Advancing Precision Medicine in Degenerative Cervical Myelopathy
Abstract
1. Introduction
2. Methodology
3. Epidemiology and Risk Factors
4. Pathophysiology of DCM
5. Clinical Presentation and Diagnosis
6. Disease Severity Classification and Natural History
7. Current Management Challenges and Predictive Tools
8. Conventional Prognostic Factors
9. Advanced Imaging Markers in DCM
10. Machine Learning and Predictive Models in DCM
Current Model Limitations
11. Discussion
11.1. Unmet Clinical Needs
11.2. Controversy in the Timing of Mild DCM Intervention
11.3. Barriers to the Development of Novel Tools
11.4. Evolution in DCM Management
11.5. Limitations
12. Future Direction
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC | Area Under the Curve |
| CSA | Cross-Sectional Area |
| CSORN | Canadian Spine Outcomes and Research Network |
| CT | Computed Tomography |
| DCM | Degenerative Cervical Myelopathy |
| DTI | Diffusion Tensor Imaging |
| EMG | Electromyography |
| FA | Fractional Anisotropy |
| MD | Mean Diffusivity |
| MEPs | Motor Evoked Potentials |
| mJOA | Modified Japanese Orthopaedic Association |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| MT | Magnetization Transfer |
| MTR | Magnetization Transfer Ratio |
| MWI | Myelin Water Imaging |
| OPLL | Ossification of the Posterior Longitudinal Ligament |
| qMRI | Quantitative MRI |
| SF-36 | 36-Item Short Form Health Survey |
| SSEPs | Somatosensory Evoked Potentials |
| T1w | T1-Weighted |
| T2w | T2-Weighted |
References
- Davies, B.M.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative Cervical Myelopathy. BMJ 2018, 360, k186. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tetreault, L.; Singh, A.; Karadimas, S.K.; Fehlings, M.G. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine 2015, 40, E675–E693. [Google Scholar] [CrossRef]
- Thompson, K.; Travers, H.; Ngan, A.; Reed, T.; Shahsavarani, S.; Verma, R.B. Updates in Current Concepts in Degenerative Cervical Myelopathy: A Systematic Review. J. Spine Surg. 2024, 10, 313–326. [Google Scholar] [CrossRef]
- Wu, J.-C.; Ko, C.-C.; Yen, Y.-S.; Huang, W.-C.; Chen, Y.-C.; Liu, L.; Tu, T.-H.; Lo, S.-S.; Cheng, H. Epidemiology of Cervical Spondylotic Myelopathy and Its Risk of Causing Spinal Cord Injury: A National Cohort Study. Neurosurg. Focus 2013, 35, E10. [Google Scholar] [CrossRef]
- Nurick, S. The Pathogenesis of the Spinal Cord Disorder Associated with Cervical Spondylosis. Brain 1972, 95, 87–100. [Google Scholar] [CrossRef]
- Tetreault, L.A.; Karadimas, S.; Wilson, J.R.; Arnold, P.M.; Kurpad, S.; Dettori, J.R.; Fehlings, M.G. The Natural History of Degenerative Cervical Myelopathy and the Rate of Hospitalization Following Spinal Cord Injury: An Updated Systematic Review. Glob. Spine J. 2017, 7, 28S–34S. [Google Scholar] [CrossRef]
- Smith, S.S.; Stewart, M.E.; Davies, B.M.; Kotter, M.R.N. The Prevalence of Asymptomatic and Symptomatic Spinal Cord Compression on Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Glob. Spine J. 2021, 11, 597–607. [Google Scholar] [CrossRef]
- Tetreault, L.A.; Kopjar, B.; Vaccaro, A.; Yoon, S.T.; Arnold, P.M.; Massicotte, E.M.; Fehlings, M.G. A Clinical Prediction Model to Determine Outcomes in Patients with Cervical Spondylotic Myelopathy Undergoing Surgical Treatment: Data from the Prospective, Multi-Center AOSpine North America Study. J. Bone Jt. Surg. Am. 2013, 95, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.F.; Badhiwala, J.H.; Moghaddamjou, A.; Vaccaro, A.R.; Arnold, P.M.; Bartels, R.H.M.A.; Barbagallo, G.; Fehlings, M.G. Adverse Effects of Frailty on the Outcomes of Surgery for Degenerative Cervical Myelopathy: Results from a Prospective Multicenter International Data Set of 757 Patients. J. Neurosurg. Spine 2023, 39, 815–821. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj, V.G.; Gustafsson, M.R.V.; Fariña Nuñez, M.T.; Staartjes, V.E.; Edström, E.; Elmi-Terander, A. Return to Work after Surgery for Degenerative Cervical Myelopathy: Prospective Data from a Swedish Nationwide Cohort of 789 Patients. Neurosurgery 2025. [Google Scholar] [CrossRef]
- Wilson, J.R.; Patel, A.A.; Brodt, E.D.; Dettori, J.R.; Brodke, D.S.; Fehlings, M.G. Genetics and Heritability of Cervical Spondylotic Myelopathy and Ossification of the Posterior Longitudinal Ligament: Results of a Systematic Review. Spine 2013, 38, S123–S146. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, B.; Tetreault, L.; Kalsi-Ryan, S.; Fehlings, M. Psychometric Properties of the Modified Japanese Orthopaedic Association Scale in Patients with Cervical Spondylotic Myelopathy. Spine 2015, 40, E23–E28. [Google Scholar] [CrossRef] [PubMed]
- Puntumetakul, R.; Chatprem, T.; Saiklang, P.; Phadungkit, S.; Kamruecha, W.; Sae-Jung, S. Prevalence and Associated Factors of Clinical Myelopathy Signs in Smartphone-Using University Students with Neck Pain. Int. J. Environ. Res. Public Health 2022, 19, 4890. [Google Scholar] [CrossRef]
- Ariëns, G.A.; Bongers, P.M.; Douwes, M.; Miedema, M.C.; Hoogendoorn, W.E.; van der Wal, G.; Bouter, L.M.; van Mechelen, W. Are Neck Flexion, Neck Rotation, and Sitting at Work Risk Factors for Neck Pain? Results of a Prospective Cohort Study. Occup. Environ. Med. 2001, 58, 200–207. [Google Scholar] [CrossRef]
- Rajesh, N.; Moudgil-Joshi, J.; Kaliaperumal, C. Smoking and Degenerative Spinal Disease: A Systematic Review. Brain Spine 2022, 2, 100916. [Google Scholar] [CrossRef]
- Tetreault, L.A.; Karpova, A.; Fehlings, M.G. Predictors of Outcome in Patients with Degenerative Cervical Spondylotic Myelopathy Undergoing Surgical Treatment: Results of a Systematic Review. Eur. Spine J. 2015, 24 (Suppl. S2), 236–251. [Google Scholar] [CrossRef] [PubMed]
- Witiw, C.D.; Mathieu, F.; Nouri, A.; Fehlings, M.G. Clinico-Radiographic Discordance: An Evidence-Based Commentary on the Management of Degenerative Cervical Spinal Cord Compression in the Absence of Symptoms or with Only Mild Symptoms of Myelopathy. Glob. Spine J. 2018, 8, 527–534. [Google Scholar] [CrossRef]
- Phan, K.; Kim, J.S.; Somani, S.; Di Capua, J.; Kim, R.; Shin, J.; Cho, S.K. Impact of Age on 30-Day Complications After Adult Deformity Surgery. Spine 2018, 43, 120–126. [Google Scholar] [CrossRef]
- Takeshima, Y.; Okamoto, A.; Yokoyama, S.; Nishimura, F.; Nakagawa, I.; Park, Y.-S.; Nakase, H. Facet Articular Irregularity Is the Most Relevant Risk Factor for Rapidly Progressive Degenerative Cervical Myelopathy. Neurospine 2023, 20, 365–373. [Google Scholar] [CrossRef]
- Ames, C.P.; Blondel, B.; Scheer, J.K.; Schwab, F.J.; Le Huec, J.-C.; Massicotte, E.M.; Patel, A.A.; Traynelis, V.C.; Kim, H.J.; Shaffrey, C.I.; et al. Cervical Radiographical Alignment: Comprehensive Assessment Techniques and Potential Importance in Cervical Myelopathy. Spine 2013, 38, S149–S160. [Google Scholar] [CrossRef]
- Lin, T.; Shangguan, Z.; Xiao, Z.; Wu, R.; Zhao, Y.; Chen, D.; Zhou, L.; Wang, Z.; Liu, W. Whether the Potential Degree of Cervical Instability and Cervical Muscle Degeneration in Patients with Cervical Spondylosis Radicular Affect the Efficacy of Cervical Traction. Sci. Rep. 2024, 14, 20467. [Google Scholar] [CrossRef]
- Tu, J.; Vargas Castillo, J.; Das, A.; Diwan, A.D. Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms. J. Clin. Med. 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Baptiste, D.C.; Fehlings, M.G. Pathophysiology of Cervical Myelopathy. Spine J. 2006, 6, 190S–197S. [Google Scholar] [CrossRef] [PubMed]
- Buell, T.J.; Buchholz, A.L.; Quinn, J.C.; Shaffrey, C.I.; Smith, J.S. Importance of Sagittal Alignment of the Cervical Spine in the Management of Degenerative Cervical Myelopathy. Neurosurg. Clin. N. Am. 2018, 29, 69–82. [Google Scholar] [CrossRef]
- Nouri, A.; Martin, A.R.; Mikulis, D.; Fehlings, M.G. Magnetic Resonance Imaging Assessment of Degenerative Cervical Myelopathy: A Review of Structural Changes and Measurement Techniques. Neurosurg. Focus 2016, 40, E5. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Qian, M.; Qin, T.; Liu, M.; Xu, H.; Xu, B. Increased Expression of Inflammatory Cytokines and Discogenic Neck Pain. Orthop. Surg. 2024, 16, 227–233. [Google Scholar] [CrossRef]
- Zdunczyk, A.; Schwarzer, V.; Mikhailov, M.; Bagley, B.; Rosenstock, T.; Picht, T.; Vajkoczy, P. The Corticospinal Reserve Capacity: Reorganization of Motor Area and Excitability As a Novel Pathophysiological Concept in Cervical Myelopathy. Neurosurgery 2018, 83, 810–818. [Google Scholar] [CrossRef]
- Wang, C.; Laiwalla, A.; Salamon, N.; Ellingson, B.M.; Holly, L.T. Compensatory Brainstem Functional and Structural Connectivity in Patients with Degenerative Cervical Myelopathy by Probabilistic Tractography and Functional MRI. Brain Res. 2020, 1749, 147129. [Google Scholar] [CrossRef]
- Wang, C.; Ellingson, B.M.; Oughourlian, T.C.; Salamon, N.; Holly, L.T. Evolution of Brain Functional Plasticity Associated with Increasing Symptom Severity in Degenerative Cervical Myelopathy. eBioMedicine 2022, 84, 104255. [Google Scholar] [CrossRef]
- Lannon, M.; Kachur, E. Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History. J. Clin. Med. 2021, 10, 3626. [Google Scholar] [CrossRef]
- Balmaceno-Criss, M.; Singh, M.; Daher, M.; Buchbinder, R.; Diebo, B.G.; Daniels, A.H. Degenerative Cervical Myelopathy: History, Physical Examination, and Diagnosis. J. Clin. Med. 2024, 13, 7139. [Google Scholar] [CrossRef]
- Toledano, M.; Bartleson, J.D. Cervical Spondylotic Myelopathy. Neurol. Clin. 2013, 31, 287–305. [Google Scholar] [CrossRef]
- Ono, K.; Ebara, S.; Fuji, T.; Yonenobu, K.; Fujiwara, K.; Yamashita, K. Myelopathy Hand. New Clinical Signs of Cervical Cord Damage. J. Bone Jt. Surg. Br. 1987, 69, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.; Lenza, E.; Gupta, G.; Jarzem, P.; Dasgupta, K.; Radhakrishna, M. A Systematic Review of the Utility of the Hoffmann Sign for the Diagnosis of Degenerative Cervical Myelopathy. Spine 2018, 43, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Tejus, M.N.; Singh, V.; Ramesh, A.; Kumar, V.R.R.; Maurya, V.P.; Madhugiri, V.S. An Evaluation of the Finger Flexion, Hoffman’s and Plantar Reflexes as Markers of Cervical Spinal Cord Compression—A Comparative Clinical Study. Clin. Neurol. Neurosurg. 2015, 134, 12–16. [Google Scholar] [CrossRef]
- Tetreault, L.A.; Dettori, J.R.; Wilson, J.R.; Singh, A.; Nouri, A.; Fehlings, M.G.; Brodt, E.D.; Jacobs, W.B. Systematic Review of Magnetic Resonance Imaging Characteristics That Affect Treatment Decision Making and Predict Clinical Outcome in Patients with Cervical Spondylotic Myelopathy. Spine 2013, 38, S89–S110. [Google Scholar] [CrossRef]
- Chen, H.; Pan, J.; Nisar, M.; Zeng, H.B.; Dai, L.F.; Lou, C.; Zhu, S.P.; Dai, B.; Xiang, G.H. The Value of Preoperative Magnetic Resonance Imaging in Predicting Postoperative Recovery in Patients with Cervical Spondylosis Myelopathy: A Meta-Analysis. Clinics 2016, 71, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Höller, Y.; Brigo, F.; Frey, V.N.; Lochner, P.; Leis, S.; Golaszewski, S.; Trinka, E. The Contribution of Neurophysiology in the Diagnosis and Management of Cervical Spondylotic Myelopathy: A Review. Spinal Cord 2016, 54, 756–766. [Google Scholar] [CrossRef]
- Feng, X.; Hu, Y.; Ma, X. Progression Prediction of Mild Cervical Spondylotic Myelopathy by Somatosensory-Evoked Potentials. Spine 2020, 45, E560–E567. [Google Scholar] [CrossRef]
- Lanza, G.; Puglisi, V.; Vinciguerra, L.; Fisicaro, F.; Vagli, C.; Cantone, M.; Pennisi, G.; Pennisi, M.; Bella, R. TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy. Brain Sci. 2020, 10, 806. [Google Scholar] [CrossRef]
- Tetreault, L.; Nouri, A.; Kopjar, B.; Côté, P.; Fehlings, M.G. The Minimum Clinically Important Difference of the Modified Japanese Orthopaedic Association Scale in Patients with Degenerative Cervical Myelopathy. Spine 2015, 40, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Carette, S.; Chen, R.; et al. A Clinical Practice Guideline for the Management of Patients with Degenerative Cervical Myelopathy: Recommendations for Patients with Mild, Moderate, and Severe Disease and Nonmyelopathic Patients with Evidence of Cord Compression. Glob. Spine J. 2017, 7, 70S–83S. [Google Scholar] [CrossRef]
- Al-Shawwa, A.; Craig, M.; Ost, K.; Anderson, D.; Casha, S.; Jacobs, W.B.; Evaniew, N.; Tripathy, S.; Bouchard, J.; Lewkonia, P.; et al. Spinal Cord Demyelination Predicts Neurological Deterioration in Patients with Mild Degenerative Cervical Myelopathy. BMJ Neurol. Open 2025, 7, e000940. [Google Scholar] [CrossRef]
- Clarke, E.; Robinson, P.K. Cervical Myelopathy: A Complication of Cervical Spondylosis. Brain 1956, 79, 483–510. [Google Scholar] [CrossRef]
- Kadanka, Z., Jr.; Adamova, B.; Kerkovsky, M.; Kadanka, Z.; Dusek, L.; Jurova, B.; Vlckova, E.; Bednarik, J. Predictors of Symptomatic Myelopathy in Degenerative Cervical Spinal Cord Compression. Brain Behav. 2017, 7, e00797. [Google Scholar] [CrossRef] [PubMed]
- Kadanka, Z.; Mares, M.; Bednarík, J.; Smrcka, V.; Krbec, M.; Chaloupka, R.; Dusek, L. Predictive Factors for Mild Forms of Spondylotic Cervical Myelopathy Treated Conservatively or Surgically. Eur. J. Neurol. 2005, 12, 16–24. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Evaniew, N.; Ter Wengel, P.V.; Vedantam, A.; Guha, D.; Margetis, K.; Nouri, A.; Ahmed, A.I.; Neal, C.J.; Davies, B.M.; et al. AO Spine Clinical Practice Recommendations for Diagnosis and Management of Degenerative Cervical Myelopathy: Evidence Based Decision Making—A Review of Cutting Edge Recent Literature Related to Degenerative Cervical Myelopathy. Glob. Spine J. 2025, 15, 2585–2593. [Google Scholar] [CrossRef]
- Brannigan, J.F.M.; Davies, B.M.; Mowforth, O.D.; Yurac, R.; Kumar, V.; Dejaegher, J.; Zamorano, J.J.; Murphy, R.K.J.; Tripathi, M.; Anderson, D.B.; et al. Management of Mild Degenerative Cervical Myelopathy and Asymptomatic Spinal Cord Compression: An International Survey. Spinal Cord 2024, 62, 51–58. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Ibrahim, A.; Tetreault, L.; Albanese, V.; Alvarado, M.; Arnold, P.; Barbagallo, G.; Bartels, R.; Bolger, C.; Defino, H.; et al. A Global Perspective on the Outcomes of Surgical Decompression in Patients with Cervical Spondylotic Myelopathy: Results from the Prospective Multicenter AOSpine International Study on 479 Patients. Spine 2015, 40, 1322–1328. [Google Scholar] [CrossRef]
- Rhee, J.M.; Shamji, M.F.; Erwin, W.M.; Bransford, R.J.; Yoon, S.T.; Smith, J.S.; Kim, H.J.; Ely, C.G.; Dettori, J.R.; Patel, A.A.; et al. Nonoperative Management of Cervical Myelopathy. Spine 2013, 38, S55–S67. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Phillips, R.; Clarke, D.; Furlan, J.C.; Demetriades, A.K.; Milligan, J.; Witiw, C.D.; Harrop, J.S.; Aarabi, B.; Kurpad, S.N.; et al. Establishing the Socio-Economic Impact of Degenerative Cervical Myelopathy Is Fundamental to Improving Outcomes [AO Spine RECODE-DCM Research Priority Number 8]. Glob. Spine J. 2022, 12, 122S–129S. [Google Scholar] [CrossRef]
- Malhotra, A.K.; Shakil, H.; Harrington, E.M.; Fehlings, M.G.; Wilson, J.R.; Witiw, C.D. Early Surgery Compared to Nonoperative Management for Mild Degenerative Cervical Myelopathy: A Cost-Utility Analysis. Spine J. 2024, 24, 21–31. [Google Scholar] [CrossRef]
- Witiw, C.D.; Tetreault, L.A.; Smieliauskas, F.; Kopjar, B.; Massicotte, E.M.; Fehlings, M.G. Surgery for Degenerative Cervical Myelopathy: A Patient-Centered Quality of Life and Health Economic Evaluation. Spine J. 2017, 17, 15–25. [Google Scholar] [CrossRef]
- Stephens, M.E.; O’Neal, C.M.; Westrup, A.M.; Muhammad, F.Y.; McKenzie, D.M.; Fagg, A.H.; Smith, Z.A. Utility of Machine Learning Algorithms in Degenerative Cervical and Lumbar Spine Disease: A Systematic Review. Neurosurg. Rev. 2022, 45, 965–978. [Google Scholar] [CrossRef]
- Al-Shawwa, A.; Craig, M.; Ost, K.; Anderson, D.; Jacobs, W.B.; Evaniew, N.; Tripathy, S.; Bouchard, J.; Casha, S.; Cho, R.; et al. Focal Compression of the Cervical Spinal Cord Alone Does Not Indicate High Risk of Neurological Deterioration in Patients with a Diagnosis of Mild Degenerative Cervical Myelopathy. J. Neurol. Sci. 2024, 461, 123042. [Google Scholar] [CrossRef] [PubMed]
- Kusin, D.J.; Ahn, U.M.; Ahn, N.U. The Effect of Smoking on Spinal Cord Healing Following Surgical Treatment of Cervical Myelopathy. Spine 2015, 40, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Zileli, M.; Borkar, S.A.; Sinha, S.; Reinas, R.; Alves, Ó.L.; Kim, S.-H.; Pawar, S.; Murali, B.; Parthiban, J. Cervical Spondylotic Myelopathy: Natural Course and the Value of Diagnostic Techniques -WFNS Spine Committee Recommendations. Neurospine 2019, 16, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Tang, J.A.; Smith, J.S.; Acosta, F.L.; Protopsaltis, T.S.; Blondel, B.; Bess, S.; Shaffrey, C.I.; Deviren, V.; Lafage, V.; et al. Cervical Spine Alignment, Sagittal Deformity, and Clinical Implications. J. Neurosurg. Spine 2013, 19, 141–159. [Google Scholar] [CrossRef]
- Shamji, M.F.; Mohanty, C.; Massicotte, E.M.; Fehlings, M.G. The Association of Cervical Spine Alignment with Neurologic Recovery in a Prospective Cohort of Patients with Surgical Myelopathy: Analysis of a Series of 124 Cases. World Neurosurg. 2016, 86, 112–119. [Google Scholar] [CrossRef]
- Fan, X.; Chen, R.; Huang, H.; Zhang, G.; Zhou, S.; Chen, X.; Zhao, Y.; Diao, Y.; Pan, S.; Zhang, F.; et al. Classification and Prognostic Factors of Patients with Cervical Spondylotic Myelopathy after Surgical Treatment: A Cluster Analysis. Sci. Rep. 2024, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Zileli, M.; Maheshwari, S.; Kale, S.S.; Garg, K.; Menon, S.K.; Parthiban, J. Outcome Measures and Variables Affecting Prognosis of Cervical Spondylotic Myelopathy: WFNS Spine Committee Recommendations. Neurospine 2019, 16, 435–447. [Google Scholar] [CrossRef]
- Dokai, T.; Nagashima, H.; Nanjo, Y.; Tanida, A.; Teshima, R. Surgical Outcomes and Prognostic Factors of Cervical Spondylotic Myelopathy in Diabetic Patients. Arch. Orthop. Trauma Surg. 2012, 132, 577–582. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Kalsi-Ryan, S.; Cadotte, D.W.; Wilson, J.R.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D.J.; et al. Monitoring for Myelopathic Progression with Multiparametric Quantitative MRI. PLoS ONE 2018, 13, e0195733. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Salamon, N.; Grinstead, J.W.; Holly, L.T. Diffusion Tensor Imaging Predicts Functional Impairment in Mild-to-Moderate Cervical Spondylotic Myelopathy. Spine J. 2014, 14, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.A.; Cen, S.Y.; Lebel, R.M.; Hsieh, P.C.; Law, M. Diffusion Tensor Imaging Correlates with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy and Predicts Outcome Following Surgery. AJNR Am. J. Neuroradiol. 2013, 34, 471–478. [Google Scholar] [CrossRef]
- Cohen-Adad, J.; El Mendili, M.-M.; Lehéricy, S.; Pradat, P.-F.; Blancho, S.; Rossignol, S.; Benali, H. Demyelination and Degeneration in the Injured Human Spinal Cord Detected with Diffusion and Magnetization Transfer MRI. NeuroImage 2011, 55, 1024–1033. [Google Scholar] [CrossRef]
- Haynes, G.; Muhammad, F.; Weber, K.A., 2nd; Khan, A.F.; Hameed, S.; Shakir, H.; Van Hal, M.; Dickson, D.; Rohan, M.; Dhaher, Y.; et al. Tract-Specific Magnetization Transfer Ratio Provides Insights into the Severity of Degenerative Cervical Myelopathy. Spinal Cord 2024, 62, 700–707. [Google Scholar] [CrossRef]
- Sharma, S.; Sial, A.; Bright, G.E.; O’Hare Doig, R.; Diwan, A.D. Diffusion Tensor Imaging in Degenerative Cervical Myelopathy: Clinical Translation Opportunities for Cause of Pain Detection and Potentially Early Diagnoses. Appl. Sci. 2025, 15, 11607. [Google Scholar] [CrossRef]
- Al-Shawwa, A.; Ost, K.; Anderson, D.; Cho, N.; Evaniew, N.; Jacobs, W.B.; Martin, A.R.; Gaekwad, R.; Tripathy, S.; Bouchard, J.; et al. Advanced MRI Metrics Improve the Prediction of Baseline Disease Severity for Individuals with Degenerative Cervical Myelopathy. Spine J. 2024, 24, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; MacMillan, E.L.; Jutzeler, C.R.; Ljungberg, E.; MacKay, A.L.; Kolind, S.H.; Mädler, B.; Li, D.K.B.; Dvorak, M.F.; Curt, A.; et al. Assessing Structure and Function of Myelin in Cervical Spondylotic Myelopathy: Evidence of Demyelination. Neurology 2017, 89, 602–610. [Google Scholar] [CrossRef]
- De Leener, B.; Lévy, S.; Dupont, S.M.; Fonov, V.S.; Stikov, N.; Louis Collins, D.; Callot, V.; Cohen-Adad, J. SCT: Spinal Cord Toolbox, an Open-Source Software for Processing Spinal Cord MRI Data. NeuroImage 2017, 145, 24–43. [Google Scholar] [CrossRef]
- Cohen-Adad, J.; Alonso-Ortiz, E.; Abramovic, M.; Arneitz, C.; Atcheson, N.; Barlow, L.; Barry, R.L.; Barth, M.; Battiston, M.; Büchel, C.; et al. Generic Acquisition Protocol for Quantitative MRI of the Spinal Cord. Nat. Protoc. 2021, 16, 4611–4632. [Google Scholar] [CrossRef]
- Khan, O.; Badhiwala, J.H.; Wilson, J.R.F.; Jiang, F.; Martin, A.R.; Fehlings, M.G. Predictive Modeling of Outcomes after Traumatic and Nontraumatic Spinal Cord Injury Using Machine Learning: Review of Current Progress and Future Directions. Neurospine 2019, 16, 678–685. [Google Scholar] [CrossRef]
- Khan, O.; Badhiwala, J.H.; Witiw, C.D.; Wilson, J.R.; Fehlings, M.G. Machine Learning Algorithms for Prediction of Health-Related Quality-of-Life After Surgery for Mild Degenerative Cervical Myelopathy. Spine J. 2021, 21, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Witiw, C.D.; Badhiwala, J.H.; Wilson, J.R.; Fehlings, M.G. Using a Machine Learning Approach to Predict Outcome after Surgery for Degenerative Cervical Myelopathy. PLoS ONE 2019, 14, e0215133. [Google Scholar] [CrossRef]
- Toop, N.; Gifford, C.S.; McGahan, B.G.; Gibbs, D.; Miracle, S.; Schwab, J.M.; Motiei-Langroudi, R.; Farhadi, H.F. Influence of Clinical and Radiological Parameters on the Likelihood of Neurological Improvement after Surgery for Degenerative Cervical Myelopathy. J. Neurosurg. Spine 2023, 38, 14–23. [Google Scholar] [CrossRef]
- Park, D.; Cho, J.M.; Yang, J.W.; Yang, D.; Kim, M.; Oh, G.; Kwon, H.D. Classification of Expert-Level Therapeutic Decisions for Degenerative Cervical Myelopathy Using Ensemble Machine Learning Algorithms. Front. Surg. 2022, 9, 1010420. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Z.; Huang, H.; Xi, H.; Fan, X.; Zhao, Y.; Chen, X.; Diao, Y.; Sun, Y.; Ji, H.; et al. Predicting Postoperative Neurological Outcomes of Degenerative Cervical Myelopathy Based on Machine Learning. Front. Bioeng. Biotechnol. 2025, 13, 1529545. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, R.A.; Karar, M.E.; Coburger, J.; Wirtz, C.R.; Burgert, O. DeepSeg: Deep Neural Network Framework for Automatic Brain Tumor Segmentation Using Magnetic Resonance FLAIR Images. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 909–920. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Badhiwala, J.H.; Ahn, H.; Farhadi, H.F.; Shaffrey, C.I.; Nassr, A.; Mummaneni, P.; Arnold, P.M.; Jacobs, W.B.; Riew, K.D.; et al. Safety and Efficacy of Riluzole in Patients Undergoing Decompressive Surgery for Degenerative Cervical Myelopathy (CSM-Protect): A Multicentre, Double-Blind, Placebo-Controlled, Randomised, Phase 3 Trial. Lancet Neurol. 2021, 20, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Khan, D.Z.; Mowforth, O.D.; McNair, A.G.K.; Gronlund, T.; Kolias, A.G.; Tetreault, L.; Starkey, M.L.; Sadler, I.; Sarewitz, E.; et al. RE-CODE DCM (REsearch Objectives and Common Data Elements for Degenerative Cervical Myelopathy): A Consensus Process to Improve Research Efficiency in DCM, through Establishment of a Standardized Dataset for Clinical Research and the Definition of the Research Priorities. Glob. Spine J. 2019, 9, 65S–76S. [Google Scholar] [CrossRef] [PubMed]
- Treanor, C.; Gallagher, C.; Lenehan, W.; Gantly, H.; Bolger, C.; Malone, A. Flipping the MJOA: Clinical Utility of the Modified Japanese Orthopaedic Association Score as a Tool for Detecting Degenerative Cervical Myelopathy. Brain Spine 2024, 4, 102853. [Google Scholar] [CrossRef]
- Irie, K.; Iseki, H.; Okamoto, K.; Nishimura, S.; Kagechika, K. Introduction of the Purdue Pegboard Test for Fine Assessment of Severity of Cervical Myelopathy before and after Surgery. J. Phys. Ther. Sci. 2020, 32, 210–214. [Google Scholar] [CrossRef]
- Mihara, H.; Kondo, S.; Murata, A.; Ishida, K.; Niimura, T.; Hachiya, M. A New Performance Test for Cervical Myelopathy: The Triangle Step Test. Spine 2010, 35, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, J.; Rochette, A.; Hébert, R.; Bravo, G. The Minnesota Manual Dexterity Test: Reliability, Validity and Reference Values Studies with Healthy Elderly People. Can. J. Occup. Ther. 1997, 64, 270–276. [Google Scholar] [CrossRef]
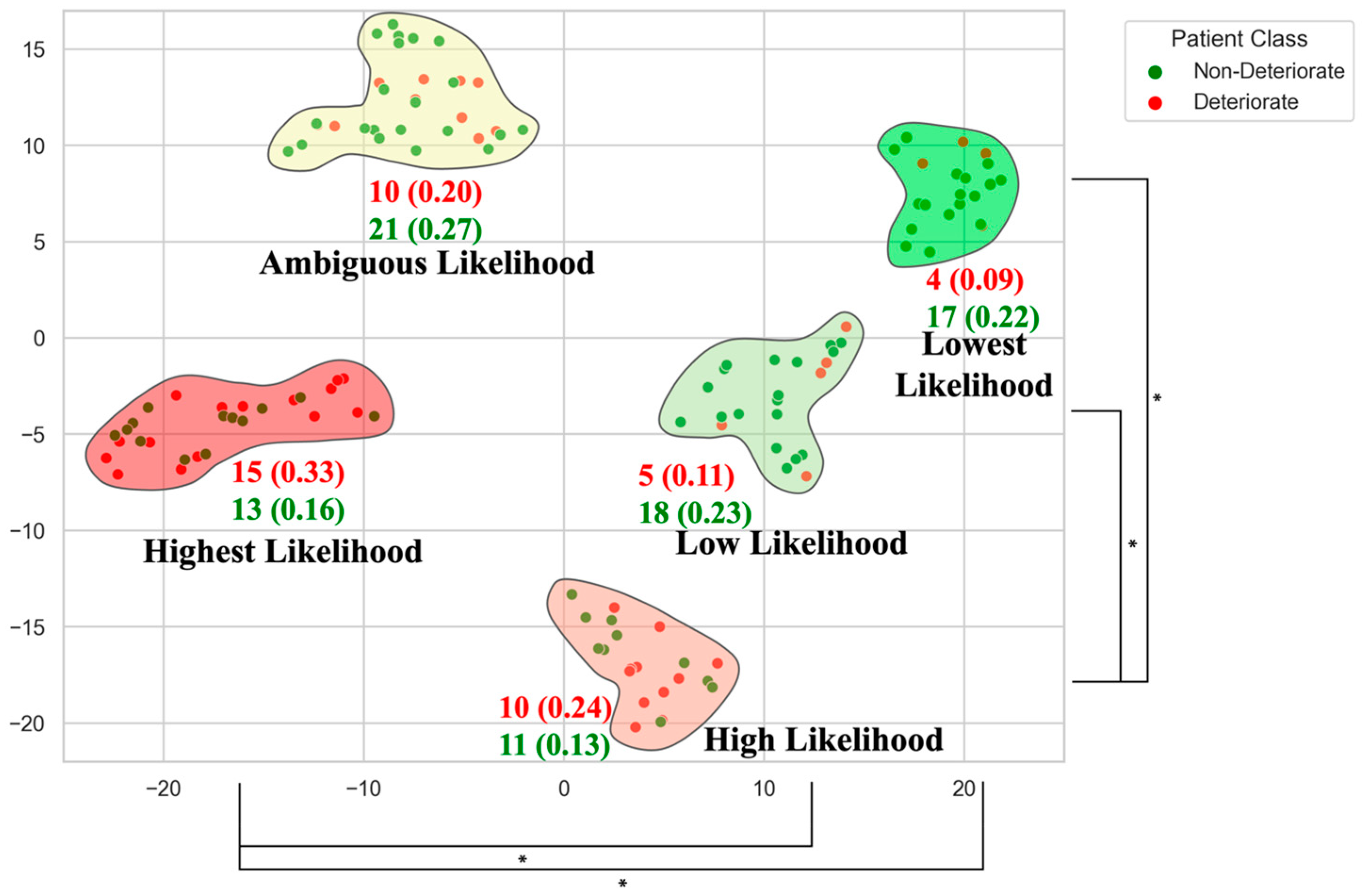
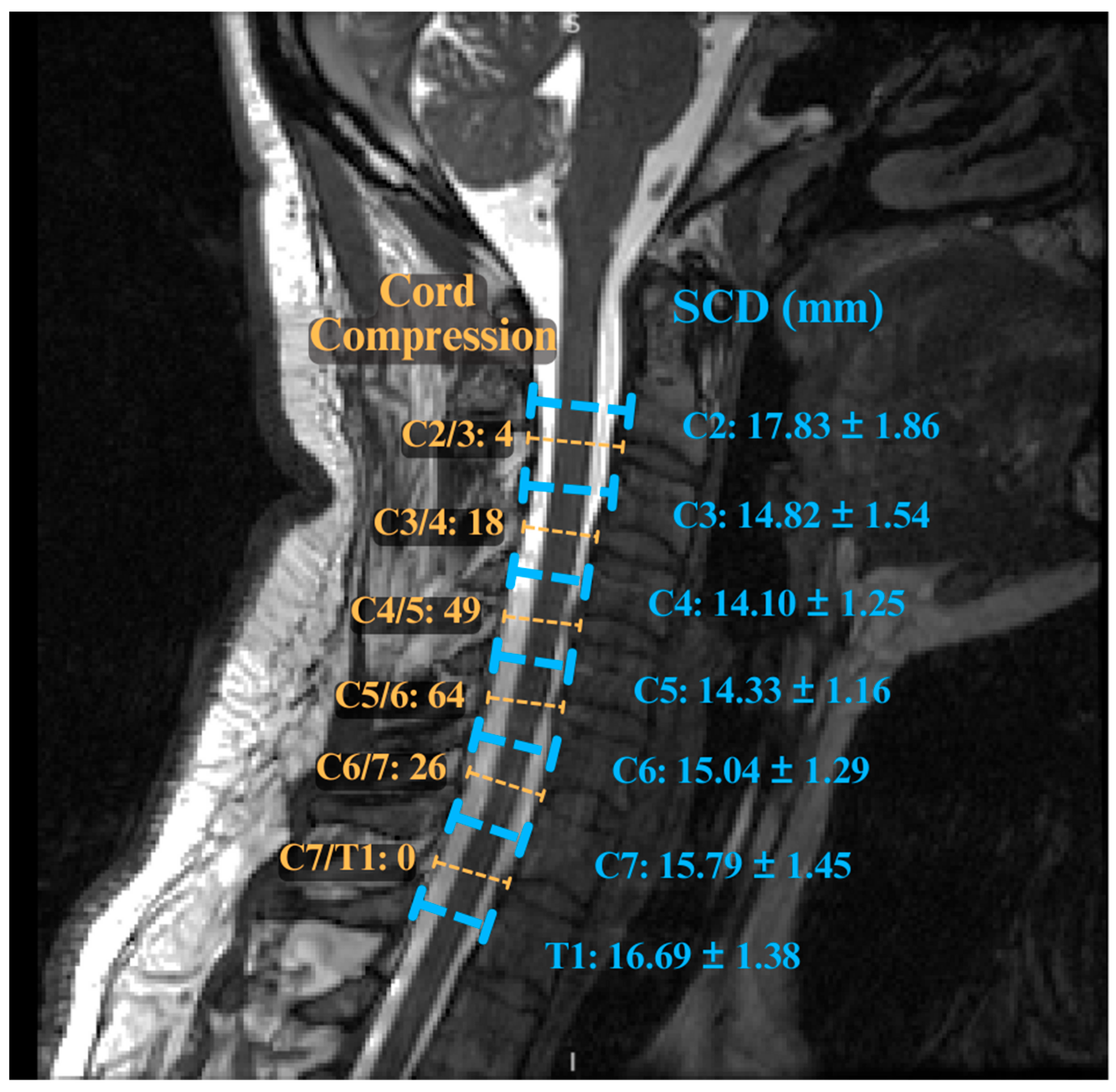
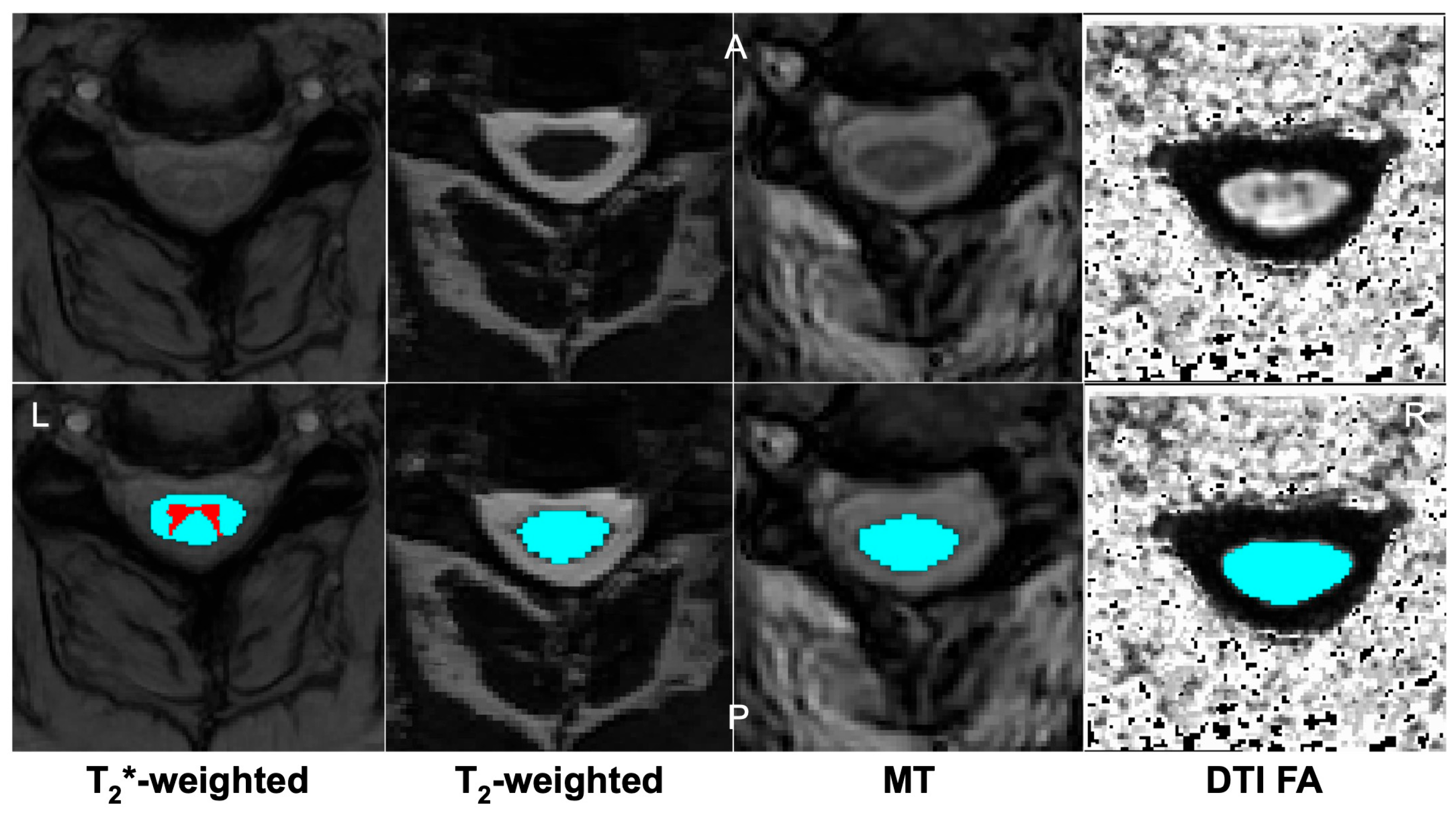
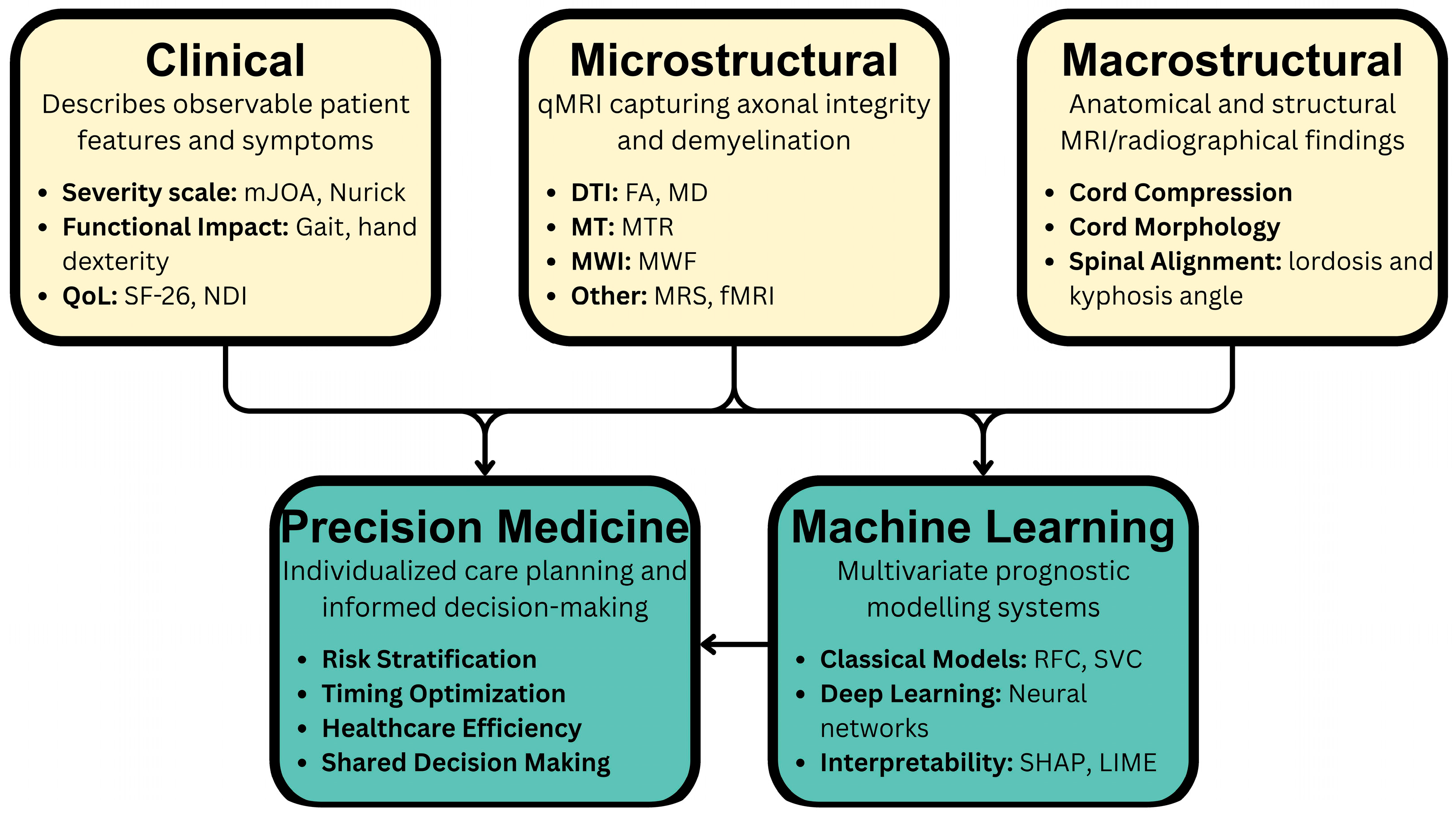
| Imaging Modality | Acquisition Considerations | Key Biomarkers | Findings/Utility in DCM |
|---|---|---|---|
| Diffusion Tensor Imaging (DTI) | Echo planar imaging sequence; moderate scan time (5–10 min); prone to motion and distortion artefacts. | Fractional anisotropy (FA), mean diffusivity (MD), etc., reflecting white matter axonal integrity and water diffusion. | Lower FA and higher MD at the compression level correlate to greater neurological impairment [64]. Low FA has been associated with poorer postoperative improvement [65] |
| Magnetization Transfer (MT) | Specialized pulse sequence with MT saturation; ~5 min scan time; requires off-resonance pulse calibration. | Magnetization transfer Ratio (MTR), indicating myelin and macromolecular content. | Compressed cord regions show reduced MTR, indicating demyelination [66]. MTR changes can appear in mild DCM before DTI changes [70]. |
| Myelin Water Imaging (MWI) | Multi-echo MRI; long acquisition (>10–15 min); high technical expertise needed. | Myelin water fraction relates to the proportion of water trapped between myelin layers. | Studies report lower myelin water fraction in patients with DCM [70], consistent with demyelination. Limited data for this imaging type. |
| Study (Year) | Sample (n, Population) | Main Predictors | Outcome Predicted | Performance (AUC, Best Model) |
|---|---|---|---|---|
| Merali et al. (2019) [75] | n ≈ 600 (multi-center surgical DCM cohort; data from AOSpine CSM trials) | Clinical and demographic features | Improvement in health-related quality of life (SF-36) after surgery | ~0.70 (Random Forest model) |
| Khan et al. (2021) [74] | n = 193 (mild DCM patients, AOSpine CSM trials) | Clinical only | Clinically meaningful improvement in SF-36 score 1-year post-surgery | 0.78 (ensemble classifier) |
| Toop et al. (2023) [76] | n = 183 (mixed severity, single center) | Clinical + conventional MRI metrics | Failure to improve neurologically after surgery | 0.82 (Logistic regression model) |
| Zhou et al. (2025) [78] | n = 672 (mixed severity, single-center retrospective) | Clinical + MRI signal change | Short-term postoperative outcomes | 0.745 (LightGBM model) |
| Park et al. (2022) [77] | n = 304 (mixed severity, single-center retrospective) | Clinical + radiologic metrics | Classification of treatment decision (conservative vs. surgery) | 0.92 (Gradient Boosting Model) |
| Al-Shawwa et al. (2024) [69] | n = 524 (mixed severity, single-center retrospective) | Conventional and qMRI metrics | Prediction of disease severity class | AUC not reported. 0.418 balanced accuracy (conventional MRI) 0.733 balanced accuracy (advanced MRI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shawwa, A.; Cadotte, D.W. Advancing Precision Medicine in Degenerative Cervical Myelopathy. J. Clin. Med. 2025, 14, 8344. https://doi.org/10.3390/jcm14238344
Al-Shawwa A, Cadotte DW. Advancing Precision Medicine in Degenerative Cervical Myelopathy. Journal of Clinical Medicine. 2025; 14(23):8344. https://doi.org/10.3390/jcm14238344
Chicago/Turabian StyleAl-Shawwa, Abdul, and David W. Cadotte. 2025. "Advancing Precision Medicine in Degenerative Cervical Myelopathy" Journal of Clinical Medicine 14, no. 23: 8344. https://doi.org/10.3390/jcm14238344
APA StyleAl-Shawwa, A., & Cadotte, D. W. (2025). Advancing Precision Medicine in Degenerative Cervical Myelopathy. Journal of Clinical Medicine, 14(23), 8344. https://doi.org/10.3390/jcm14238344






