Revealing Subtle Age-Related Balance Differences: Applying Stock Market Indicators to Posturographic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B2 | Regression line coefficient for the Phase II limit of stability test, indicating the speed of leaning forward |
| COP | Center of foot pressure |
| LOS | Limit of stability test |
| R1 | Limit of stability range calculated from the mean COP position |
| stdCOP | Standard deviation of COP position, |
| TCI | Trend change index |
| TCI [j] | Total number of trend changes during the whole test |
| TCI_per_s[j] | Trend change index per second |
| TCI_dS | Mean displacement between trend changes |
| TCI_dT | Mean time between trend changes |
| TCI_dV | Mean velocity between trend changes |
| vCOP | Velocity of COP |
References
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, 7–11. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. Postural control in the older adult. Clin. Geriatr. Med. 1996, 12, 635–658. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Baudry, S. Age-related changes in leg proprioception: Implications for postural control. J. Neurophysiol. 2019, 122, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Laughton, C.A.; Slavin, M.; Katdare, K.; Nolan, L.; Bean, J.F.; Kerrigan, D.C.; Phillips, E.; Lipsitz, L.A.; Collins, J.J. Aging, muscle activity, and balance control: Physiologic changes associated with balance impairment. Gait Posture 2003, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- De Blasiis, P.; Caravaggi, P.; Fullin, A.; Leardini, A.; Lucariello, A.; Perna, A.; Guerra, G.; De Luca, A. Postural stability and plantar pressure parameters in healthy subjects: Variability, correlation analysis and differences under open and closed eye conditions. Front. Bioeng. Biotechnol. 2023, 11, 1198120. [Google Scholar] [CrossRef]
- Ivanenko, Y.; Gurfinkel, V.S. Human postural control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Quijoux, F.; Nicolaï, A.; Chairi, I.; Bargiotas, I.; Ricard, D.; Yelnik, A.; Oudre, L.; Bertin-Hugault, F.; Vidal, P.; Vayatis, N.; et al. A review of center of pressure (COP) variables to quantify standing balance in elderly people: Algorithms and open-access code. Physiol. Rep. 2021, 9, e15067. [Google Scholar] [CrossRef]
- Słomka, K.J.; Michalska, J.; Marszałek, W.; Bacik, B.; Juras, G. Forward functional stability indicator (FFSI) as a reliable measure of limits of stability. MethodsX 2020, 7, 10–16. [Google Scholar] [CrossRef]
- Nolan, L.; Kerrigan, D.C. Postural control: Toe-standing versus heel-toe standing. Gait Posture 2004, 19, 11–15. [Google Scholar] [CrossRef]
- Bolgla, L.A.; Malone, T.R. Plantar Fasciitis and the Windlass Mechanism: A Biomechanical Link to Clinical Practice. J. Athl. Train. 2004, 39, 77–82. [Google Scholar]
- Williams, L.R.; Ridge, S.T.; Johnson, A.W.; Arch, E.S.; Bruening, D.A. The influence of the windlass mechanism on kinematic and kinetic foot joint coupling. J. Foot Ankle Res. 2022, 15, 16. [Google Scholar] [CrossRef]
- Rouhani, H.; Favre, J.; Crevoisier, X.; Aminian, K. Measurement of multi-segment foot joint angles during gait using a wearable system. J. Biomech. Eng. 2012, 134, 061006. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Allum, J.H.J.; Carpenter, M.G.; Honegger, F. Is lower leg proprioception essential for triggering human automatic postural responses? Exp. Brain Res. 2000, 130, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Vol, B. The stock market indexes in research on human balance. Acta Bioeng. Biomech. 2022, 24, 163–176. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R.; Stirling, L.A.; Lipsitz, L.A.; Qureshi, M.; Kelty-stephen, D.G.; Goldberger, A.L.; Costa, M.D.; Biol, J.G.A.; Med, S.; et al. Physiological time-series analysis using approximate entropy and sample entropy methods patterns Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Wodarski, P.; Id, M.C.; Szl, M.; Bajor, G.; Gzik, M.; Jurkoj, J. Trend change analysis in the assessment of body balance during posture adjustment in reaction to anterior-posterior ground perturbation. PLoS ONE 2024, 19, e0301227. [Google Scholar] [CrossRef]
- Wodarski, P.; Chmura, M.; Jurkojć, J. Impact of Visual Disturbances on the Trend Changes of COP Displacement Courses Using Stock Exchange Indices. Appl. Sci. 2024, 14, 4953. [Google Scholar] [CrossRef]
- Winter, D.A. Human balance and posture standing and walking control during. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Duarte, M.; Zatsiorsky, V.M. Effects of body lean and visual information on the equilibrium maintenance during stance. Exp. Brain Res. 2002, 146, 60–69. [Google Scholar] [CrossRef]
- Chiari, L.; Rocchi, L.; Cappello, A. Stabilometric parameters are affected by anthropometry and foot placement. Clin. Biomech. 2002, 17, 666–677. [Google Scholar] [CrossRef]
- Wodarski, P. Trend Change Analysis as a New Tool to Complement the Evaluation of Human Body Balance in the Time and Frequency Domains. J. Hum. Kinet. 2023, 88, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.B.; Rodacki, A.L.F.; Pereira, G.; Bento, P.C.B. Does functional capacity, fall risk awareness and physical activity level predict falls in older adults in different age groups? Arch. Gerontol. Geriatr. 2018, 77, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, H.; Maeo, S.; Kusagawa, Y.; Ono, M.; Watanabe, K.; Isaka, T. Plantar intrinsic foot muscle activity and its relationship with postural sway during tiptoe standing in ballet dancers and non-dancers. Gait Posture 2024, 108, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mockford, K.A.; Vanicek, N.; Jordan, A.; Chetter, I.C.; Coughlin, P.A. Kinematic adaptations to ischemic pain in claudicants during continuous walking. Gait Posture 2010, 32, 395–399. [Google Scholar] [CrossRef]
- Wodarski, P.; Jurkojć, J.; Chmura, M.; Warmerdam, E.; Romijnders, R.; Hobert, M.A.; Maetzler, W.; Cygoń, K.; Hansen, C. Trend change analysis of postural balance in Parkinson’s disease discriminates between medication state. J. Neuroeng. Rehabil. 2024, 21, 112. [Google Scholar] [CrossRef]
- Wodarski, P.; Jurkojć, J.; Michalska, J.; Kamieniarz, A.; Juras, G.; Gzik, M. Balance assessment in selected stages of Parkinson’s disease using trend change analysis. J. Neuroeng. Rehabil. 2023, 20, 99. [Google Scholar] [CrossRef]
- Zelik, K.E.; La Scaleia, V.; Ivanenko, Y.P.; Lacquaniti, F. Coordination of intrinsic and extrinsic foot muscles during walking. Eur. J. Appl. Physiol. 2015, 115, 691–701. [Google Scholar] [CrossRef]
- Ferrazzoli, D.; Fasano, A.; Maestri, R.; Bera, R.; Palamara, G.; Ghilardi, M.F.; Pezzoli, G.; Frazzitta, G. Balance dysfunction in Parkinson’s disease: The role of posturography in developing a rehabilitation program. Parkinsons Dis. 2015, 2015, 520128. [Google Scholar] [CrossRef]
- Whitney, S.L.; Marchetti, G.F.; Schade, A.I. The relationship between falls history and computerized dynamic posturography in persons with balance and vestibular disorders. Arch. Phys. Med. Rehabil. 2006, 87, 402–407. [Google Scholar] [CrossRef]
- Spink, M.J.; Fotoohabadi, M.R.; Wee, E.; Hill, K.D.; Lord, S.R.; Menz, H.B. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch. Phys. Med. Rehabil. 2011, 92, 68–75. [Google Scholar] [CrossRef]
- Judge, J.O. Balance training to maintain mobility and prevent disability. Am. J. Prev. Med. 2003, 25, 150–156. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Goodman, S.; Zatsiorsky, V.; Latash, M.L. Muscle synergies during shifts of the center of pressure by standing persons: Identification of muscle modes. Biol. Cybern. 2003, 89, 152–161. [Google Scholar] [CrossRef]
- Trabassi, D.; Castiglia, S.F.; Bini, F.; Marinozzi, F.; Ajoudani, A.; Lorenzini, M.; Chini, G.; Varrecchia, T.; Ranavolo, A.; De Icco, R.; et al. Optimizing Rare Disease Gait Classification through Data Balancing and Generative AI: Insights from Hereditary Cerebellar Ataxia. Sensors 2024, 24, 3613. [Google Scholar] [CrossRef]

| Young Adults (n = 20) | Older Adults (n = 24) | |
|---|---|---|
| age [years] | 20 ± 2 | 65 ± 5 * |
| height [cm] | 174.55 ± 10.90 | 163.17 ± 6.60 * |
| weight [kg] | 70.83 ± 19.91 | 71.86 ± 11.40 |
| foot lenght [cm] | 24.50 ± 1.79 | 25 ± 1.45 |
| metatarsal length [cm] | 13.26 ± 1.63 | 12.61 ± 0.80 |
| big toe length [cm] | 5.2 ± 0.7 | 6.98 ± 0.77 * |
| stability margin of LOS [%] | 12.39 ± 12.55 | 18.20 ± 15.08 |
| stability margin of Tiptoe Rising [%] | 93.17 ± 10.26 | 90.7 ± 8.72 |
| Variable | Phase | Young Adults M ± SD Mdn (Min–Max) | Older Adults M ± SD Mdn (Min–Max) | T/Z p Value | Cohen’s d /r_pb |
|---|---|---|---|---|---|
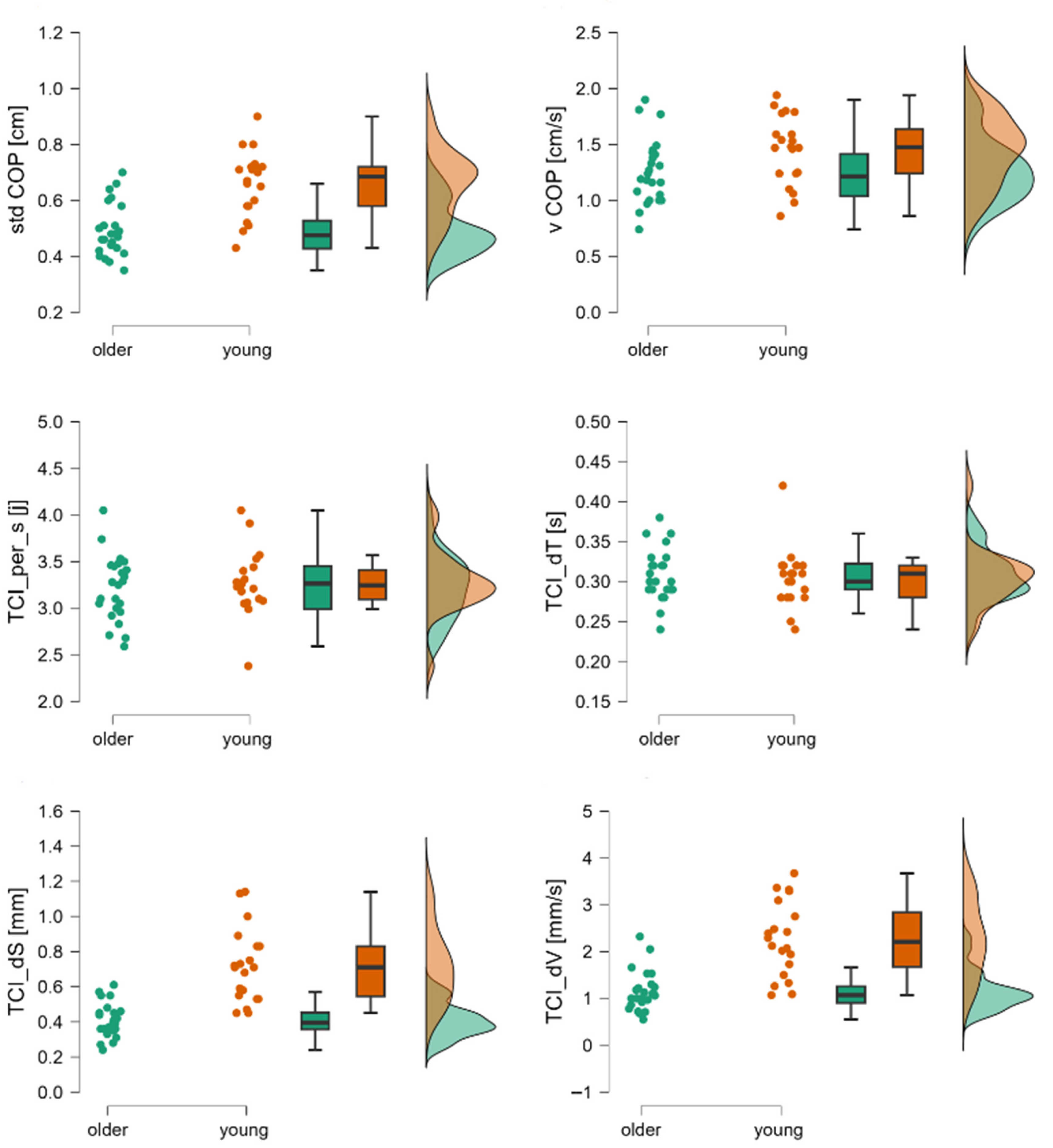
| stdCOP [cm] | 1st | 0.47 (0.19–0.77) | 0.45 (0.26–0.89) | Z = 0.72; p = 0.48 | 0.13 |
| vCOP [cm/s] | 1.15 (0.52–1.69) | 1.28 (0.72–2.30) | Z = −1.24; p = 0.22 | 0.23 | |
| TCI [j] | 34.67 (31.33–45.33) | 35.33 (26–43.33) | Z = −0.45; p = 0.65 | 0.08 | |
| TCI per s [j] | 36.6 ± 0.27 | 3.27 ± 0.40 | t = 0.85; p = 0.40 | −0.26 | |
| TCI dS [mm] | 0.42 ± 0.18 | 0.37 ± 0.12 | t = 1.18; p > 0.24 | −0.35 | |
| TCI dT [s] | 0.29 ± 0.02 | 0.3 ± 0.04 | t = −1.15; p > 0.26 | 0.32 | |
| TCI dV [cm/s] | 1.3 ± 0.61 | 0.96 ± 0.32 | t = 2.35; p < 0.02 | −0.79 | |
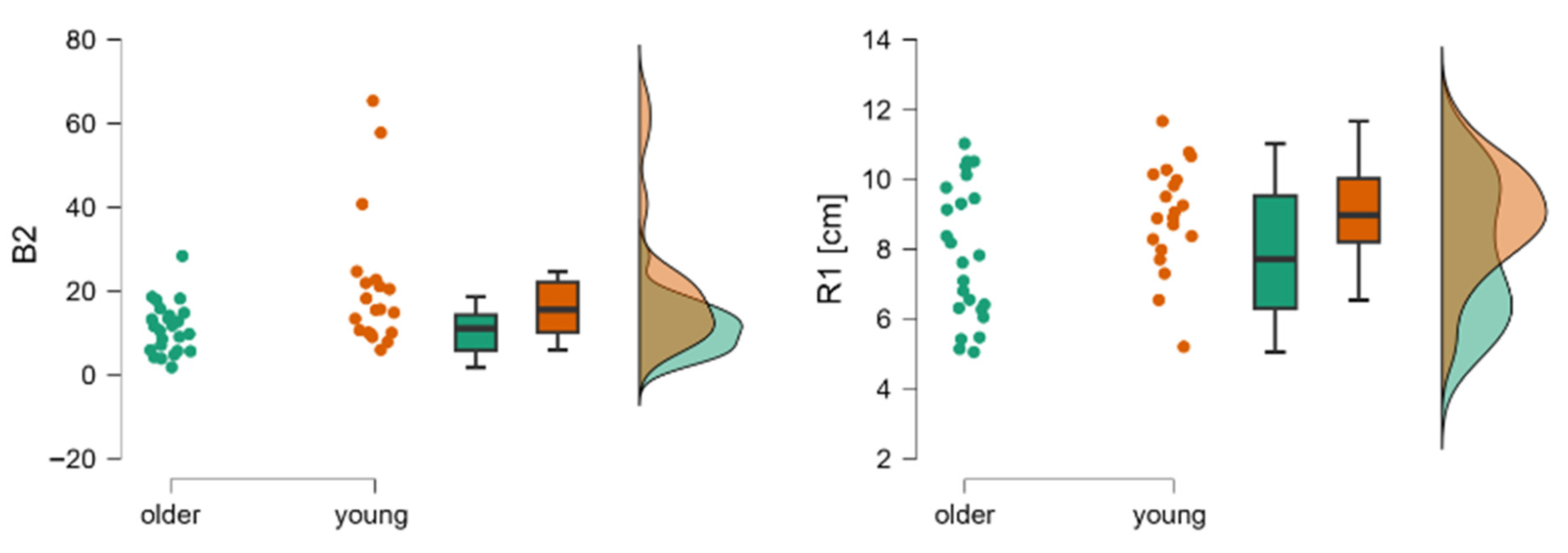
| R1 [cm] | 2nd | 7.51 ± 1.83 | 5.61 ± 184 | t = 3.20; p < 0.00 | −0.96 |
| B2 | 16.39 (4.65–33.66) | 7.53 (2.70–24.83) | Z = 3.43; p < 0.00 | −1.04 | |
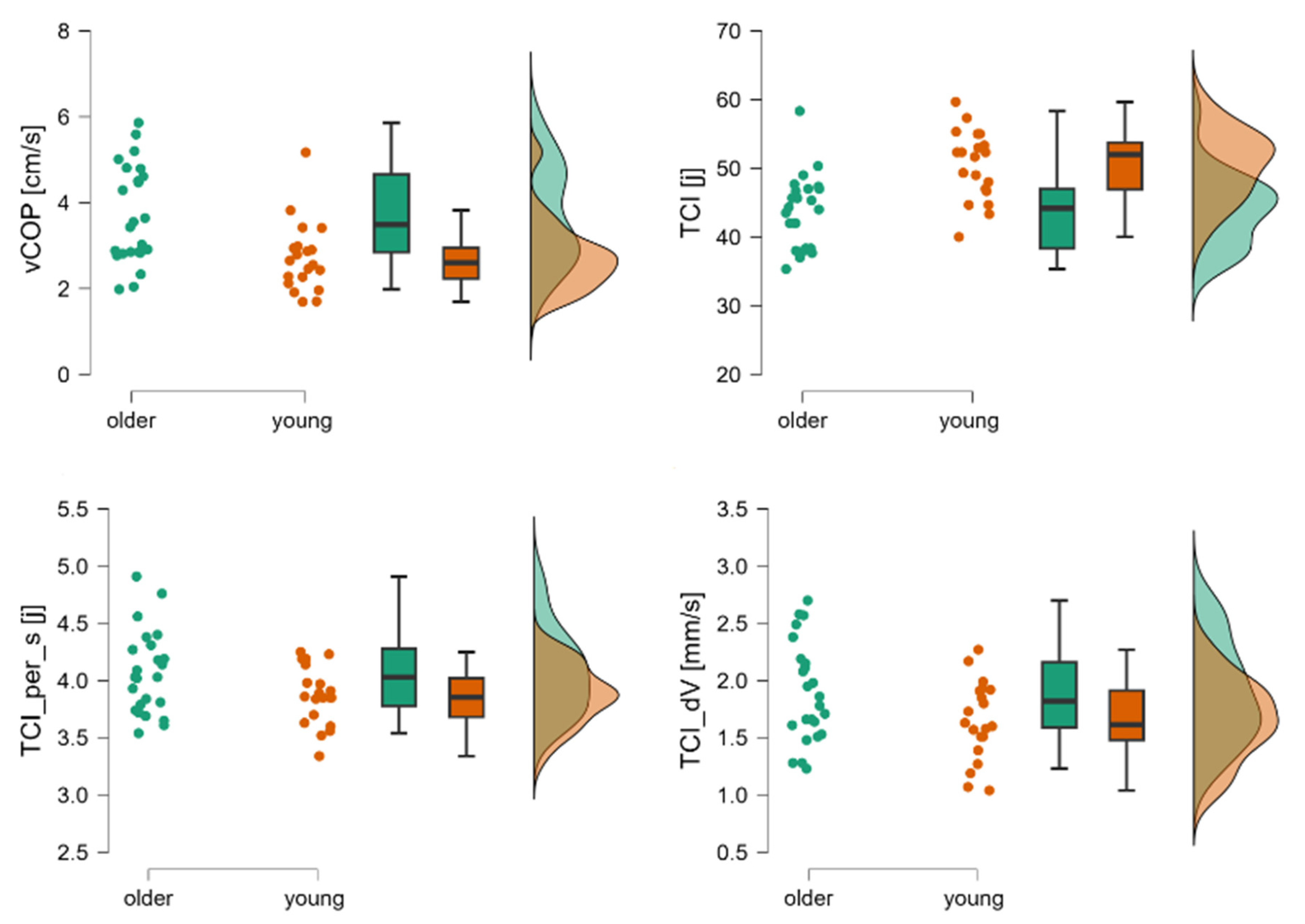
| stdCOP [cm] | 3rd | 0.56 (0.36–0.86) | 0.57 (0.28–1.31) | Z = −0.39; p = 0.69 | 0.07 |
| vCOP [cm/s] | 1.70 (0.90–2.58) | 1.57 (0.81–3.86) | Z = 0.44; p = 0.65 | 0.07 | |
| TCI [j] | 48.08 (40–66.33) | 44.67 (35–57.67) | Z = 2.49; p < 0.01 | 0.44 | |
| TCI per s [j] | 4.02 ± 0.59 | 3.68 ± 0.40 | t = 2.25; p < 0.05 | −0.68 | |
| TCI dS [mm] | 0.4 ± 0.11 | 0.40 ± 0.14 | t = −1.98; p = 0.16 | −0.02 | |
| TCI d T [s] | 0.25 ± 0.04 | 0.27 ± 0.03 | t = 0.05; p = 0.06 | 0.06 | |
| TCI dV [cm/s] | 1.26 ± 0.32 | 1.22 ±0.40 | t = 0.38; p < 0.05 | −0.11 |
| Variable | Phase | Young Adults M ± SD Mdn (Min–Max) | Older Adults M ± SD Mdn (Min–Max) | T/Z p Value | Cohen’s d /r_pb |
|---|---|---|---|---|---|
| stdCOP [cm] | 1st | 0.66 ± 012 | 0.49 ± 0.09 | t = −5.28; p < 0.001 | −1.60 |
| vCOP [cm/s] | 1.45 ± 0.31 | 1.26 ± 0.29 | t = −2.14; p = 0.039 | −0.65 | |
| TCI [j] | 33.77 ± 3.74 | 38.86 ±5.20 | t = 3.66; p <0.001 | 1.11 | |
| TCI per s [j] | 3.28 ± 0.35 | 3.21 ± 0.35 | t = −059; p = 0.56 | −0.18 | |
| TCI dS [mm] | 0.71 ± 0.21 | 0.41 ± 0.10 | t −6.44; p < 0.001 | −1.95 | |
| TCI d T [s] | 0.31 (0.24–0.42) | 0.30 (0.25–0.38) | Z = −2.34; p = 0.002 | 0.07 | |
| TCI dV [cm/s] | 2.21 (1.07–3.67) | 1.07 (0.55–2.32) | Z = 4.47; p < 0.001 | 0.80 | |
| R1 [cm] | 2nd | 8.95 ± 1.54 | 7.86 ± 1.94 | t = −2.02; p = 0.049 | −0.61 |
| B2 | 15.53 (5.94–65.38) | 11.05 (1.78–28.35) | Z = −2.58; p < 0.001 | 0.46 | |
| stdCOP [cm] | 3rd | 0.67 ± 0.14 | 070 ± 0.15 | t = 0.86; p = 0.40 | 0.26 |
| vCOP [cm/s] | 2.60 (1.69–5.17) | 3.49 (1.98–5.86) | Z = −2.91; p = 0.003 | 0.52 | |
| TCI [j] | 50.50 ± 5.60 | 43.77 ± 5.27 | t = −4.29; p < 0.001 | −1.3 | |
| TCI per s [j] | 3.87 ± 0.26 | 4.07 ± 0.36 | t = 2.06; p = 0.05 | 0.62 | |
| TCI dS [mm] | 0.54 ± 0.12 | 0.60 ± 0.15 | t = 1.43; p = 0.16 | 0.43 | |
| TCI d T [s] | 0.26 ± 0.02 | 0.25 ± 0.02 | t = −1.90; p = 0.06 | −0.58 | |
| TCI dV [cm/s] | 1.65 ± 0.34 | 1.89 ± 0.44 | t = 2.04; p = 0.05 | 0.62 |
| Test | Phase | Cohen’s d COP | Cohen’s d TCI | Cohen Δd |
|---|---|---|---|---|
| LOS | 1 st | −0.23 | −0.20 | +0.03 |
| 2 nd | −0.21 | −0.15 | +0.07 | |
| TIPTOE | 1 st | −1.12 | −0.53 | +0.59 |
| 2 nd | 0.62 | 0.04 | −0.579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska, J.; Wodarski, P.; Jurkojć, J.; Słomka, K.J. Revealing Subtle Age-Related Balance Differences: Applying Stock Market Indicators to Posturographic Analysis. J. Clin. Med. 2025, 14, 8346. https://doi.org/10.3390/jcm14238346
Michalska J, Wodarski P, Jurkojć J, Słomka KJ. Revealing Subtle Age-Related Balance Differences: Applying Stock Market Indicators to Posturographic Analysis. Journal of Clinical Medicine. 2025; 14(23):8346. https://doi.org/10.3390/jcm14238346
Chicago/Turabian StyleMichalska, Justyna, Piotr Wodarski, Jacek Jurkojć, and Kajetan J. Słomka. 2025. "Revealing Subtle Age-Related Balance Differences: Applying Stock Market Indicators to Posturographic Analysis" Journal of Clinical Medicine 14, no. 23: 8346. https://doi.org/10.3390/jcm14238346
APA StyleMichalska, J., Wodarski, P., Jurkojć, J., & Słomka, K. J. (2025). Revealing Subtle Age-Related Balance Differences: Applying Stock Market Indicators to Posturographic Analysis. Journal of Clinical Medicine, 14(23), 8346. https://doi.org/10.3390/jcm14238346








