High-Flow Nasal Oxygenation During Sedation for Transcatheter Aortic Valve Replacement: The HIGH-OXY-TAVR Randomised–Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Trial Design
2.2. Study Population
2.3. Randomisation and Blinding
2.4. Trial Intervention
2.5. Endpoints
2.6. Statistical Analysis
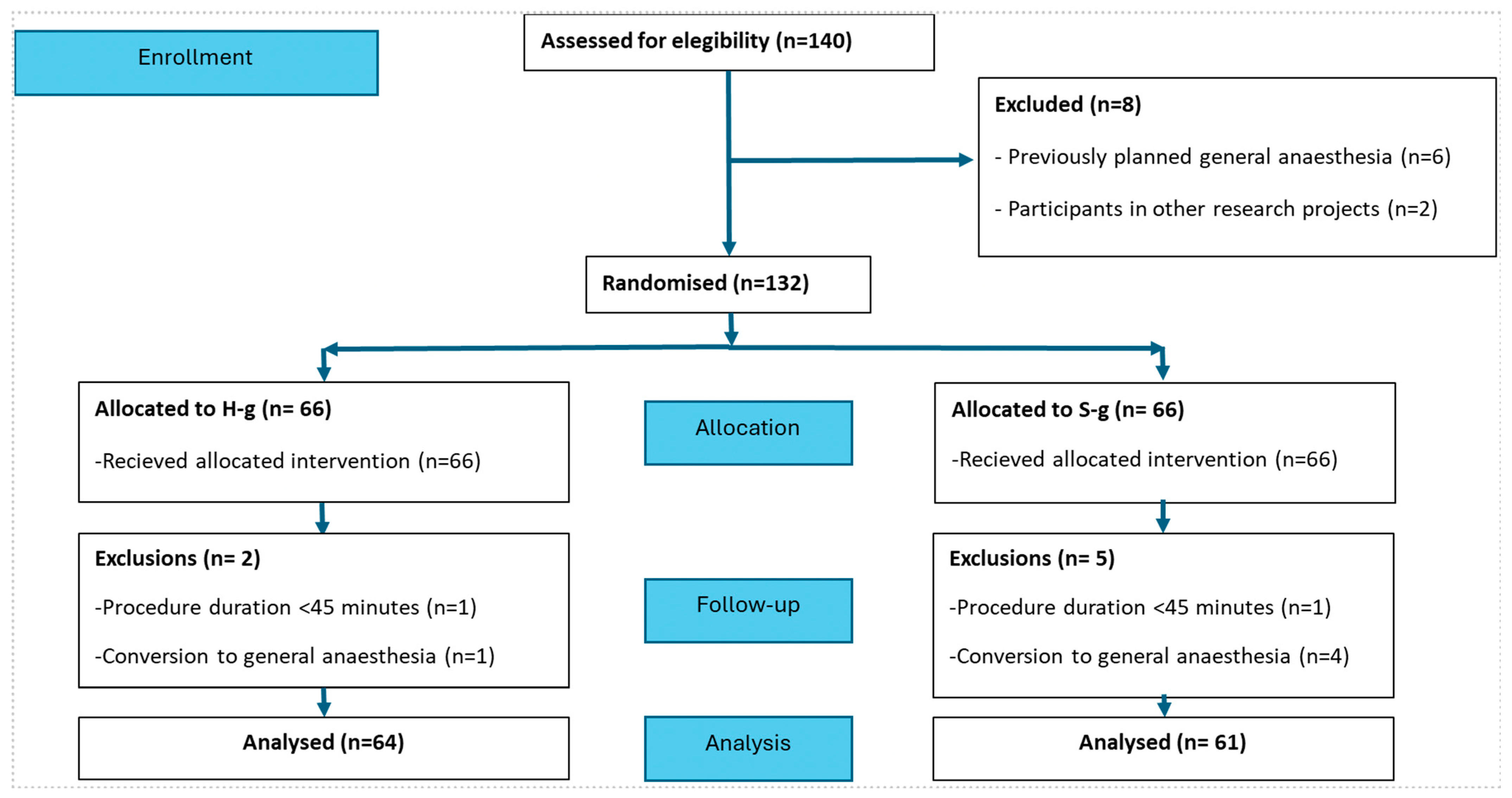
3. Results
3.1. Baseline Characteristics
3.2. Procedural Characteristics
3.3. Endpoints
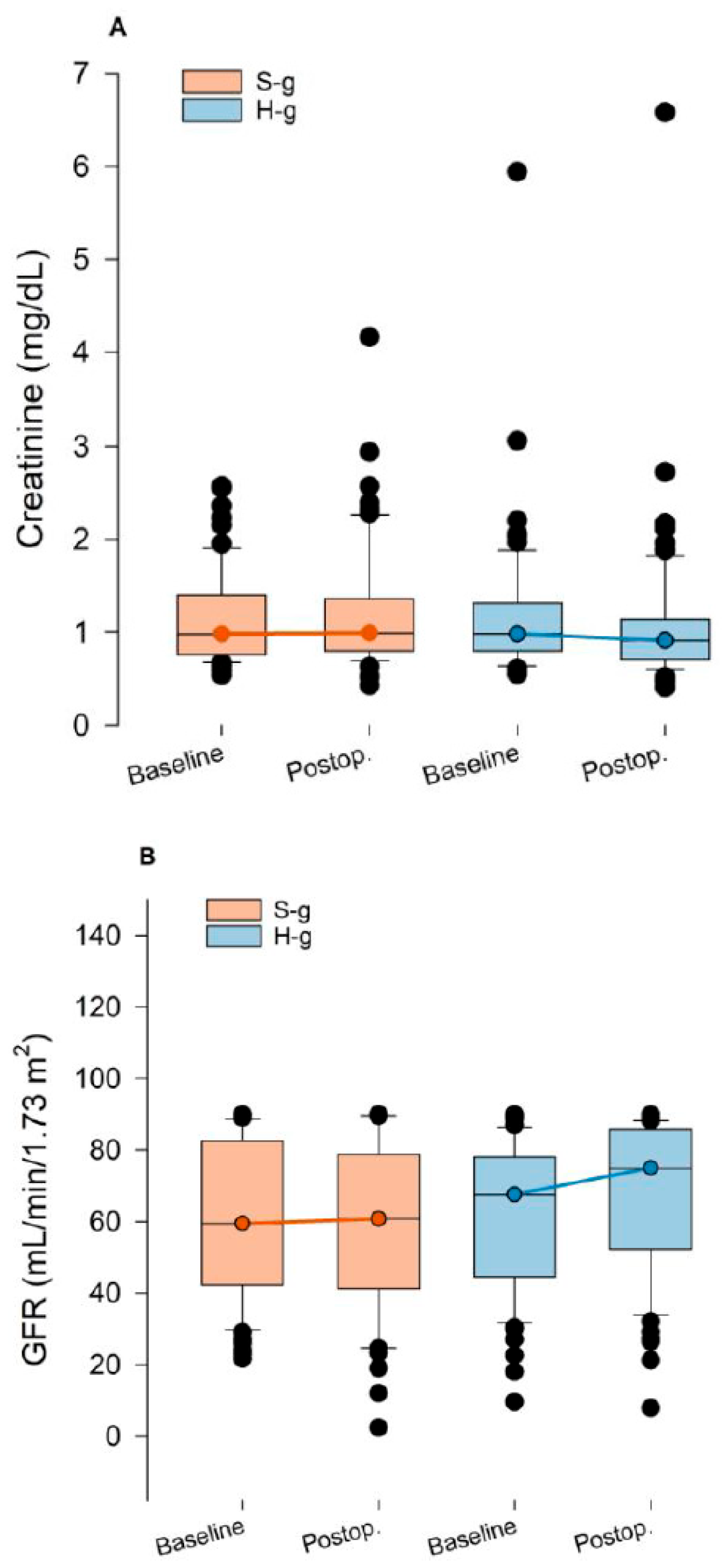
3.4. End-Organ Damage Biomarkers
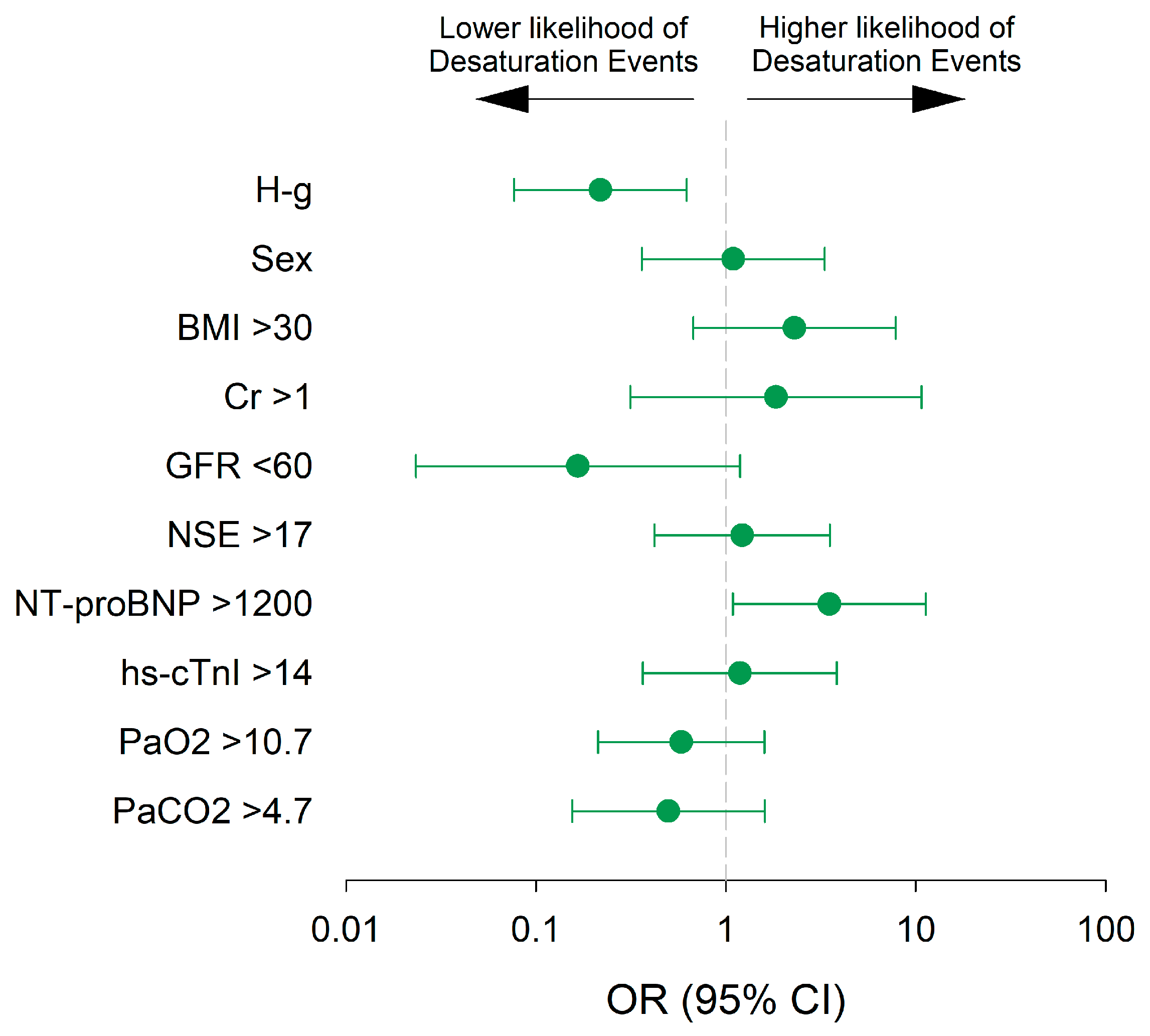
3.5. Predictors of Desaturation Episodes
3.6. Safety and Mortality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
References
- Miles, L.F.; Joshi, K.R.; Ogilvie, E.H.; Densem, C.G.; Klein, A.A.; O’Sullivan, M.; Martinez, G.; Sudarshan, C.D.; Abu-Omar, Y.; Irons, J.F. General anaesthesia vs. conscious sedation for transfemoral aortic valve implantation: A single UK centre before-and-after study. Anaesthesia 2016, 71, 892–900. [Google Scholar] [CrossRef]
- Mayr, N.P.; Michel, J.; Bleiziffer, S.; Tassani, P.; Martin, K. Sedation or general anesthesia for transcatheter aortic valve implantation (TAVI). J. Thorac. Dis. 2015, 7, 1518–1526. [Google Scholar]
- Ehret, C.; Rossaint, R.; Foldenauer, A.C.; Stoppe, C.; Stevanovic, A.; Dohms, K.; Hein, M.; Schälte, G. Is local anaesthesia a favourable approach for transcatheter aortic valve implantation? A systematic review and meta-analysis comparing local and general anaesthesia. BMJ Open 2017, 7, e016321. [Google Scholar] [CrossRef] [PubMed]
- Coté, G.A.; Hovis, R.M.; Ansstas, M.A.; Waldbaum, L.; Azar, R.R.; Early, D.S.; Edmundowicz, S.A.; Mullady, D.K.; Jonnalagadda, S.S. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin. Gastroenterol. Hepatol. 2010, 8, 137–142. [Google Scholar] [CrossRef]
- van Schaik, E.P.C.; Blankman, P.; van Klei, W.A.; Knape, H.J.T.A.; Vaessen, P.H.H.B.; Braithwaite, S.A.; van Wolfswinkel, L.; Schellekens, W.M. Hypoxemia during procedural sedation in adult patients: A retrospective observational study. Can. J. Anaesth. 2021, 68, 1349. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, M.A.; Rocio Lopez, A.; Dumot, J.A.; Vargo, J.J. Risk factors for hypoxemia during ambulatory gastrointestinal endoscopy in ASA I-II patients. Dig. Dis. Sci. 2009, 54, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Trauzeddel, R.F.; Nordine, M.; Balanika, M.; Bence, J.; Bouchez, S.; Ender, J.; Erb, J.M.; Fassl, J.; Fletcher, N.; Mukherjee, C.; et al. Current anesthetic care of patients undergoing transcatheter aortic valve replacement in Europe: Results of an online survey. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1737–1746. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ling, L.C.; Cardosa, M.S.; Wong, A.K.H.; Wong, N.W. Hypoxia during upper gastrointestinal endoscopy with and without sedation and the effect of pre-oxygenation on oxygen saturation. Anaesthesia 2000, 55, 654–658. [Google Scholar] [CrossRef]
- Wagstaff, T.A.J.; Soni, N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia 2007, 62, 492–503. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, W.; Zhang, H.; Wu, C.; Ding, J.; Shen, M. Is humidified better than non-humidified low-flow oxygen therapy? A systematic review and meta-analysis. J. Adv. Nurs. 2017, 73, 2522–2533. [Google Scholar] [CrossRef]
- Ischaki, E.; Pantazopoulos, I.; Zakynthinos, S. Nasal high flow therapy: A novel treatment rather than a more expensive oxygen device. Eur. Respir. Rev. 2017, 26, 170028. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Respir. Care 2016, 61, 529–541. [Google Scholar] [CrossRef]
- Teng, W.N.; Ting, C.K.; Wang, Y.T.; Hou, M.C.; Chang, W.K.; Tsou, M.Y.; Chiang, H.; Lin, C.L. High-Flow Nasal Cannula and Mandibular Advancement Bite Block Decrease Hypoxic Events during Sedative Esophagogastroduodenoscopy: A Randomized Clinical Trial. Biomed. Res. Int. 2019, 2019, 4206795. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, X.; Li, L.; Wei, M.; Zhao, B.; Wang, X.; Pan, Z.; Tian, J.; Yu, W.; Su, D. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: A randomized multicenter clinical trial. Gastrointest. Endosc. 2019, 90, 591–601. [Google Scholar] [CrossRef]
- Hung, K.C.; Chang, Y.J.; Chen, I.W.; Soong, T.C.; Ho, C.N.; Hsing, C.H.; Chu, C.-C.; Chen, J.-Y.; Sun, C.-K. Efficacy of high flow nasal oxygenation against hypoxemia in sedated patients receiving gastrointestinal endoscopic procedures: A systematic review and meta-analysis. J. Clin. Anesth. 2022, 77, 110651. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, S.; Tan, A.; Govender, P.; Mckie, M.; Pack, J.; Martinez, G.; Falter, F.; George, S.; AKlein, A. High-flow nasal oxygen vs. standard oxygen therapy for patients undergoing transcatheter aortic valve replacement with conscious sedation: A randomised controlled trial. Perioper. Med. 2023, 12, 11. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; McKenzie, J.; O’Sullivan, C.; Havryk, A.P. High-flow nasal oxygen versus standard oxygen during flexible bronchoscopy in lung transplant patients: A randomized controlled trial. J. Bronchol. Interv. Pulmonol. 2020, 27, 259–265. [Google Scholar] [CrossRef]
- Lucangelo, U.; Vassallo, F.G.; Marras, E.; Ferluga, M.; Beziza, E.; Comuzzi, L.; Berlot, G.; Zin, W.A. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit. Care Res. Prac. 2012, 2012, 506382. [Google Scholar] [CrossRef]
- Mazzeffi, M.A.; Petrick, K.M.; Magder, L.; Greenwald, B.D.; Darwin, P.; Goldberg, E.M.; Bigeleisen, P.; Chow, J.H.; Anders, M.; Boyd, C.M.; et al. High-flow nasal cannula oxygen in patients having anesthesia for advanced esophagogastroduodenoscopy: HIFLOW-ENDO, a randomized clinical trial. Anesth. Analg. 2020, 132, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, N.; Thiruvenkatarajan, V.; Brown-Beresford, K.; Liu, W.M.; Gupta, D.; Van Wijk, R.M.; Ludbrook, G.L. Incidence and predictors of airway obstruction during high-flow nasal oxygen assisted procedural sedation during gastrointestinal interventions: A prospective observational study. J. Clin. Anesth. 2023, 89, 111160. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Greenwald, B.D.; Darwin, P.; Goldberg, E.M.; Bigeleisen, P.; Chow, J.H.; Anders, M.; Boyd, C.M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet. Infect. Dis. 2016, 3099, e276–e287. [Google Scholar] [CrossRef]
- Wetterslev, J.; Meyhoff, C.S.; Jorgensen, L.N. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. Cochrane Database Syst. Rev. 2015, 6, CD008884. [Google Scholar] [CrossRef]
- Cohen, C.; Schacham, Y.N.; Ruetzler, K.; Ahuja, S.; Yang, D.; Mascha, E.J.; Barclay, A.B.; Hung, M.H.; Sessler, D.I. Effect of intraoperative hyperoxia on the incidence of surgical site infections: A meta-analysis. Br. J. Anaesth. 2018, 120, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.; Xiong, J. The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease. Molecules 2022, 27, 7318. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Bragadottir, G.; Sellgren, J.; Swärd, K.; Ricksten, S.E. Acute renal failure is NOT an ‘acute renal success’—A clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit. Care Med. 2010, 38, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tanaka, T.; Nangaku, M. Hypoxia and Dysregulated Angiogenesis in Kidney Disease. Kidney Dis. 2015, 1, 80–89. [Google Scholar] [CrossRef]
- Barbash, I.M.; Ben-Dor, I.; Dvir, D.; Maluenda, G.; Xue, Z.; Torguson, R.; Satler, L.F.; Pichard, A.D.; Waksman, R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am. Heart J. 2012, 163, 1031–1036. [Google Scholar] [CrossRef]
- Julien, H.M.; Stebbins, A.; Vemulapalli, S.; Nathan, A.S.; Eneanya, N.D.; Groeneveld, P.; Fiorilli, P.N.; Herrmann, H.C.; Szeto, W.Y.; Desai, N.D.; et al. Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights from the Society of Thoracic Surgeons/American College of Cardiology National Cardiovascular Data Registry-Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2021, 14, e010032. [Google Scholar]
- Ye, C.; Ma, X.; Shi, B.; Yan, R.; Fu, S.; Wang, K.; Yan, R.; Jia, S.; Yang, S.; Cong, G. Acute kidney injury and in-hospital outcomes after transcatheter aortic valve replacement in patients without chronic kidney disease: Insights from the national inpatient sample. BMC Cardiovasc. Disord. 2024, 24, 706. [Google Scholar] [CrossRef]
- Matteucci, A.; Pandozo, C.; Bonanni, M.; Mariani, M.V.; Sgarra, L.; Nesti, L.; Pierucci, N.; Mirco La Fazia, V.; Lavalle, C.; Nardi, F.; et al. Impact of empagliflozin and dapagliflozin on sudden cardiac death: A systematic review and meta-analysis of adjudicated randomized evidence. Heart Rhythm. 2025, in press. [CrossRef]
- Raposeiras-Roubin, S.; Amat-Santos, I.J.; Rossello, X.; González Ferreiro, R.; González Bermúdez, I.; Lopez Otero, D.; Nombela-Franco, L.; Gheorghe, L.; Diez, J.L.; Baladrón Zorita, C.; et al. Dapagliflozin in patients undergoing transcatheter aortic-valve implantation. N. Engl. J. Med. 2025, 392, 1396–1405. [Google Scholar] [CrossRef]
- Shao, L.J.; Hong, F.X.; Liu, F.K.; Wan, L.; Xue, F.S. Prospective, randomized comparison of two supplemental oxygen methods during gastroscopy with propofol mono-sedation in obese patients. World J. Clin. Cases 2021, 9, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Han, D.; Wei, M.; Zhang, J.; Cheng, X.; Zhang, Y.; Shi, M.; Song, Z.; Wang, X.; et al. Effect of high flow nasal cannula oxygenation on incidence of hypoxia during sedated gastrointestinal endoscopy in patients with obesity: Multicentre randomised controlled trial. BMJ 2025, 388, e080795. [Google Scholar] [CrossRef] [PubMed]
- Saia, F.; Lauck, S.; Durand, E.; Muri, D.F.; Spence, M.; Vasa-Nicotera, M.; Wood, D.; Urbano-Carrillo, C.A.; Bouchayer, D.; Iliescu, V.A.; et al. The implementation of a streamlined TAVI patient pathway across five European countries: BENCHMARK registry. Clin. Res. Cardiol. 2025, in press. [CrossRef] [PubMed]
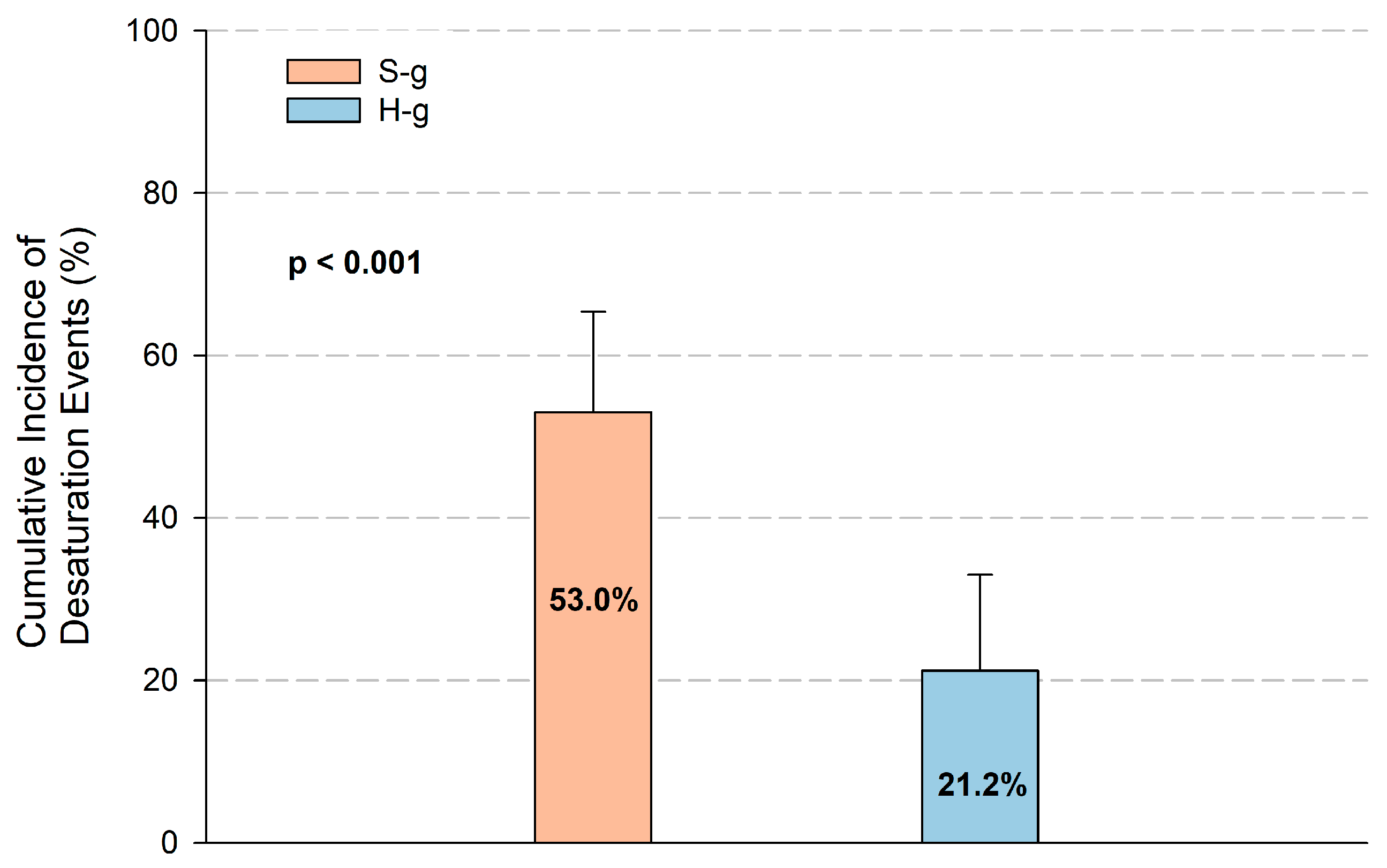
| Variable | H-g (n = 66) | S-g (n = 66) | SMD |
|---|---|---|---|
| Age (years), mean (SD) | 80.6 (5.2) | 81.5 (5.1) | 0.175 |
| Female sex, n (%) | 25 (38) | 25 (38) | <0.001 |
| Height (cm), mean (SD) | 164 (9.6) | 163 (9.6) | 0.061 |
| Weight (Kg), mean (SD) | 71.5 (12.0) | 71.5 (11.9) | 0.001 |
| BMI (Kg m−2), mean (SD) | 26.3 (3.9) | 27.1 (4.7) | 0.170 |
| Euroscore-II, median (IQR) | 2.7 (1.8–4.4) | 3.2 (1.9–5.2) | 0.247 |
| STS Score, median (IQR) | 3.6 (2.8–6.2) | 4.7 (2.7–7.7) | 0.291 |
| Hypertension, n (%) | 52 (79) | 52 (79) | <0.001 |
| Dyslipidaemia, n (%) | 40 (61) | 43 (65) | 0.094 |
| Diabetes, n (%) | 19 (29) | 17 (26) | 0.068 |
| Atrial fibrillation, n (%) | 18 (27) | 27 (41) | 0.291 |
| COPD, n (%) | 13 (20) | 10 (15) | 0.120 |
| CKD, n (%) | 30 (46) | 29 (44) | 0.030 |
| Previous myocardial ischemia, n (%) | 15 (23) | 15 (23) | <0.001 |
| Previous PCI, n (%) | 11 (17) | 8 (12) | 0.130 |
| PCI during TAVR, n (%) | 3 (5) | 5 (8) | 0.127 |
| pO2 (kPa), mean (SD) | 10.8 (1.9) | 10.8 (1.7) | 0.006 |
| pCO2 (kPa), mean (SD) | 4.8 (0.6) | 4.7 (0.6) | 0.211 |
| Aortic gradient (mmHg), mean (SD) | 47.7 (14.5) | 46.7 (14.2) | 0.073 |
| Left ventricle EF (%), mean (SD) | 56 (10.0) | 55 (10.8) | 0.155 |
| Variable | H-g n = 64 | S-g n = 61 | p-Value |
|---|---|---|---|
| BEV, n (%) | 17 (26.6) | 14 (23.0) | 0.253 |
| Sapien™ | 13 (20.3) | 8 (13.1) | |
| Myval™ | 4 (6.3) | 6 (9.8) | |
| SEV, n (%) | 47 (73.4) | 47 (77.1) | 0.253 |
| Evolut™ | 26 (40.6) | 25 (41.0) | |
| Navitor™ | 17 (26.6) | 17 (27.9) | |
| Acurate™ | 4 (6.3) | 5 (8.2) | |
| Pre-dilatation, n (%) | 52 (81.3) | 51 (83.6) | 0.729 |
| Post-dilatation, n (%) | 8 (12.5) | 8 (13.1) | 0.918 |
| Intervention time, min, median (IQR) | 53 (40–134) | 61 (43–142) | 0.089 |
| Sedation time, min, median (IQR) | 67.5 (46–144) | 76 (47–152) | 0.066 |
| Amount of iodinated contrast (mL) median (IQR) | 130 (45–320) | 135 (50–350) | 0.485 |
| Length of stay, days, median (IQR) | 4 (1–28) | 5 (2–85) | 0.085 |
| Pre-TAVR Procedure | Post-TAVR Procedure (8–12 h After) | |||||
|---|---|---|---|---|---|---|
|
H-g
n = 64 |
S-g
n = 61 | p -Value |
H-g
n = 64 |
S-g
n = 61 | p -Value | |
| Creatinine (mg dL−1) | 0.99 (0.55–5.94) | 0.98 (0.57–2.57) | 0.672 | 0.93 (0.41–6.41) | 0.99 (0.43–4.16) | 0.082 |
| Glomerular Filtration Rate (mL min−1 1.73 m−2) | 67.13 (9.6–90) | 57.94 (21.7–90) | 0.532 | 71.73 (7.84–90) | 59.66 (2.39–90) | 0.029 |
| NSE * (ng mL−1) | 17 (10–41) | 17 (9–36) | 0.716 | 22 (11–44) | 23 (12–40) | 0.517 |
| hs-cTnI † (ng L−1) | 57.17 (3–1425) | 83.98 (3–2858) | 0.964 | 840.6 (5–9118) | 945.3 (45–3746) | 0.722 |
| NT-proBNP ‡ (pg mL−1) | 4082.8 (80–47,886) | 3281.3 (60–18,012) | 0.767 | 1778 (206–41,240) | 1995 (64–31,917) | 0.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Milà, M.; Manzano-Valls, A.; Abdul-Jawad, O.; Arguis, M.J.; Brugaletta, S.; Carnaval, T.; Carretero, M.J.; Flores-Umanzor, E.; Freixa, X.; Ibañez, C.; et al. High-Flow Nasal Oxygenation During Sedation for Transcatheter Aortic Valve Replacement: The HIGH-OXY-TAVR Randomised–Controlled Trial. J. Clin. Med. 2025, 14, 8347. https://doi.org/10.3390/jcm14238347
Giménez-Milà M, Manzano-Valls A, Abdul-Jawad O, Arguis MJ, Brugaletta S, Carnaval T, Carretero MJ, Flores-Umanzor E, Freixa X, Ibañez C, et al. High-Flow Nasal Oxygenation During Sedation for Transcatheter Aortic Valve Replacement: The HIGH-OXY-TAVR Randomised–Controlled Trial. Journal of Clinical Medicine. 2025; 14(23):8347. https://doi.org/10.3390/jcm14238347
Chicago/Turabian StyleGiménez-Milà, Marc, Antoni Manzano-Valls, Omar Abdul-Jawad, María José Arguis, Salvatore Brugaletta, Thiago Carnaval, Maria José Carretero, Eduardo Flores-Umanzor, Xavier Freixa, Cristina Ibañez, and et al. 2025. "High-Flow Nasal Oxygenation During Sedation for Transcatheter Aortic Valve Replacement: The HIGH-OXY-TAVR Randomised–Controlled Trial" Journal of Clinical Medicine 14, no. 23: 8347. https://doi.org/10.3390/jcm14238347
APA StyleGiménez-Milà, M., Manzano-Valls, A., Abdul-Jawad, O., Arguis, M. J., Brugaletta, S., Carnaval, T., Carretero, M. J., Flores-Umanzor, E., Freixa, X., Ibañez, C., Italiano, S., López-Baamonde, M., Martínez-Otero, S., Matute, P., Pozo, M., Navarro-Ripoll, R., Perdomo, J. M., Regueiro, A., Rovira, I., ... Sabaté, M. (2025). High-Flow Nasal Oxygenation During Sedation for Transcatheter Aortic Valve Replacement: The HIGH-OXY-TAVR Randomised–Controlled Trial. Journal of Clinical Medicine, 14(23), 8347. https://doi.org/10.3390/jcm14238347






