A Novel Protocol for Feasibility and Safety in Early Discharge with ACS
Highlights
- Early discharge in low-risk patients with ACS is safe and feasible.
- The LATE2ACS protocol is a novel easy checklist to define low risk for early discharge after ACS.
- A bidirectional management track between CRH and primary care centers is crucial.
- Follow-up after early discharge can be performed telematically without prognostic worsening.
- This new tool will need prospective evaluation to determine its true validation for prognostic value, beyond logistical and functional considerations.
Abstract
1. Introduction: Background of Early Discharge in ACS
2. Risk Scores to Establish Early Discharge in ACS
3. LATE2ACS Protocol to Achieve Early Discharge in ACS
- Lungs, which is represented by the letter “L” for lungs. It represents the presence of pulmonary congestion which, in short, defines the congestive state of the patient with ACS and is evaluated using the Killip-Kimball scale (KK) proposed in the 1980s and which has shown its prognostic value in patients with ACS in whom early discharge is considered. Thus, in order to consider early discharge, our patient should be in a stable situation in the absence of HF: Killip-Kimball class I.
- Age and autonomy, which is represented by the first “A” in the acronym. It refers to the age of the patient and baseline status. Age is a clear CVRF and studies have shown that it is safe to propose early discharge at ages below 75–80 years. Although the first scores proposed more restrictive ages, with the aging of the population and better access to primary PCI, the overall prognosis of these patients has improved. On the other hand, another relevant factor that defines this criterion is the baseline situation, “autonomy”; early access to CRH should be guaranteed, as well as care and maintenance of optimal medical treatment during follow-up. Therefore, all patients who are considered for early discharge should have acceptable independence for activities of daily living without significant signs of frailty and acceptable social and/or family support.
- TIMI final flow, represented by the letter “T” of TIMI flow, which defines the final flow state after PCI. From the pathophysiological point of view, it is essential to obtain TIMI 3 final flow, which indicates complete restoration of flow in the culprit coronary artery and, in short, acute treatment of this ACS, limiting the ischemia time.
- Ejection fraction, represented by the letter “E”, with LVEF being the main prognostic factor in patients with ACS during follow-up. This is so much the case that those patients with LVEF ≤ 35% present a significantly higher risk of debuting with sudden cardiac death in the first month of follow-up after the index event. Thus, it is essential that all patients considered for early discharge have at least LVEF > 45% estimated by transthoracic echocardiography [11,29,30,32].
- 2A comes to represent the second letter “A” which has a double meaning (2A), “the 2 absences”:
- Absence of anterior wall akinesia, thus ruling out those patients with indirect data of extensive AMI, prolonged ischemia with possible adverse remodeling, which will probably be accompanied by a significant decrease in LVEF and therefore should be monitored and cared for longer due to the inherent risk of complications. Thus, a patient with akinesia in the theoretical territory of the left anterior descending (LAD) artery cannot be admitted for early discharge [21,24]. This item reflects its historical origin in the Zwolle index (anterior AMI); recognizing inter-observer variability across imaging metrics, contemporary practice may reasonably rely on standardized early TTE with LVEF (e.g., ≥45%) and/or GLS as pragmatic surrogates where available.
- Absence of laboratory abnormalities, which is basically based on the indirect detection of comorbidities that may presage greater risk secondary to ACS therapies or that go beyond the coronary event itself. Of the parameters and comorbidities considered, only three have been shown to be related to the low-risk profile: hemoglobin, glycemia, and renal filtration rate. Paradoxically, hemorrhagic events have not been shown to be associated with early discharge protocols. Thus, according to the literature [11,24,29,35], early discharge may be considered in those patients who do NOT present anemia with Hb < 11 g/dL, glycemia > 270 mg/dL (>15 mmol/L), and eGFR < 60 mL/min/1.73 m2 [2]. Another important aspect from a laboratory perspective is that the patient has reached peak troponin, which is a marker of myocardial damage. In the setting of STEMI, those who died at 30 days and at 1 year had significantly higher peak troponin levels than those who survived. Peak troponin is also inversely proportional to LVEF, with higher troponin levels associated with lower LVEF [35]. In the context of ACS, blood troponin levels are elevated with a time lag. Therefore, it is highly likely that ongoing ischemia has largely stopped after coronary flow has been restored after PCI, when peak troponin has been reached at the moment when discharge is considered. Studies published previously have not identified a troponin level cut-off value to indicate higher or lower risk in early discharge. However, elevated blood troponin is an adverse prognostic parameter itself. In stable patients with STEMI who have undergone successful primary PCI, the long-term LVEF and infarct size are closely correlated with both serial and peak concentrations of high-sensitivity cardiac troponin T [36]. Operationally, documenting a reached peak followed by an early down-trend (e.g., one value 6–12 h post-PCI, or two values 3–6 h apart) supports that acute injury is not ongoing and can aid next-day discharge decisions in otherwise suitable cases. “Peak reached” is defined by one sample 6–12 h post-PCI showing an absolute decrease from the prior value or two samples 3–6 h apart with a downward trend. No fixed cut-off is used; the criterion captures post-reperfusion decline as a signal of stabilized myocardial injury.
- Coronary complications, represented by the “C” of “ACS”, which makes it very intuitive. This item defines a situation of successful PCI (which was already defined by the TIMI 3, described as a “double check”) but also rules out the presence of complications derived from the procedure such as slow/no-reflow phenomena, coronary dissection requiring more stent placements, or a conservative approach and subsequent re-evaluation. There should be complete revascularization, and it can be considered in the case of incomplete revascularization as long as the remaining lesions are not significant (stenosis < 70%) and revascularization is not pending a second time in an imminent fashion. Patients with culprit lesions in the left main coronary artery (LMCA) should be ruled out for early discharge. Arrhythmias derived from the procedure are not considered a complication. Planned staged PCI or CABG in the near term excludes early discharge; non-culprit residual disease can be acceptable when it is angiographically non-significant (e.g., <70%) and clinically non-ischemic. Periprocedural myocardial infarction (type 4a), frequently related to distal embolization or microvascular obstruction, carries adverse short- and long-term prognostic implications even after technically successful PCI and should exclude candidates for early discharge [37].
- Shock and support, represented by the “S”, referring to the shock situation encompassing all those situations in which the patient is in clinical and/or hemodynamic instability (HDI) requiring medical and/or mechanical support by cardiopulmonary resuscitation (CPR) maneuvers due to cardiopulmonary arrest (CPA), inotropic treatment, vasoactivity, appearance of ventricular arrhythmias > 24 h of PCI, or persistent arrhythmias, and ventilatory and/or mechanical circulatory support. In short, it is, as a whole, again a “double check” for all those processes that go beyond the definition of KK-IV.

3.1. LATE2ACS: How Was It Made?
3.2. Operationalization of LATE2ACS
3.3. Safety and Feasibility in Early Discharge
4. Discussion and Limitations
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| AMI | acute myocardial infarction |
| BP | blood pressure |
| CAD | coronary artery disease |
| CPA | cardiopulmonary arrest |
| CPR | cardiopulmonary resuscitation |
| CRH | cardiac rehabilitation |
| CVRF | cardiovascular risk factor |
| ED | emergency department |
| eGFR | estimated glomerular filtration rate |
| ESC | European Society of Cardiology |
| GRACE | Global Registry Acute Coronary Events |
| Hb | hemoglobin |
| HDI | hemodynamic instability |
| HF | heart failure |
| HR | heart rate |
| KDIGO | Kidney Disease: Improving Global Outcomes guidelines |
| KK | Killip–Kimball class |
| LBBB | left bundle branch block |
| LMCA | left main coronary artery |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| MACE | major adverse cardiac events |
| NIMV | non-invasive mechanical ventilation |
| NSTEMI | non-ST-elevation myocardial infarction |
| PCI | percutaneous coronary intervention |
| SBP | systolic blood pressure |
| STEMI | ST-elevation myocardial infarction |
| TIMI | thrombolysis in myocardial infarction, a grade of coronary flow |
| UA | unstable angina |
References
- Califf, R.M.; Mark, D.B.; Harrell, F.E.; Hlatky, M.A.; Lee, K.L.; Rosati, R.A.; Pryor, D.B. Importance of clinical measures of ischemia in the prognosis of patients with documented coronary artery disease. J. Am. Coll. Cardiol. 1988, 11, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H.; Braunwald, E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Cohen, M.; Bernink, P.J.L.M.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. J. Am. Med. Assoc. 2000, 284, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.W.; Wong, C.K.; Herbison, P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am. Heart J. 2007, 153, 29–35. [Google Scholar] [CrossRef]
- Peterson, E.D.; Ohman, E.M.; Brindis, R.G.; Cohen, D.J.; Magid, D.J. Development of systems of care for ST-elevation myocardial infarction patients: Evaluation and outcomes. Circulation 2007, 116, e64–e67. [Google Scholar] [CrossRef] [PubMed]
- Spencer, F.A.; Lessard, D.; Gore, J.M.; Yarzebski, J.; Goldberg, R.J. Declining length of hospital stay for acute myocardial infarction and postdischarge outcomes: A community-wide perspective. Arch. Intern. Med. 2004, 164, 733–740. [Google Scholar] [CrossRef]
- French, J.K.; Hellkamp, A.S.; Armstrong, P.W.; Cohen, E.; Kleiman, N.S.; O’Connor, C.M.; Holmes, D.R.; Hochman, J.S.; Granger, C.B.; Mahaffey, K.W. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEXAMI). Am. J. Cardiol. 2010, 105, 59–63. [Google Scholar] [CrossRef]
- Puerto, E.; Viana-Tejedor, A.; Martínez-Sellés, M.; Domínguez-Pérez, L.; Moreno, G.; Martín-Asenjo, R.; Bueno, H. Temporal Trends in Mechanical Complications of Acute Myocardial Infarction in the Elderly. J. Am. Coll. Cardiol. 2018, 72, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Li, A.; Ai, H.; Shi, H.; Wang, X.; Nie, S. Safety of early discharge after primary angioplasty in low-risk patients with ST-segment elevation myocardial infarction: A meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 2018, 25, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Comer, K.; Casey-Gillman, O.; Moore, L.; Mills, G.; Ferguson, G.; Antoniou, S.; Patel, R.; Fhadil, S.; Damani, T.; et al. Early Hospital Discharge Following PCI for Patients With STEMI. J. Am. Coll. Cardiol. 2021, 78, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Rathod, K.S.; Howard, J.P.; Gallagher, S.; Antoniou, S.; De Palma, R.; Guttmann, O.; Cliffe, S.; Colley, J.; Butler, J.; et al. Safety and feasibility of hospital discharge 2 days following primary percutaneous intervention for ST-segment elevation myocardial infarction. Heart 2012, 98, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.P.; Jones, D.A.; Gallagher, S.; Rathod, K.; Jain, A.; Mohiddin, S.; Knight, C.; Mathur, A.; Smith, E.J.; Wragg, A. Is it safe to discharge patients 24 h after uncomplicated successful primary percutaneous coronary intervention? Heart 2012, 98, A29–A30. [Google Scholar] [CrossRef]
- Jirmár, R.; Widimský, P.; Capek, J.; Hlinomaz, O.; Groch, L. Next day discharge after successful primary angioplasty for acute ST elevation myocardial infarction. An open randomized study “Prague-5”. Int. Heart J. 2008, 49, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G. PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.; Sgura, F.; Rossi, R.; Monopoli, D.; Guerri, E.; Leuzzi, C.; Bursi, F.; Sangiorgi, G.M.; Modena, M.G. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: Major adverse cardiac events during long-term follow-up. Heart 2010, 96, 662–667. [Google Scholar] [CrossRef]
- Di Mario, C.; Mara, S.; Flavio, A.; Imad, S.; Antonio, M.; Anna, P.; Emanuela, P.; Stefano, D.S.; Angelo, R.; Stefania, C.; et al. Single vs. multivessel treatment during primary angioplasty: Results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int. J. Cardiovasc. Interv. 2004, 6, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kotowycz, M.A.; Cosman, T.L.; Tartaglia, C.; Afzal, R.; Syal, R.P.; Natarajan, M.K. Safety and feasibility of early hospital discharge in STsegment elevation myocardial infarction—A prospective and randomized trial in low-risk primary percutaneous coronary intervention patients (the Safe-Depart Trial). Am. Heart J. 2010, 159, 117.e1–117.e6. [Google Scholar] [CrossRef]
- Barchielli, A.; Balzi, D.; Marchionni, N.; Carrabba, N.; Margheri, M.; Santoro, G.M.; Olivotto, I.; Buiatti, E.; AMI-Florence Working Group. Early discharge after acute myocardial infarction in the current clinical practice. Community data from the AMIFlorence Registry, Italy. Int. J. Cardiol. 2007, 114, 57–63. [Google Scholar] [CrossRef]
- Kociol, R.D.; Lopes, R.D.; Clare, R.; Thomas, L.; Mehta, R.H.; Kaul, P.; Pieper, K.S.; Hochman, J.S.; Weaver, W.D.; Armstrong, P.W.; et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA 2012, 307, 66–74. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Suryapranata, H.; van ‘t Hof, A.W.; de Boer, M.J.; Hoorntje, J.C.; Dambrink, J.H.; Gosselink, A.T.; Ottervanger, J.P.; Zijlstra, F. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: Implications for early discharge. Circulation 2004, 109, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Solé, E.; Sans, J.; Vila, M.; Durán, A.; Gil-Alonso, D.; Santaló, M.; Garcia-Moll, X.; Sionis, A. Feasibility and safety of an early discharge strategy after low-risk acute myocardial infarction treated with primary percutaneous coronary intervention: The EDAMI pilot trial. Cardiology 2015, 130, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Melberg, T.; Jørgensen, M.; Ørn, S.; Solli, T.; Edland, U.; Dickstein, K. Safety and health status following early discharge in patients with acute myocardial infarction treated with primary PCI: A randomized trial. Eur. J. Prev. Cardiol. 2015, 22, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Laurencet, M.E.; Girardin, F.; Rigamonti, F.; Bevand, A.; Meyer, P.; Carballo, D.; Roffi, M.; Noble, S.; Mach, F.; Gencer, B. Early Discharge in Low-Risk Patients Hospitalized for Acute Coronary Syndromes: Feasibility, Safety and Reasons for Prolonged Length of Stay. PLoS ONE 2016, 11, e0161493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2019, 14, 1435–1534. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef]
- Gershlick, A.H.; Banning, A.S.; Parker, E.; Wang, D.; Budgeon, C.A.; Kelly, D.J.; Kane, P.O.; Dalby, M.; Hetherington, S.L.; McCann, G.P.; et al. Long-Term Follow-Up of Complete Versus Lesion-Only Revascularization in STEMI and Multivessel Disease: The CvLPRIT Trial. J. Am. Coll. Cardiol. 2019, 74, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Neuberg, M.; Nováčková, M.; Mašek, P.; Kočka, V.; Moťovská, Z.; Toušek, P. Predictors allowing early discharge after interventional treatment of acute coronary syndrome patients. Eur. Heart J. Suppl. 2022, 24, B10–B15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halkin, A.; Singh, M.; Nikolsky, E.; Grines, C.L.; Tcheng, J.E.; Garcia, E.; Cox, D.A.; Turco, M.; Stuckey, T.D.; Na, Y.; et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: The CADILLAC risk score. J. Am. Coll. Cardiol. 2005, 45, 1397–1405. [Google Scholar] [CrossRef]
- Palmerini, T.; Genereux, P.; Caixeta, A.; Cristea, E.; Lansky, A.; Mehran, R.; Della Riva, D.; Fahy, M.; Xu, K.; Stone, G.W. A new score for risk stratification of patients with acute coronary syndromes undergoing percutaneous coronary intervention: The ACUITYPCI (Acute Catheterization and Urgent Intervention Triage Strategy-Percutaneous Coronary Intervention) risk score. JACC Cardiovasc. Interv. 2012, 5, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Marco Del Castillo, Á.; Sanmartín Fernández, M.; Jiménez Mena, M.; Camino López, A.; Zamorano Gómez, J.L. Safety of a Very Early Discharge Strategy for ST-segment Elevation Acute Coronary Syndrome. Rev. Esp. Cardiol. 2019, 72, 874–875. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Díez-Delhoyo, F.; Valero-Masa, M.J.; Velásquez-Rodríguez, J.; Devesa-Cordero, C.; Sousa-Casasnovas, I.; Juárez, M.; Angulo-Llanos, R.; Fernández-Avilés, F.; Martínez-Sellés, M. Very low risk STsegment elevation myocardial infarction? It exists and may be easily identified. Int. J. Cardiol. 2017, 228, 615–620. [Google Scholar] [CrossRef]
- Alvarez Alvarez, B.; Cid Alvarez, A.B.; Redondo Dieguez, A.; Sanmartin Pena, X.; Lopez Otero, D.; Avila Carrillo, A.; Gomez Peña, F.; Trillo Nouche, R.; Martinez Selles, M.; Gonzalez-Juanatey, J. Short-term and long-term validation of the fastest score in patients with ST-elevation myocardial infarction after primary angioplasty. Int. J. Cardiol. 2018, 269, 19–22. [Google Scholar] [CrossRef]
- Khullar, N.; Buckley, A.J.; O’Connor, C.; Ibrahim, A.; Ibrahim, A.; Ahern, C.; Cahill, C.; Arnous, S.; Kiernan, T.J. Peak troponin T in STEMI: A predictor of all-cause mortality and left ventricular function. Open Heart 2022, 9, e001863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinstadler, S.J.; Feistritzer, H.J.; Klug, G.; Mair, J.; Tu, A.M.; Kofler, M.; Henninger, B.; Franz, W.M.; Metzler, B. High-sensitivity troponin T for prediction of left ventricular function and infarct size one year following ST-elevation myocardial infarction. Int. J. Cardiol. 2016, 202, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients With Non-ST-Segment-Elevation Myocardial Infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carda Barrio, R.; de Agustín, J.A.; Manzano, M.C.; García-Rubira, J.C.; Fernández-Ortiz, A.; Vilacosta, I.; Macaya, C. Valor pronóstico intrahospitalario del filtrado glomerular en pacientes con síndrome coronario agudo y creatinina normal [In-hospital prognostic value of glomerular filtration rate in patients with acute coronary syndrome and a normal creatinine level]. Rev. Esp. Cardiol. 2007, 60, 714–719. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Grines, C.L.; Marsalese, D.L.; Brodie, B.; Griffin, J.; Donohue, B.; Costantini, C.R.; Balestrini, C.; Stone, G.; Wharton, T.; Esente, P.; et al. Safety and costeffectiveness of early discharge after primary angioplasty in low risk patients with acute myocardial infarction. J. Am. Coll. Cardiol. 1998, 31, 967–972. [Google Scholar] [CrossRef]
- Newby, L.K.; Hasselblad, V.; Armstrong, P.W.; Van De Werf, F.; Mark, D.B.; White, H.D.; Topol, E.J.; Califf, R.M. Time-based risk assessment after myocardial infarction. Implications for timing of discharge and applications to medical decision-making. Eur. Heart J. 2003, 24, 182–189. [Google Scholar] [CrossRef]
- Yndigegn, T.; Gilje, P.; Dankiewicz, J.; Mokhtari, A.; Isma, N.; Holmqvist, J.; Schiopu, A.; Ravn-Fischer, A.; Hofmann, R.; Szummer, K.; et al. Safety of early hospital discharge following admission with ST-elevation myocardial infarction treated with percutaneous coronary intervention: A nationwide cohort study. EuroIntervention 2022, 17, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, J.; Ahmed Solangi, B.; Batra, M.K.; Khan, K.A.; Shah, G.A.; Ali, G.; Zehra, M.; Hassan, M.; Zubair, M.; Karim, M. Zwolle Risk Score for Safety Assessment of Same-day Discharge after Primary Percutaneous Coronary Intervention. J. Saudi Heart Assoc. 2021, 33, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Rymer, J.A.; Tempelhof, M.W.; Clare, R.M.; Pieper, K.S.; Granger, C.B.; Van de Werf, F.; Moliterno, D.J.; Harrington, R.A.; White, H.D.; Armstrong, P.W.; et al. Discharge timing and outcomes after uncomplicated non-ST-segment elevation acute myocardial infarction. Am. Heart J. 2018, 201, 103–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- John, A.K.; Norbert, L.; Peter, A.; Rashad, S.B.; Emmanuel, A.B.; Stuart, L.G.; Charles, A.H.; Michael, J.; Andreas, K.; Andrew, S.L. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- John, N.; Jones, D. Early Hospital Discharge Following Primary Percutaneous Coronary Intervention (PCI): A Delivery Guide Based on Work from Barts Heart Centre, London; Getting It Right First Time (GIRFT); NHS England: London, UK, 2023; Available online: https://gettingitrightfirsttime.co.uk/wp-content/uploads/2023/05/Early-Hospital-Discharge-Pathway-May-2023-FINAL-V1.pdf (accessed on 30 October 2025).
- Alshahrani, N.; Hartley, A.; Howard, J.; Hajhosseiny, R.; Khawaja, S.; Seligman, H.; Akbari, T.; Alharbi, B.A.; Bassett, P.; Al-Lamee, R.; et al. Randomized Trial of Remote Assessment of Patients After an Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2024, 83, 2250–2259. [Google Scholar] [CrossRef]

| TIMI Risk Score | UA/NSTEMI 0–2 points: low risk 3–5 points: intermediate risk ≥6 points: high risk | |||
| UA/NSTEMI | STEMI | |||
| POINTS | POINTS | |||
| Age ≥ 65 years | 1 | Age 65–74/>75 | 2/3 | |
| ≥3 CVRF | 1 | SBP < 100 mmHg | 3 | |
| Used ASA last 7 days | 1 | Heart rate > 100 | 2 | |
| Prior CAD > 50% stenosis | 1 | Killip class ≥ 2 | 2 | STEMI 0–4 points: low risk 5–8 points: intermediate risk ≥9 points: high risk |
| >1 rest angina episode in <24 h | 1 | Anterior STEMI or LBBB | 1 | |
| ST-segment deviation | 1 | Diabetes, HTA, angina | 1 | |
| Elevated cardiac markers | 1 | Weight < 67 Kg | 1 | |
| Total points | 0–7 | Time to treat > 4 h | 1 | |
| Total points | 0–14 | |||
| Zwolle Risk Score | ||
|---|---|---|
| Score | ||
| Killip class | 1 | +0 |
| 2 | +4 | |
| 3–4 | +9 | |
| Final TIMI | 0–1 | +2 |
| 2 | +1 | |
| 3 | +0 | |
| Age | ≥60 | +2 |
| 3v CAD | NO | +0 |
| YES | +1 | |
| Anterior AMI | NO | +0 |
| YES | +1 | |
| >4 h ischemia | NO | +0 |
| YES | +1 | |
| Low risk: 0–3 points High risk: ≥ 4 points | ||
| Low-Risk Profile Criteria in ACS |
|---|
| Age < 80 years KK-I at admission No CPR or NIMV Successful uncomplicated PCI with TIMI3 flow without significant residual stenosis (>90%) Absence of lesion in LMCA or 3v CAD No VT after 24 h from PCI LVEF ≥ 50% Hb > 11 g/dL at admission Glycemia < 270 mg/dL (≈15 mmol/L) eGFR > 60 mL/min/1.73 m2 Good social situation: close family support |
| CADILLAC ACS Risk Score | ||
|---|---|---|
| Points | Patients are stratified into three risk groups that predict 30-day and 1-year mortality: | |
| LVEF < 40% | 4 | |
| Renal failure | 3 | |
| Killip class 2 or 3 | 3 | |
| Final TIMI < 3 | 2 | Low risk: 0–2 points Up to 0.2% mortality at 30 days and up to 0–9% at 1 year Intermediate risk: 3–5 points Up to 1.9% mortality at 30 days and up to 4.5% at 1 year High risk: ≥6 points Up to 8.1% mortality at 30 days and up to 13.2% at 1 year |
| Age > 65 years | 2 | |
| Anemia | 2 | |
| 3-vessel CAD | 2 | |
| Total points | 0–14 | |
| ACUITY-PCI Risk Score (NSTEMI) | ||
|---|---|---|
| Points | ||
| Insulin-treated diabetes | 12 | |
| Renal insufficiency | 12 | |
| Troponin elevation or ST-segment deviation | 8 | |
| Bifurcation lesion | 4 | |
| Small-vessel/diffuse CAD | 2 | |
| Extent of CAD (1 point for each 10 mm of disease) | 1 | |
| Low-Risk Criteria in STEMI (ESC) |
|---|
| LVEF > 40% by TTE or ventriculography. Successful primary angioplasty (TIMI 3). Absence of pending revascularization (surgical or percutaneous). Absence of persistent ischemia symptoms. Absence of HF (Killip–Kimball I). Absence of significant arrhythmias. Absence of hemodynamic instability. Absence of significant comorbidities. Circumstances of mobility and acceptable socio-family support. |
| Low-Risk Profile in STEMI |
|---|
| <65 years Complete or incomplete revascularization in case of distal disease or small caliber LVEF > 45% KK-I classification Admission to CRH program or early review (<3 weeks) Absence of CPA Absence of acute stent thrombosis |
| GRACE 2007 [4] | TIMI 2000 [2,30] | ZWOLLE 2004 [21] | CADILLAC 2005 [30] | ACUITY 2012 [31] | Marco Del Castillo 2019 [32] | ESC Criteria 2019 [26] | Bauer 2022 [29] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L | Lungs | Absence of heart failure (KK I) | |||||||||
| A | Age and autonomy | <80 years with favorable social situation | |||||||||
| T | Final TIMI flow | Restored final TIMI flow | |||||||||
| E | Ejection fraction | ≥45% | |||||||||
| 2A | Absence of | Anterior akinesia | |||||||||
| Analysis abnormalities | Glycemia > 270 mg/dL | ||||||||||
| Hb < 11 g/dL | |||||||||||
| eGFR < 60 mL/min/1.73m2 | |||||||||||
| Troponin peak reached | |||||||||||
| C | Coronary complication | No complication related to PCI procedure | |||||||||
| S | Shock and support | NO shock, arrhythmia, arrest, support required | |||||||||
| Item | Threshold/Definition | Category | How to Verify | If NO |
|---|---|---|---|---|
| Clinical stability | Killip–Kimball I; no shock/NIMV/VT-VF | M | Clinical exam/monitoring | Not eligible |
| Final TIMI flow | TIMI 3 in culprit vessel | M | Cath report | Not eligible |
| Procedural complications | None (no-reflow sustained, perforation, acute ST, type 4a MI, major access) | M | Cath report | Not eligible |
| Planned procedure | No staged PCI/CABG | M | Team plan | Not eligible |
| LVEF | ≥45% | M | TTE ≤24 h | Not eligible |
| Renal function | eGFR > 60 mL/min/1.73 m2 | M | Same-day labs | Not eligible |
| Anterior wall | No anterior akinesia | C | TTE ≤24 h | Reassess 24 h |
| Labs stability | Hb ≥11 g/dL, glucose <270 mg/dL | C | Same-day labs | Correct/observe and re-check |
| Troponin trend | Peak reached (declining trend) | C | 6–12 h post-PCI or 2× at 3–6 h | Repeat and defer |
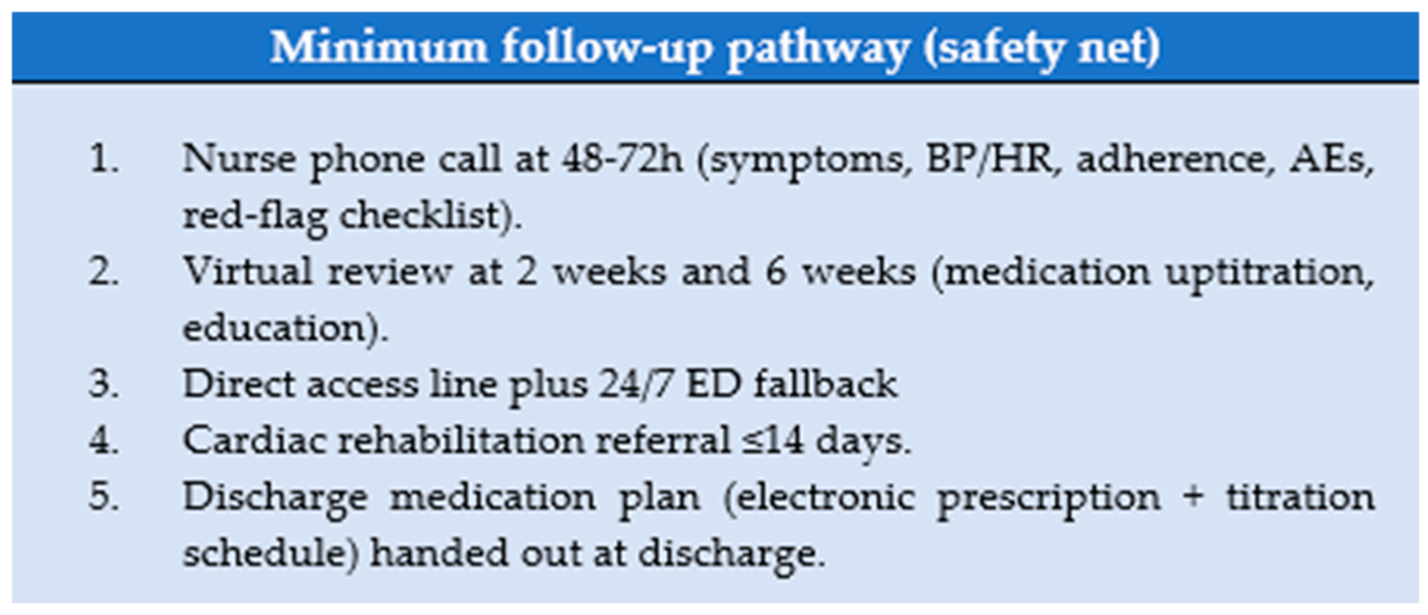
| Safety net | 48–72 h call; week-2 & week-6 reviews; CRH ≤ 14 d; 24/7 ED fallback | M-org | Appointments booked | Not eligible |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamhour-Chelh, K. A Novel Protocol for Feasibility and Safety in Early Discharge with ACS. J. Clin. Med. 2025, 14, 8373. https://doi.org/10.3390/jcm14238373
Jamhour-Chelh K. A Novel Protocol for Feasibility and Safety in Early Discharge with ACS. Journal of Clinical Medicine. 2025; 14(23):8373. https://doi.org/10.3390/jcm14238373
Chicago/Turabian StyleJamhour-Chelh, Karim. 2025. "A Novel Protocol for Feasibility and Safety in Early Discharge with ACS" Journal of Clinical Medicine 14, no. 23: 8373. https://doi.org/10.3390/jcm14238373
APA StyleJamhour-Chelh, K. (2025). A Novel Protocol for Feasibility and Safety in Early Discharge with ACS. Journal of Clinical Medicine, 14(23), 8373. https://doi.org/10.3390/jcm14238373







