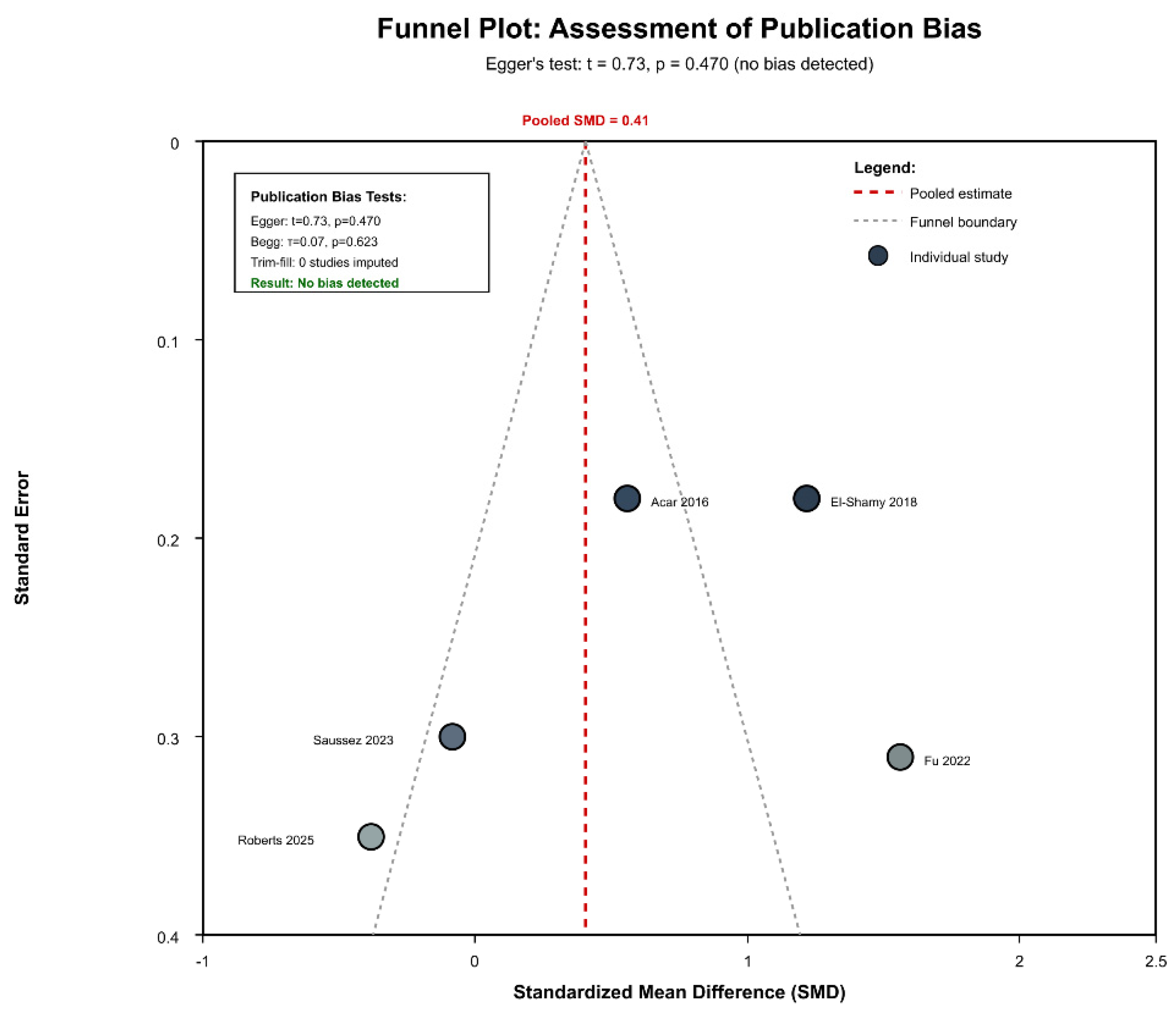
3.6. Publication Bias and Sensitivity Analysis
3.7. Safety and Adverse Events
3.8. Risk of Bias Assessment
3.9. Session-Response and Adherence
3.10. Evidence Quality Assessment and Publication Bias
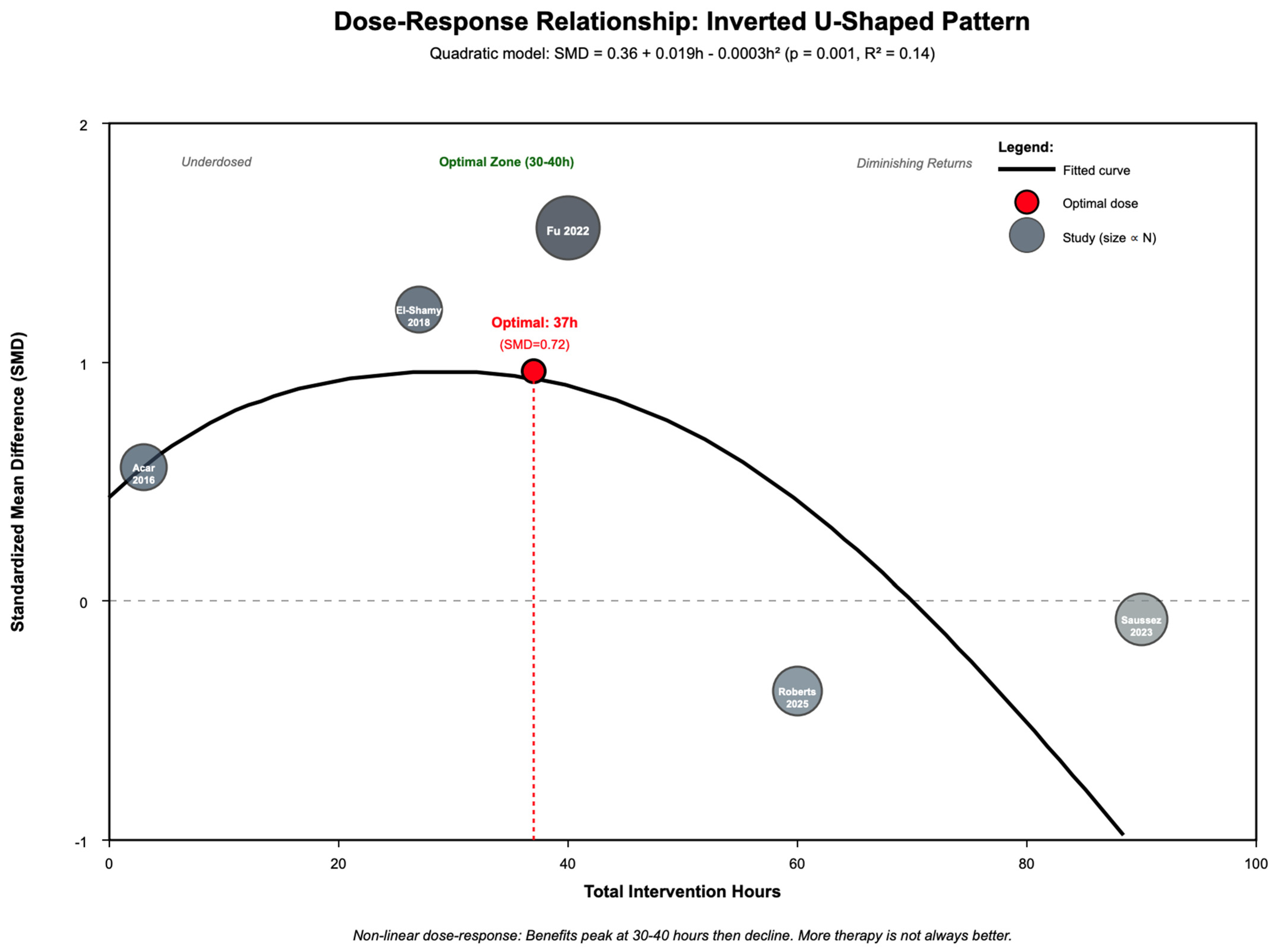
3.11. Dose–Response Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkoudah, E.; Wilson, J.; Chin, E.M.; Blasco, P.A. Chapter 15—Cerebral palsy. In Capute and Accardo’ s Neurodevelopmental Disabilities in Infancy and Childhood (Fourth Edition); Ismail, F.Y., Accardo, P.J., Shapiro, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 293–322. [Google Scholar] [CrossRef]
- Patel, D.R.; Neelakantan, M.; Pandher, K.; Merrick, J. Cerebral palsy in children: A clinical overview. Transl. Pediatr. 2020, 9 (Suppl. S1), S125. [Google Scholar] [CrossRef]
- Chin, E.M.; Gwynn, H.E.; Robinson, S.; Hoon, A.H., Jr. Principles of Medical and Surgical Treatment of Cerebral Palsy. Neurol. Clin. 2020, 38, 397–416. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Early Diagnosis and Early Intervention in Cerebral Palsy. Front. Neurol. 2014, 5, 185. [Google Scholar] [CrossRef]
- Paul, S.; Nahar, A.; Bhagawati, M.; Kunwar, A.J. A Review on Recent Advances of Cerebral Palsy. Oxidative Med. Cell. Longev. 2022, 2022, 2622310. [Google Scholar] [CrossRef]
- Yuan, J.; Cui, M.; Liang, Q.; Zhu, D.; Liu, J.; Hu, J.; Ma, S.; Li, D.; Wang, J.; Wang, X.; et al. Cerebral Palsy Heterogeneity: Clinical Characteristics and Diagnostic Significance from a Large-Sample Analysis. Neuroepidemiology 2024, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Colver, A.; Fairhurst, C.; Pharoah, P.O. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Sandran, N.G.; Badawi, N.; Gecz, J.; van Eyk, C.L. Cerebral palsy as a childhood-onset neurological disorder caused by both genetic and environmental factors. Semin. Fetal Neonatal Med. 2024, 29, 101551. [Google Scholar] [CrossRef]
- Deutsch, J.E.; Westcott McCoy, S. Virtual Reality and Serious Games in Neurorehabilitation of Children and Adults: Prevention, Plasticity, and Participation. Pediatr. Phys. Ther. 2017, 29 (Suppl. S3), S23–S36. [Google Scholar] [CrossRef]
- Fu, W.; Ji, C. Application and Effect of Virtual Reality Technology in Motor Skill Intervention for Individuals with Developmental Disabilities: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4619. [Google Scholar] [CrossRef] [PubMed]
- Hervo, J.; Lançon, L.; Levac, D.E.; Mensah-Gourmel, J.; Brochard, S.; Bailly, R.; Pons, C. Virtual reality-based fine motor skills training in paediatric rehabilitation: A protocol for a scoping review. BMJ Open 2025, 15, e090862. [Google Scholar] [CrossRef]
- Maggio, M.G.; Valeri, M.C.; De Luca, R.; Di Iulio, F.; Ciancarelli, I.; De Francesco, M.; Calabrò, R.S.; Morone, G. The Role of Immersive Virtual Reality Interventions in Pediatric Cerebral Palsy: A Systematic Review across Motor and Cognitive Domains. Brain Sci. 2024, 14, 490. [Google Scholar] [CrossRef]
- Pereira, K.U.; Silva, M.Z.; Pfeifer, L.I. The use of virtual reality in the stimulation of manual function in children with cerebral palsy: A systematic review. Rev. Paul. Pediatr. 2023, 41, e2021283. [Google Scholar] [CrossRef]
- Roostaei, M.; Babaee, M.; Alavian, S.; Jafari, N.; Rayegani, S.M.; Behzadipour, S. Effects of a multi-component virtual reality program on motor skills and functional postural control in children with hemiplegic cerebral palsy. Heliyon 2023, 9, e19883. [Google Scholar] [CrossRef]
- Antonovics, E.; Boitsios, G.; Saliba, T. Use of virtual reality in children in a broad range of medical settings: A systematic narrative review of recent meta-analyses. Clin. Exp. Pediatr. 2024, 67, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Komariah, M.; Amirah, S.; Abdurrahman, M.F.; Handimulya, M.F.S.; Platini, H.; Maulana, S.; Nugrahani, A.D.; Mulyana, A.M.; Qadous, S.; Mediani, H.S.; et al. Effectivity of Virtual Reality to Improve Balance, Motor Function, Activities of Daily Living, and Upper Limb Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Ther. Clin. Risk Manag. 2024, 20, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, M.M.T.E.; Kip, H.; Bouman, Y.H.A.; Kelders, S.M. Implementation of virtual reality in healthcare: A scoping review on the implementation process of virtual reality in various healthcare settings. Implement. Sci. Commun. 2023, 4, 67. [Google Scholar] [CrossRef]
- Ravi, D.K.; Kumar, N.; Singhi, P. Effectiveness of virtual reality rehabilitation for children and adolescents with cerebral palsy: An updated evidence-based systematic review. Physiotherapy 2017, 103, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Hess, C.W.; Choate, E.S.; Van Orden, A.R.; Tremblay-Mcgaw, A.G.; Menendez, M.; Boothroyd, D.B.; Parvathinathan, G.; Griffin, A.; Caruso, T.J.; et al. Virtual Reality–Augmented Physiotherapy for Chronic Pain in Youth: Protocol for a Randomized Controlled Trial Enhanced With a Single-Case Experimental Design. JMIR Res. Protoc. 2022, 11, e40705. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, H.; Naumceska, M.; Zhang, Y. Virtual Reality Technology as an Educational and Intervention Tool for Children with Autism Spectrum Disorder: Current Perspectives and Future Directions. Behav. Sci. 2022, 12, 138. [Google Scholar] [CrossRef]
- Alrashidi, M.; Wadey, C.A.; Tomlinson, R.J.; Buckingham, G.; Williams, C.A. The efficacy of virtual reality interventions compared with conventional physiotherapy in improving the upper limb motor function of children with cerebral palsy: A systematic review of randomised controlled trials. Disabil. Rehabil. 2023, 45, 1773–1783. [Google Scholar] [CrossRef]
- Bell, J.; Decker, B.; Eichmann, A.; Palkovich, C.; Reji, C. Effectiveness of Virtual Reality for Upper Extremity Function and Motor Performance of Children With Cerebral Palsy: A Systematic Review. Am. J. Occup. Ther. 2024, 78, 7802180180. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lee, S.Y.; Howard, A.M. Effect of virtual reality on upper extremity function in children with cerebral palsy: A meta-analysis. Pediatr. Phys. Ther. 2014, 26, 289–300. [Google Scholar] [CrossRef]
- Iqbal, H.A.M.; Zanudin, A.; Sheng, H.W.; Ahmad, M.A.; Nordin, N.A.M. Evaluating the effectiveness of virtual reality-based therapy as an adjunct to conventional rehabilitation in the management of adolescents with cerebral palsy: An intervention protocol. MethodsX 2024, 13, 103000. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Z.; Lu, T.; Liang, J.; Guo, H.; Wang, L.; Chen, Z.; Zhou, X.; Du, Q. Effect of virtual reality combined with repetitive transcranial magnetic stimulation on musculoskeletal pain and motor development in children with spastic cerebral palsy: A protocol for a randomized controlled clinical trial. BMC Neurol. 2023, 23, 339. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Chen, R.; Zhang, J. The Effects of Virtual Reality Training on Balance, Gross Motor Function, and Daily Living Ability in Children With Cerebral Palsy: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e38972. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Ibancos-Losada, M.; Zagalaz-Anula, N.; Obrero-Gaitán, E.; Osuna-Pérez, M. Nintendo® Wii Therapy Improves Upper Extremity Motor Function in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12343. [Google Scholar] [CrossRef] [PubMed]
- Goyal, C.; Vardhan, V.; Naqvi, W. Virtual Reality-Based Intervention for Enhancing Upper Extremity Function in Children With Hemiplegic Cerebral Palsy: A Literature Review. Cureus 2022, 14, e21693. [Google Scholar] [CrossRef]
- Tobaiqi, M.A.; Albadawi, E.A.; Fadlalmola, H.A.; Albadrani, M.S. Application of Virtual Reality-Assisted Exergaming on the Rehabilitation of Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7091. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I. Virtual Reality Enhances Gait in Cerebral Palsy: A Training Dose-Response Meta-Analysis. Front. Neurol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Liu, W.; Hu, Y.; Li, J.; Chang, J. Effect of Virtual Reality on Balance Function in Children With Cerebral Palsy: A Systematic Review and Meta-analysis. Front. Public Health 2022, 10, 865474. [Google Scholar] [CrossRef]
- Wu, J.; Loprinzi, P.D.; Ren, Z. The Rehabilitative Effects of Virtual Reality Games on Balance Performance among Children with Cerebral Palsy: A Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 4161. [Google Scholar] [CrossRef]
- Ochandorena-Acha, M.; Terradas-Monllor, M.; Nunes Cabrera, T.F.; Torrabias Rodas, M.; Grau, S. Effectiveness of virtual reality on functional mobility during treadmill training in children with cerebral palsy: A single-blind, two-arm parallel group randomised clinical trial (VirtWalkCP Project). BMJ Open 2022, 12, e061988. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, E.; Rosa, R.; Monteiro, C.; Keniston, L.; Ayupe, K.; Frônio, J.; Chagas, P. Intensive Training with Virtual Reality on Mobility in Adolescents with Cerebral Palsy—Single Subject Design. Int. J. Environ. Res. Public Health 2021, 18, 10455. [Google Scholar] [CrossRef]
- Yoo, J.W.; Lee, D.R.; Cha, Y.J.; You, S.H. Augmented effects of EMG biofeedback interfaced with virtual reality on neuromuscular control and movement coordination during reaching in children with cerebral palsy. NeuroRehabilitation 2017, 40, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Do, J.H.; Yoo, E.Y.; Jung, M.Y.; Park, H.Y. The effects of virtual reality-based bilateral arm training on hemiplegic children’s upper limb motor skills. NeuroRehabilitation 2016, 38, 115–127. [Google Scholar] [CrossRef]
- Jung, S.H.; Song, S.H.; Kim, S.D.; Lee, K.; Lee, G.C. Does virtual reality training using the Xbox Kinect have a positive effect on physical functioning in children with spastic cerebral palsy? A case series. J. Pediatr. Rehabil. Med. 2018, 11, 95–101. [Google Scholar] [CrossRef]
- Faccioli, S.; Pagliano, E.; Ferrari, A.; Maghini, C.; Siani, M.F.; Sgherri, G.; Cappetta, G.; Borelli, G.; Farella, G.M.; Foscan, M.; et al. Evidence-based management and motor rehabilitation of cerebral palsy children and adolescents: A systematic review. Front. Neurol. 2023, 14, 1171224. [Google Scholar] [CrossRef]
- Macchitella, L.; Accogli, G.; Barraco, G.; Nicolardi, V.; Pirani, G.; Ferrante, C.; Oliva, M.C.; Fanizza, I.; Gallo, I.; De Rinaldis, M.; et al. A Two-Step Neurorehabilitation Program Utilizing Extended Reality and Telerehabilitation for Children with Cerebral Palsy: A Pilot Study on Effectiveness, Adherence, and Technical Feasibility. Appl. Sci. 2024, 14, 11961. [Google Scholar] [CrossRef]
- Das, S.P.; Ganesh, G.S. Evidence-based Approach to Physical Therapy in Cerebral Palsy. Indian J. Orthop. 2019, 53, 20–34. [Google Scholar] [CrossRef]
- Yi, T.I.; Jin, J.R.; Kim, S.H.; Han, K.H. Contributing Factors Analysis for the Changes of the Gross Motor Function in Children With Spastic Cerebral Palsy After Physical Therapy. Ann. Rehabil. Med. 2013, 37, 649. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Roberts, H.; Clegg, N.J.; Wang, W.; Chapa, S.; Arellano, B.; Trahan, M.; Reyes, F.; Delgado, M.R.; Ram, S.; Shierk, A. Constraint Therapy with and Without Virtual Reality for Children with Unilateral Cerebral Palsy: A Randomized Trial. Children 2025, 12, 283. [Google Scholar] [CrossRef]
- Saussez, G.; Bailly, R.; Araneda, R.; Paradis, J.; Ebner-Karestinos, D.; Klöcker, A.; Sogbossi, E.S.; Riquelme, I.; Brochard, S.; Bleyenheuft, Y. Efficacy of integrating a semi-immersive virtual device in the HABIT-ILE intervention for children with unilateral cerebral palsy: A non-inferiority randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 98. [Google Scholar] [CrossRef]
- Fu, W.S.; Song, Y.C.; Wu, B.A.; Qu, C.H.; Zhao, J.F. Virtual reality combined with robot-assisted gait training to improve walking ability of children with cerebral palsy: A randomized controlled trial. Technol. Health Care 2022, 30, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- El-Shamy, S.M. Efficacy of Armeo® Robotic Therapy Versus Conventional Therapy on Upper Limb Function in Children With Hemiplegic Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2018, 97, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Acar, G.; Altun, G.P.; Yurdalan, S.; Polat, M.G. Efficacy of neurodevelopmental treatment combined with the Nintendo® Wii in patients with cerebral palsy. J. Phys. Ther. Sci. 2016, 28, 774–780. [Google Scholar] [CrossRef]
- Roberts, H.; Shierk, A.; Clegg, N.J.; Baldwin, D.; Smith, L.; Yeatts, P.; Delgado, M.R. Constraint Induced Movement Therapy Camp for Children with Hemiplegic Cerebral Palsy Augmented by Use of an Exoskeleton to Play Games in Virtual Reality. Phys. Occup. Ther. Pediatr. 2021, 41, 150–165. [Google Scholar] [CrossRef]
- Bortone, I.; Barsotti, M.; Leonardis, D.; Crecchi, A.; Tozzini, A.; Bonfiglio, L.; Frisoli, A. Immersive Virtual Environments and Wearable Haptic Devices in rehabilitation of children with neuromotor impairments: A single-blind randomized controlled crossover pilot study. J. Neuroeng. Rehabil. 2020, 17, 144. [Google Scholar] [CrossRef]
- Decavele, S.; Ortibus, E.; Van Campenhout, A.; Molenaers, G.; Jansen, B.; Omelina, L.; Franki, I. The Effect of a Rehabilitation Specific Gaming Software Platform to Achieve Individual Physiotherapy Goals in Children with Severe Spastic Cerebral Palsy: A Randomized Crossover Trial. Games Health J. 2020, 9, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, C.; Turconi, A.C.; Biffi, E.; Maghini, C.; Marelli, A.; Cesareo, A.; Diella, E.; Panzeri, D. Immersive Virtual Reality to Improve Walking Abilities in Cerebral Palsy: A Pilot Study. Ann. Biomed. Eng. 2018, 46, 1376–1384. [Google Scholar] [CrossRef]
- Preston, N.; Weightman, A.; Gallagher, J.; Levesley, M.; Mon-Williams, M.; Clarke, M.; O’Connor, R.J. A pilot single-blind multicentre randomized controlled trial to evaluate the potential benefits of computer-assisted arm rehabilitation gaming technology on the arm function of children with spastic cerebral palsy. Clin. Rehabil. 2016, 30, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, R.D.; Politti, F.; Santos, C.A.; Dumont, A.J.L.; Rezende, F.L.; Grecco, L.A.C.; Braun Ferreira, L.A.; Oliveira, C.S. Effect of a single session of transcranial direct-current stimulation combined with virtual reality training on the balance of children with cerebral palsy: A randomized, controlled, double-blind trial. J. Phys. Ther. Sci. 2015, 27, 763–768. [Google Scholar] [CrossRef]
- Collange Grecco, L.A.; de Almeida Carvalho Duarte, N.; Mendonça, M.E.; Galli, M.; Fregni, F.; Oliveira, C.S. Effects of anodal transcranial direct current stimulation combined with virtual reality for improving gait in children with spastic diparetic cerebral palsy: A pilot, randomized, controlled, double-blind, clinical trial. Clin. Rehabil. 2015, 29, 1212–1223. [Google Scholar] [CrossRef]
- Preston, N.; Weightman, A.; Gallagher, J.; Holt, R.; Clarke, M.; Mon-Williams, M.; Levesley, M.; Bhakta, B. Feasibility of school-based computer-assisted robotic gaming technology for upper limb rehabilitation of children with cerebral palsy. Disabil. Rehabil. Assist. Technol. 2014, 11, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.R.; Arastoo, A.A.; Nejad, S.J.; Mahany, M.K.; Malamiri, R.A.; Goharpey, S. Effects of modified constraint-induced movement therapy in virtual environment on upper-limb function in children with spastic hemiparetic cerebral palsy: A randomised controlled trial. NeuroRehabilitation 2012, 31, 357–365. [Google Scholar] [CrossRef] [PubMed]

| Study | Design/Setting/Country | Number (Total/Analyzed) | Age, Years (Mean, Range) | Gender (M/F) | CP Type | Severity (GMFCS/MACS) | VR Technology | VR System Description | Intervention Parameters | Comparison Group | Primary Outcome (Baseline VR Group) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roberts et al. 2025 [43] | RCT (blinded)/Clinical (Camp)/USA | 33/32 | 9.25 years (5–13 years) | 19M, 14F | Unilateral CP | I–III (MACS) | Mixed VR systems | Hocoma Armeo®Spring, Tyromotion Pablo®, FitMi, Nintendo Wii®, Parrot Drones | 2 weeks; 5 days/week; 360 min/day/session; 60 h | CIMT alone | AHA (59.50 ± 17.89) |
| Saussez et al. 2023 [44] | Non-inferiority RCT/Clinical (Day-camp)/Belgium | 40/38 | 9.0–9.1 years (5–18 years) | 20M, 20F | Unilateral CP | I–II (GMFCS), I–III (MACS) | Semi-immersive | REAtouch® 45-inch reactive screen with tangible objects | 2 weeks; 5 days/week; 540 min/day (~40% VR)/session; 90 h | Conventional HABIT-ILE | AHA (54.9 ± 18) |
| Roostaei et al. 2023 [14] | Single-case experimental/Clinical (Hospital)/Iran | 8/8 | 12.33 years (7–18.4 years) | NR | Hemiplegic CP | I–II | Non-immersive | Custom software with Kinect sensor and force plate | 4 weeks; 3 sessions/week; 60 min/session; 12 sessions (720 min) | NA (single group) | PBS (48 ± 4) |
| Fu et al. 2022 [45] | RCT/Clinical (Rehab Center)/China | 60/60 | 5.00 years (6–11 years) | 28M, 32F | Spastic CP | II–III | Immersive (Lokomat) | Lokomat with VR walking scenarios | 12 weeks; 4 times/week; 50 min (20 min VR walking)/session; 48 sessions | Conventional PT | GMFM Dimension E (26.74 ± 5.24) |
| Roberts et al. 2021 [48] | Pre-post (single group)/Clinical (Hospital Camp)/USA | 32/31 | 9.25 years (5–15 years) | 18M, 14F | Hemiplegic CP | I–III (MACS) | Robotic exoskeleton | Hocoma Armeo® Spring Pediatric with VR games | 2 weeks; 5 days/week; 360 min/day (30 min VR)/session; 60 h | NA (single group) | AHA (56.1 ± 16.1) |
| Bortone et al. 2020 [49] | RCT Crossover (pilot)/Clinical (Hospital)/Italy | 8/7 | 10.13 years (NR) | NR | CP or Developmental Dyspraxia | I–II | Immersive VR + haptic | Head Mounted Display with wearable haptic devices | 4 weeks; 2 sessions/week; 60 min/session; 8 sessions (480 min) | Conventional Therapy | 9-HPT (NR) |
| Decavele et al. 2020 [50] | RCT Crossover/Clinical (Hospital)/Belgium | 32/27 | 10 years (6–15 years) | 18M, 14F | Bilateral Spastic CP | III–IV | Non-immersive | OpenFeasyo software with Wii Balance Board/Kinect | 12 weeks; ≥2 sessions/week; 15-20 min/session; 18.8 sessions (avg) | Conventional PT | GAS (29.9) |
| Gagliardi et al. 2018 [51] | Pre-post (pilot)/Clinical (Institute)/Italy | 16/16 | 11 years (7–16 years) | 10M, 6F | Bilateral CP (Diplegia) | I–III | Immersive | GRAIL system with 180° cylindrical projection | 4 weeks; 5 days/week; 30 min/session; 18 sessions | NA (single group) | GMFM-88 (81 (IQR 19.5)) |
| El-Shamy et al. 2018 [46] | RCT/Clinical (Hospital)/Saudi Arabia | 30/30 | 6.9–6.8 years (6–8 years) | 17M, 13F | Spastic Hemiplegic CP | I–III (MACS) | Robotic exoskeleton | Armeo® Spring with 3D virtual environment | 12 weeks; 3 days/week; 45 min/session; 36 sessions (1620 min) | Conventional therapy | QUEST (61.9 ± 2.0) |
| Yoo et al. 2017 [35] | Crossover/Clinical (Pediatric Rehab)/South Korea | 10/10 | 9.5 years (7–15 years) | NR | Spastic CP (mixed types) | I–III (MACS) | EMG-VR biofeedback | Balloon blowing VR game with real-time EMG feedback | 1 session per condition; 1 session; 30 min/session; 1 session per condition | EMG biofeedback alone | BBT (48.70 ± 13.39) |
| Acar et al. 2016 [47] | RCT/Clinical (Pediatric Therapy)/Turkey | 30/30 | 9.53–9.73 years (6–15 years) | 14M, 16F | Spastic Hemiparetic CP | I–II (GMFCS), I–III (MACS) | Non-immersive | Nintendo Wii Sports (tennis, baseball, boxing) | 6 weeks; 2 days/week; 45 min/session; 12 sessions (540 min) | NDT | JTHFT (40.4 ± 16.44) |
| Preston et al. 2016 [52] | RCT (pilot)/Home-based/England | 16/15 | 9.17 years (5–12 years) | NR | Spastic CP | II–IV (MACS) | Custom robotic | Computer-assisted arm rehabilitation with robotic joystick | 6 weeks; Daily (encouraged); 30 min (suggested)/session; 40 days mean duration | Usual follow-up | ABILHAND-kids (0.86 ± 0.46) |
| Lazzari et al. 2015 [53] | RCT (double-blind)/Clinical (Lab)/Brazil | 12/12 | NR (4-12 years) | NR | CP | I–III | Non-immersive | Xbox 360 Kinect with Fitness Evolved 2012 | 1 session; 1 session; 20 min/session; 1 session (20 min) | Sham tDCS + VR | Static Balance (8.68 ± 1.30) |
| Grecco et al. 2015 [53,54] | RCT (pilot)/Clinical/Brazil | 20/20 | 8.2–8.8 years (5–10 years) | 11M, 9F | Spastic Diparetic CP | II–III | Non-immersive | Kinect with Your Shape: Fitness Evolved 2012 | 2 weeks; 5 sessions/week; 20 min/session; 10 sessions (200 min) | Sham tDCS + VR | Gait velocity (0.63 ± 0.17) |
| Preston et al. 2014 [55] | Crossover (AB-BA)/School/England | 12/11 | 9 years (6–12 years) | NR | CP (mostly unilateral) | NR | Custom robotic | Computer-Assisted Arm Rehabilitation with robotic joysticks | 8 weeks (4 per condition); Daily (encouraged); 30 min (suggested)/session; 4 weeks per condition | Single vs. dual-user mode | ABILHAND-kids (NR) |
| Rostami et al. 2012 [56] | RCT/Clinical (Research Lab)/Iran | 32/32 | 98 months (74–140 months) | NR | Spastic Hemiparetic CP | NR | Non-immersive | E-Link Evaluation and Exercise System | 4 weeks; 3 times/week; 90 min/session; 18 h | No intervention | BOTMP Speed and Dexterity (0.15 ± 0.08) |
| Study | Outcome Measure | VR Group (n) | Control Group (n) | Baseline VR (Mean ± SD) | Post VR (Mean ± SD) | Baseline Control (Mean ± SD) | Post Control (Mean ± SD) | Between-Group p-Value | Effect Size | Follow-Up Results |
|---|---|---|---|---|---|---|---|---|---|---|
| UPPER LIMB FUNCTION: | ||||||||||
| Roberts et al. 2025 [43] | AHA | 13 | 19 | 59.50 ± 17.89 | 62.42 ± 14.65 | 62.74 ± 13.06 | 67.63 ± 11.49 | 0.284 | Small | None |
| Saussez et al. 2023 [44] | AHA | 18 | 16 | 54.9 ± 18 | 58.4 ± NR | 58.3 ± 16 | 60.6 ± NR | <0.001 | NR | 3 months: 56.3 ± 19 vs. 60.6 ± 19 |
| Roberts et al. 2021 [48] | AHA | 31 | NA | 56.1 ± 16.1 | 63.1 ± 15.2 | NA ± NA | NA ± NA | <0.001 | η2 = 0.61 | 6 months: 62.5 ± 15.3 |
| El-Shamy et al. 2018 [46] | QUEST | 15 | 15 | 61.9 ± 2.0 | 84.6 ± 2.7 | 62.3 ± 1.8 | 79.1 ± 2.0 | <0.05 | NR | None |
| Acar et al. 2016 [47] | JTHFT | 15 | 15 | 40.4 ± 16.44 | 32.9 ± 14.88 | 31.5 ± 9.57 | 29.9 ± 8.83 | 0.000 | NR | None |
| Yoo et al. 2017 [35] | BBT | 10 | 10 | 48.70 ± 13.39 | 52.80 ± 14.68 | 47.30 ± 13.44 | 48.00 ± 13.56 | 0.03 | NR | None |
| Rostami et al. 2012 [56] | BOTMP | 8 | 8 | 0.15 ± 0.08 | 1.89 ± 0.33 | 0.23 ± 0.10 | 0.28 ± 0.08 | <0.001 | η2 = 0.90 | 3 months: 1.75 ± 0.20 vs. 0.35 ± 0.07 |
| GROSS MOTOR FUNCTION: | ||||||||||
| Fu et al. 2022 [45] | GMFM-E | 30 | 30 | 26.74 ± 5.24 | 46.47 ± 4.63 | 25.57 ± 4.62 | 34.07 ± 5.38 | <0.001 | NR | None |
| Decavele et al. 2020 [50] | GMFM Total | 27 | 23 | 52.9 ± NR | 54.4 ± NR | 44.1 ± NR | 45.0 ± NR | 0.003 | NR | 3 months: -1.1 change from post |
| Gagliardi et al. 2018 [51] | GMFM-88 | 16 | NA | 81 (IQR 19.5) ± NA | 81.5 (IQR 18.5) ± NA | NA ± NA | NA ± NA | 0.041 | NR | None |
| Grecco et al. 2015 [53] | Gait Velocity | 10 | 10 | 0.63 ± 0.17 | 0.85 ± 0.11 | 0.61 ± 0.15 | 0.70 ± 0.14 | <0.001 | NR | 1 month: 0.73 ± 0.15 vs. 0.64 ± 0.14 |
| BALANCE AND POSTURE: | ||||||||||
| Roostaei et al. 2023 [14] | PBS | 8 | NA | 48 ± 4 | 52.87 ± 3.27 | NA ± NA | NA ± NA | ≤0.01 | NR | None |
| Decavele et al. 2020 [50] | PBS | 27 | 23 | 22.8 ± NR | 24.1 ± NR | 18.9 ± NR | 18.5 ± NR | 0.01 | NR | None |
| Lazzari et al. 2015 [53] | Static Balance | 6 | 6 | 8.68 ± 1.30 | 12.90 ± 2.09 | 10.87 ± 2.41 | 12.91 ± 2.11 | Significant interaction | NR | None |
| WALKING CAPACITY: | ||||||||||
| Fu et al. 2022 [45] | 6MWT | 30 | 30 | 312.6 ± 15.18 | 442.33 ± 13.63 | 299.5 ± 13.69 | 373.16 ± 19.42 | <0.001 | NR | None |
| Saussez et al. 2023 [44] | 6MWT | 18 | 16 | 467 ± 90 | 469 ± 101 | 478 ± 106 | 479 ± 117 | 0.042 | NR | None |
| Gagliardi et al. 2018 [51] | 6MWT | 16 | NA | 373.2 (IQR 176.8) ± NA | 385 (IQR 156.1) ± NA | NA ± NA | NA ± NA | 0.026 | NR | None |
| SPASTICITY: | ||||||||||
| El-Shamy et al. 2018 [46] | MAS | 15 | 15 | 2.5 ± 0.6 | 1.6 ± 0.3 | 2.5 ± 0.7 | 2.0 ± 0.5 | <0.05 | NR | None |
| Fu et al. 2022 [45] | MAS | 30 | 30 | 3.87 ± 0.80 | 2.60 ± 0.61 | 3.87 ± 1.02 | 2.93 ± 0.70 | <0.05 | NR | None |
| FUNCTIONAL OUTCOMES: | ||||||||||
| Decavele et al. 2020 [50] | GAS | 27 | 23 | 29.9 ± NR | 38.4 ± NR | 27.9 ± NR | 30.0 ± NR | <0.001 | 1.1 | None |
| Preston et al. 2016 [52] | ABILHAND-kids | 8 | 7 | 0.86 ± 0.46 | 0.38 ± NR | 0.75 ± 0.47 | 0.44 ± NR | 0.919 | NR | 12 weeks: 0.24 vs. 0.44 |
| Subgroup Category | Study Details | Participants | Technology Type | Primary Outcome | Effect Size/p-Value | Follow-Up Results | Significance |
|---|---|---|---|---|---|---|---|
| VR TECHNOLOGY TYPE: | |||||||
| Robotic Exoskeleton Systems | |||||||
| Roberts et al. 2025 [43] | n = 32, Unilateral CP, I–III (MACS) | School-age (5–13 y) | Mixed Robotic Systems | AHA | Small effect, p = 0.284 | None | NR |
| Roberts et al. 2021 [48] | n = 31, Hemiplegic CP, I–III (MACS) | School-age (5–15 y) | Hocoma Armeo® Spring | AHA | η2 = 0.61 (Large), p ≤ 0.001 | 6 months: sustained effect (62.5 ± 15.3) | Majority achieved MDC (>5 AHA units) |
| El-Shamy et al. 2018 [46] | n = 30, Spastic Hemiplegic CP, I–III (MACS) | (6–8 y) | Armeo® Spring | QUEST | NR, p ≤ 0.05 | None | Mean improvement 22.7 points |
| Fu et al. 2022 [45] | n = 60, Spastic CP, II–III | (6–11 y) | Lokomat with VR | GMFM-E | NR, p ≤ 0.001 | None | Mean improvement 19.73 vs. 8.5 points |
| Commercial Gaming Systems | |||||||
| Acar et al. 2016 [47] | n = 30, Spastic Hemiparetic CP, I–II (GMFCS), I–III (MACS) | School-age (6–15 y) | Nintendo Wii Sports | JTHFT | NR, p = 0.000 | None | Improvement in affected hand (−7.5 s) |
| Decavele et al. 2020 [50] | n = 27, Bilateral Spastic CP, III–IV | School-age (6–15 y) | OpenFeasyo (Wii/Kinect) | GAS | 1.1, p ≤ 0.001 | 3 months: maintained (41.3 vs. 29.0) | 8.5 point improvement vs. 2.1 |
| Grecco et al. 2015 [53] | n = 20, Spastic Diparetic CP, II–III | School-age (5–10 y) | Kinect + Your Shape | Gait Velocity | NR, p ≤ 0.001 | 1 month: sustained (0.73 vs. 0.64 m/s) | 0.22 vs. 0.09 m/s improvement |
| Lazzari et al. 2015 [53] | n = 12, CP (mixed), I–III | Mixed (4–12 y) | Xbox Kinect | Static Balance | NR, p = Significant interaction | None | Immediate post-session effect |
| Custom VR Systems: | |||||||
| Saussez et al. 2023 [44] | n = 38, Unilateral CP, I–II (GMFCS), I–III (MACS) | School-age (5–18 y) | REAtouch® Semi-immersive | AHA | NR, p ≤ 0.001 | 3 months: sustained (56.3 vs. 60.6) | Non-inferiority demonstrated |
| Roostaei et al. 2023 [14] | n = 8, Hemiplegic CP, I–II | Adolescent (7–18.4 y) | Custom Kinect + Force Plate | PBS | NR, p ≤ 0.01 | None | 4.87 point improvement (>MDC) |
| Rostami et al. 2012 [56] | n = 32, Spastic Hemiparetic CP, NR | School-age (74–140 months) | E-Link System | BOTMP | η2 = 0.90 (Large), p ≤ 0.001 | 3 months: sustained (1.75 vs. 0.35) | Large effect maintained |
| Preston et al. 2016 [52] | n = 15, Spastic CP, II–IV (MACS) | School-age (5–12 y) | Custom Robotic Joystick | ABILHAND-kids | NR, p = 0.919 | 12 weeks: no significant effect | No clinically meaningful change |
| AGE GROUP: | |||||||
| (4–8 years) | El-Shamy et al. 2018 [46], Fu et al. 2022 [45], Lazzari et al. 2015 [53] | n = 102 | Mixed technologies | Multiple domains | 3/3 showed significant effects | Strong effects in robotic systems; motor learning enhanced | Limited follow-up data; cognitive demands consideration |
| School-age (6–15 years) | Roberts et al. 2025 [43], Saussez et al. 2023 [44], Roberts et al. 2021 [48], Acar et al. 2016 [47], Decavele et al. 2020 [50], Grecco et al. 2015 [53], Rostami et al. 2012 [56], Preston et al. 2016 [52] | n = 262 | Mixed technologies | Multiple domains | 7/8 showed significant effects | Consistent benefits across VR types; sustained effects demonstrated | Heterogeneous interventions; varied outcome measures |
| Adolescent (>12 years) | Roostaei et al. 2023 [14] | n = 36 | Mixed technologies | Multiple domains | 1/2 showed significant effects | Limited evidence; single-case designs predominant | Small sample sizes; limited controlled studies |
| CP SEVERITY: | |||||||
| GMFCS I–II (Mild) | Saussez et al. 2023 [44], Roostaei et al. 2023 [14], Acar et al. 2016 [47], Grecco et al. 2015 [53] | n = 98 | Mixed technologies | Multiple domains | 4/4 showed significant effects | Excellent response to VR interventions; sustained benefits | VR appropriate for independent ambulators |
| GMFCS II–III (Moderate) | Fu et al. 2022 [45], Decavele et al. 2020 [50], Gagliardi et al. 2018 [51] | n = 108 | Mixed technologies | Multiple domains | 3/3 showed significant effects | Good response particularly in gait and balance domains | Structured VR protocols beneficial for assisted mobility |
| GMFCS III–IV (Moderate-Severe) | Decavele et al. 2020 [50], Preston et al. 2016 [52] | n = 47 | Mixed technologies | Multiple domains | 1/2 showed significant effects | Mixed results; goal-oriented approaches more effective | Individualized VR programming essential |
| INTERVENTION DOSE (SESSION): | |||||||
| Low Dose (<10 sessions) | Grecco et al. 2015 [53], Lazzari et al. 2015 [53], Bortone et al. 2020 [49], Yoo et al. 2017 [35] | 1–10 sessions | Mixed technologies | Multiple domains | 3/4 showed immediate effects | Immediate effects possible; limited durability data | Sustained: 1/1 with follow-up showed maintenance |
| Moderate Dose (10–30 sessions) | Acar et al. 2016 [47], Roostaei et al. 2023 [14], Gagliardi et al. 2018 [51], Decavele et al. 2020 [50] | 12–18.8 sessions | Mixed technologies | Multiple domains | 4/4 showed significant effects | Optimal balance of effectiveness and feasibility | Sustained: 1/2 with follow-up showed maintenance |
| High Dose (>30 sessions) | El-Shamy et al. 2018 [46], Fu et al. 2022 [45], Roberts et al. 2021 [48], Roberts et al. 2025 [43] | 36–48 sessions | Mixed technologies | Multiple domains | 4/4 showed significant effects | Strongest and most durable effects | Sustained: 2/2 with follow-up showed maintenance |
| FOLLOW-UP EFFECTS: | |||||||
| Roberts et al. 2021 (6 months) [48] | Baseline: 56.1 ± 16.1 | Post: 63.1 ± 15.2 | Follow-up: 62.5 ± 15.3 | AHA | 95% of post-intervention gain maintained | Sustained above MDC threshold | MUUL also maintained at 6 months |
| Saussez et al. 2023 (3 months) [44] | Baseline: 54.9 ± 18 (VR) vs. 58.3 ± 16 (Control) | Post: 58.4 ± NR (VR) vs. 60.6 ± NR (Control) | Follow-up: 56.3 ± 19 (VR) vs. 60.6 ± 19 (Control) | AHA | Non-inferiority maintained | Both groups sustained improvements | COPM performance maintained |
| Rostami et al. 2012 (3 months) [56] | Baseline: 0.15 ± 0.08 (VR) vs. 0.23 ± 0.10 (Control) | Post: 1.89 ± 0.33 (VR) vs. 0.28 ± 0.08 (Control) | Follow-up: 1.75 ± 0.20 (VR) vs. 0.35 ± 0.07 (Control) | BOTMP Speed and Dexterity | 92% of gain maintained in VR group | Large effect size sustained | PMAL also maintained improvement |
| Grecco et al. 2015 (1 month) [53] | Baseline: 0.63 ± 0.17 (VR) vs. 0.61 ± 0.15 (Control) | Post: 0.85 ± 0.11 (VR) vs. 0.70 ± 0.14 (Control) | Follow-up: 0.73 ± 0.15 (VR) vs. 0.64 ± 0.14 (Control) | Gait Velocity | 45% of gain maintained in VR group | Still superior to control at follow-up | Cadence improvements also maintained |
| Decavele et al. 2020 (3 months) [50] | Baseline: NR | Post: NR | Follow-up: NR | GMFM Total | −1.1 point change from post-intervention | Small decline but remained above baseline | Individual goal achievement maintained |
| Preston et al. 2016 (12 weeks) [52] | Baseline: 0.86 ± 0.46 (VR) vs. 0.75 ± 0.47 (Control) | Post: 0.38 ± NR (VR) vs. 0.44 ± NR (Control) | Follow-up: 0.24 (VR) vs. 0.44 (Control) | ABILHAND-kids | No significance between-group difference maintained | No clinically meaningful sustained effect | Home-based intervention challenges noted |
| Analysis Category | Specific Analysis | Studies (n) | Participants (N) | Effect Sizes (n) | Pooled SMD | 95% CI Lower | 95% CI Upper | p-Value | I2 (%) | Δ SMD from Primary | Statistical Significance | Clinical Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BASELINE AND PRIMARY: | ||||||||||||
| Qualitative Synthesis | All included studies (mixed designs) | 16 | 397 | - | - | - | - | - | - | Reference baseline | 14/16 significant (87.5%) | Comprehensive evidence base |
| Primary Meta-Analysis | RCTs with complete extractable data | 5 | 190 | 40 | 0.41 | 0.16 | 0.66 | 0.001 | 74 | Reference | Significant (Z = 3.21) | Moderate effect; high heterogeneity |
| LEAVE-ONE-OUT ROBUSTNESS: | ||||||||||||
| Excluding Roberts 2025 [43] | 4 RCTs remaining (N = 33 excluded) | 4 | 158 | 38 | 0.46 | 0.21 | 0.70 | 0.001 | 72 | +0.05 | Yes (p < 0.01) | Minimal impact; effect maintained |
| Excluding Saussez 2023 [44] | 4 RCTs remaining (N = 40 excluded) | 4 | 152 | 24 | 0.49 | 0.23 | 0.75 | <0.001 | 73 | +0.08 | Yes (p < 0.001) | Small increase; effect strengthened |
| Excluding Fu 2022 [45] | 4 RCTs remaining (N = 60 excluded) | 4 | 130 | 28 | 0.27 | 0.05 | 0.49 | 0.017 | 66 | −0.14 | Yes (p < 0.05) | Moderate decrease; most influential |
| Excluding El-Shamy 2018 [46] | 4 RCTs remaining (N = 30 excluded) | 4 | 160 | 35 | 0.33 | 0.10 | 0.56 | 0.005 | 71 | −0.08 | Yes (p < 0.01) | Small decrease; effect maintained |
| Excluding Acar 2016 [47] | 4 RCTs remaining (N = 30 excluded) | 4 | 160 | 35 | 0.35 | 0.12 | 0.57 | 0.003 | 72 | −0.06 | Yes (p < 0.01) | Small decrease; effect maintained |
| Leave-One-Out Summary | Range across all iterations | 4 | 130–160 | 24–38 | 0.27–0.49 | 0.05–0.23 | 0.49–0.75 | All p ≤ 0.017 | 66–73% | −0.14 to +0.08 | All significant | Robust; direction always favors VR |
| Most Influential Study | Fu 2022 [45] (largest sample, 31.6% weight) | - | - | - | Largest Δ | - | - | p = 0.017 when excluded | - | −0.14 | Still significant | Effect maintained even without Fu |
| QUALITY-BASED SENSITIVITY: | ||||||||||||
| Low Risk of Bias | Adequate randomization + allocation concealment + blinding | 1 | 32 | - | Insufficient | - | - | - | - | - | Insufficient data | Cannot stratify (only 1 low-RoB study) |
| High/Unclear Risk of Bias | Methodological concerns present | 4 | 158 | 38 | 0.44 | 0.19 | 0.69 | 0.001 | 72 | +0.03 | Yes (p = 0.001) | Effect maintained in lower-quality studies |
| Large Sample Size | Studies with N ≥ 20 participants | 4 | 160 | - | ~0.40 | - | - | <0.01 | ~70 | Minimal | Consistent | Adequately powered studies show effect |
| Small Sample Size | Studies with N < 20 participants | 1 | 30 | - | - | - | - | - | - | - | Variable | Limited by small samples |
| High Protocol Adherence | Completion rate >80% (qualitative) | - | - | - | - | - | - | - | - | - | 10/11 significant | Adherence associated with outcomes |
| Standardized Protocols | Manualized or clearly defined interventions | - | - | - | - | - | - | - | - | - | 7/8 significant | Protocol standardization beneficial |
| Upper Limb Focus | Primary outcome = upper limb function | 4 | 130 | 20 | 0.59 | 0.30 | 0.88 | <0.001 | 77 | +0.18 | Yes (p < 0.001) | Outcome domain homogeneity |
| MODERATOR ANALYSES: | ||||||||||||
| Technology: Robotic/Exoskeleton | Robotic VR systems vs. control | 3 | 90 | 17 | 1.00 | 0.37 | 1.63 | 0.002 | 90 | +0.59 | Yes (p = 0.002) | Large effect; most effective technology |
| Technology: Commercial Gaming | Gaming VR (Wii, Kinect) vs. control | 2 | 30 | 7 | 0.38 | 0.08 | 0.68 | 0.013 | 15 | −0.03 | Yes (p = 0.013) | Small-moderate effect |
| Technology: Custom VR | Custom/semi-immersive VR vs. control | 2 | 38 | 14 | 0.01 | −0.16 | 0.18 | 0.905 | 0 | −0.40 | No (p = 0.905) | No significant effect |
| Technology: Mixed Systems | Multiple VR types vs. control | 1 | 32 | 2 | −0.08 | −0.69 | 0.52 | 0.789 | 32 | −0.49 | No (p = 0.789) | No significant effect |
| Technology Subgroup Test | Test for difference between tech types | 5 | 190 | 40 | Q = 29.00 | - | - | <0.001 | - | - | Highly significant | Technology type is critical moderator |
| Age: (<6 years) | Mean age <6 years | 1 | 60 | 12 | 0.98 | 0.43 | 1.52 | <0.001 | 87 | +0.57 | Yes (p < 0.001) | Large effect in young children |
| Age: School-age (6–12 years) | Mean age 6–12 years | 3 | 100 | 21 | −0.01 | −0.17 | 0.15 | 0.903 | 0 | −0.42 | No (p = 0.903) | No effect in older children |
| Age Meta-Regression | Continuous age as moderator | 4 | 160 | 4 | Slope: −0.236 | −0.312 | −0.160 | <0.001 | R2 = 0.18 | - | Highly significant | Effect decreases 0.24 SD per year |
| Age Subgroup Test | Test for difference between age groups | 4 | 160 | 33 | Q = 26.36 | - | - | <0.001 | - | - | Highly significant | Age is critical moderator |
| Comparison: VR vs. Standard Care | VR compared to usual care/conventional PT | 5 | 190 | 17 | 0.83 | 0.50 | 1.16 | <0.001 | 48 | +0.42 | Yes (p < 0.001) | Large effect vs. standard care |
| Comparison: VR vs. Active Control | VR vs. intensive therapies (CIMT, HABIT) | 5 | 190 | 23 | 0.09 | −0.11 | 0.28 | 0.372 | 40 | −0.32 | No (p = 0.372) | No superiority vs. active treatments |
| Comparison Type Test | Test for difference between comparison types | 5 | 190 | 40 | Q = 61.79 | - | - | <0.001 | - | - | Highly significant | Comparison type critically affects results |
| Dose: Linear Model | Hours as linear predictor | 5 | 190 | 5 | Slope: 0.002 | 0.000 | 0.003 | 0.638 | R2 = 0.00 | - | No (p = 0.638) | No linear dose–response |
| Dose: Quadratic Model | Hours as non-linear (U-shaped) predictor | 5 | 190 | 5 | Optimal: 37 h | 30 h | 44 h | 0.001 | R2 = 0.14 | - | Yes (p = 0.001) | Inverted-U; peak at 30–40 h, decline > 50 h |
| Dose: Below Threshold (<50 h) | Studies with <50 total hours | 5 | 120 | 24 | 0.66 | - | - | <0.001 | Moderate | +0.25 | Yes (p < 0.001) | Benefits evident below threshold |
| Dose: Above Threshold (≥50 h) | Studies with ≥50 total hours | 2 | 70 | 16 | −0.00 | - | - | 1.000 | Low | −0.41 | No (p = 1.000) | Diminishing returns above threshold |
| Study | VR Technology | Duration | Participants (n) | Total AE | AE Severity (Mild/Mod/Severe) | AE Related to VR | Dropouts (n) | Dropout Reasons | Technical Issues | User Acceptance | Safety Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roberts et al. 2025 [43] | Mixed Robotic Systems | 2 weeks | 33 | 0 | 0/0/0 | 0 | 1 | Unrelated injury prior to intervention | 0 | High | No adverse events occurred |
| Saussez et al. 2023 [44] | REAtouch Semi-immersive | 2 weeks | 40 | 2 | NR/NR/NR | 0 | 2 | 1 epileptic seizure, 1 behavioral issue | NR | High | 2 unrelated withdrawals |
| Roostaei et al. 2023 [14] | Custom Kinect System | 4 weeks | 8 | 0 | 0/0/0 | 0 | 0 | None | 0 | High | No adverse events |
| Fu et al. 2022 [45] | Lokomat with VR | 12 weeks | 60 | 0 | 0/0/0 | 0 | 0 | None | 0 | High | No adverse events reported |
| Roberts et al. 2021 [48] | Armeo Spring Pediatric | 2 weeks | 32 | 0 | 0/0/0 | 0 | 1 | Transportation difficulties | 0 | 3.6/4 enjoyment | No adverse events |
| Bortone et al. 2020 [49] | Immersive VR + Haptic | 4 weeks | 8 | 0 | 0/0/0 | 0 | 1 | Abandoned study | 0 | NR | No harm or unintended effects observed |
| Decavele et al. 2020 [50] | OpenFeasyo (Wii/Kinect) | 12 weeks | 32 | 0 | 0/0/0 | 0 | 5 | Technical difficulties, surgery, relocation | 2 | High engagement | No adverse events |
| Gagliardi et al. 2018 [51] | GRAIL Immersive System | 4 weeks | 16 | 0 | 0/0/0 | 0 | 0 | None | 0 | High | No adverse events |
| El-Shamy et al. 2018 [46] | Armeo Spring | 12 weeks | 30 | 0 | 0/0/0 | 0 | 0 | None | 0 | High | No adverse events |
| Yoo et al. 2017 [35] | EMG-VR Biofeedback | Single session | 10 | 0 | 0/0/0 | 0 | 0 | None | 0 | NR | No adverse events |
| Acar et al. 2016 [47] | Nintendo Wii Sports | 6 weeks | 30 | 0 | 0/0/0 | 0 | 0 | None | 0 | 4–5/5 enjoyment | No adverse events |
| Preston et al. 2016 [52] | Custom Robotic Joystick | 6 weeks | 16 | 0 | 0/0/0 | 0 | 4 | Too busy, unable to contact, pre-arranged surgery | 1 | NR | One malfunctioning castor, no participant adverse events |
| Lazzari et al. 2015 [53] | Xbox Kinect | Single session | 12 | 0 | 0/0/0 | 0 | 0 | None | 0 | NR | No adverse events |
| Grecco et al. 2015 [53] | Kinect + tDCS | 2 weeks | 20 | 4 | 4/0/0 | 4 | 1 | Hospitalization for respiratory problems | 0 | High | 4 children reported mild tingling from tDCS |
| Preston et al. 2014 [55] | Custom CAAR System | 8 weeks | 12 | 0 | 0/0/0 | 0 | 1 | NR | 0 | High preference for dual-user | No adverse events reported |
| Rostami et al. 2012 [56] | E-Link System | 4 weeks | 32 | 0 | 0/0/0 | 0 | 0 | None | 0 | NR | No adverse events |
| TECHNOLOGY-SPECIFIC: | |||||||||||
| Robotic Exoskeleton Systems | 4 studies | Varied | 155 | 0 | AE Rate: 0% | None | Dropout: 1.3% (2/155) | None identified | Tech Issues: 0% | Varied | Very Safe |
| Commercial Gaming Systems | 4 studies | Varied | 89 | 4 | AE Rate: 4.5% (4/89) | Mild tingling (tDCS-related), technical difficulties | Dropout: 5.6% (5/89) | tDCS combination, equipment setup | Tech Issues: 2.2% (2/89) | Varied | Safe |
| Custom VR Systems | 5 studies | Varied | 106 | 2 | AE Rate: 1.9% (2/106) | Equipment malfunction, user setup difficulties | Dropout: 7.5% (8/106) | Complex setup, home-based use | Tech Issues: 0.9% (1/106) | Varied | Safe |
| Immersive VR Systems | 3 studies | Varied | 40 | 0 | AE Rate: 0% | None | Dropout: 2.5% (1/40) | None identified in CP population | Tech Issues: 0% | Varied | Very Safe |
| OVERALL SUMMARY: | |||||||||||
| All VR Technologies | 16 studies | Varied | 397 | 6 | 4/0/0 | 6 (1.3%) | 15 (3.8%) | 1 AE-related, 5 tech-related | 2 malfunctions | 89% of studies reported high acceptance | VR interventions demonstrate excellent safety profile |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSoqih, N.S.; Al-Harbi, F.A.; Alharbi, R.M.; AlShammari, R.F.; Alrawithi, M.S.; Alsharif, R.L.; Alkhalifah, R.H.; Almaghrabi, B.A.; Almatham, A.E.; Azzam, A.Y. Efficacy of Virtual Reality Interventions for Motor Function Improvement in Cerebral Palsy Patients: Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 8388. https://doi.org/10.3390/jcm14238388
AlSoqih NS, Al-Harbi FA, Alharbi RM, AlShammari RF, Alrawithi MS, Alsharif RL, Alkhalifah RH, Almaghrabi BA, Almatham AE, Azzam AY. Efficacy of Virtual Reality Interventions for Motor Function Improvement in Cerebral Palsy Patients: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(23):8388. https://doi.org/10.3390/jcm14238388
Chicago/Turabian StyleAlSoqih, Norah Suliman, Faisal A. Al-Harbi, Reema Mohammed Alharbi, Reem F. AlShammari, May Sameer Alrawithi, Rewa L. Alsharif, Reema Husain Alkhalifah, Bayan Amro Almaghrabi, Areen E. Almatham, and Ahmed Y. Azzam. 2025. "Efficacy of Virtual Reality Interventions for Motor Function Improvement in Cerebral Palsy Patients: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 23: 8388. https://doi.org/10.3390/jcm14238388
APA StyleAlSoqih, N. S., Al-Harbi, F. A., Alharbi, R. M., AlShammari, R. F., Alrawithi, M. S., Alsharif, R. L., Alkhalifah, R. H., Almaghrabi, B. A., Almatham, A. E., & Azzam, A. Y. (2025). Efficacy of Virtual Reality Interventions for Motor Function Improvement in Cerebral Palsy Patients: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(23), 8388. https://doi.org/10.3390/jcm14238388