The Results of a 12-Month Open-Label Follow-Up Study with MRI Monitoring of Patients with Parkinson’s Disease After MRI-Guided FUS
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Assessments
2.3. MRI
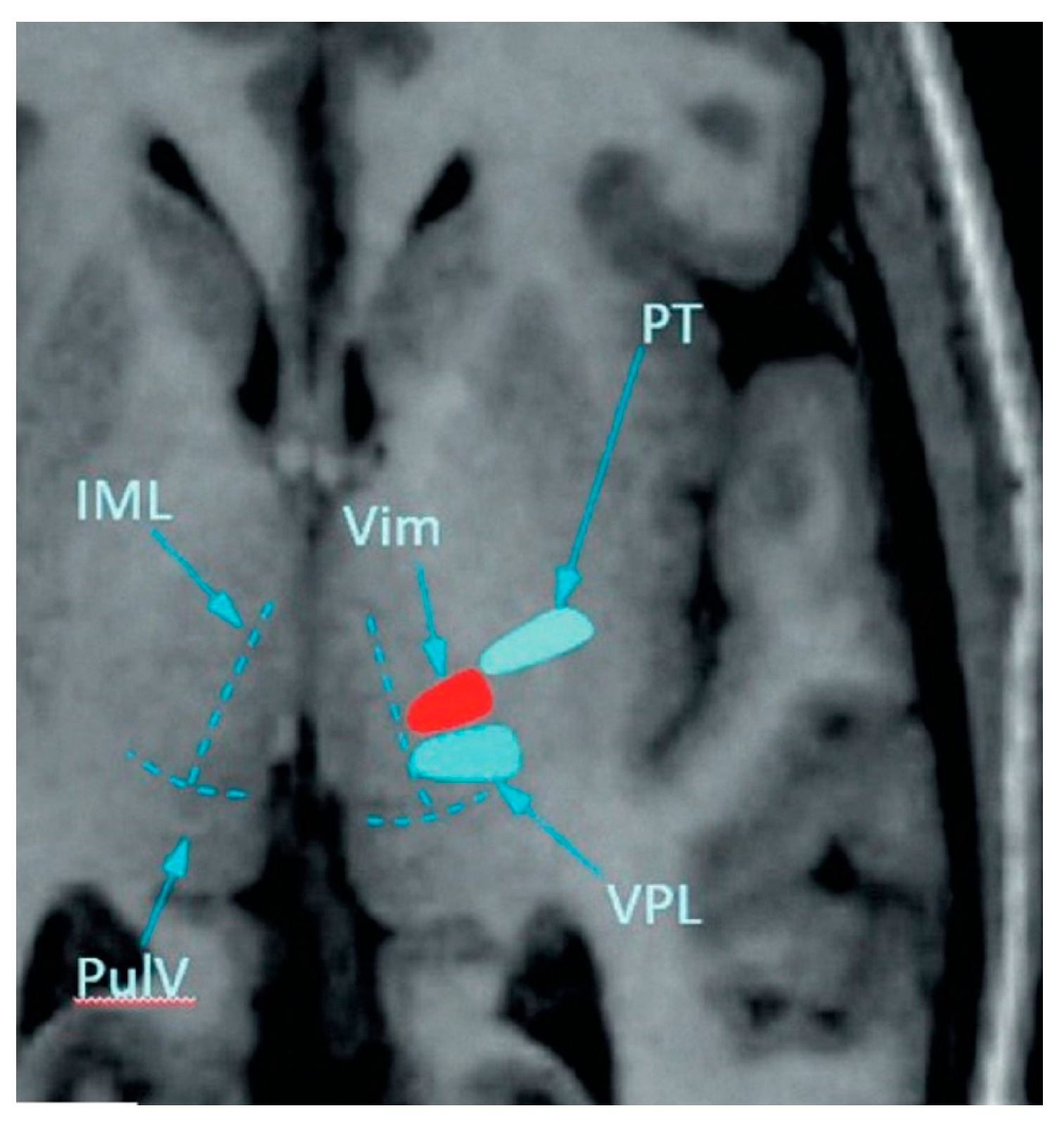
2.4. MRgFUS Treatment
2.5. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Patients
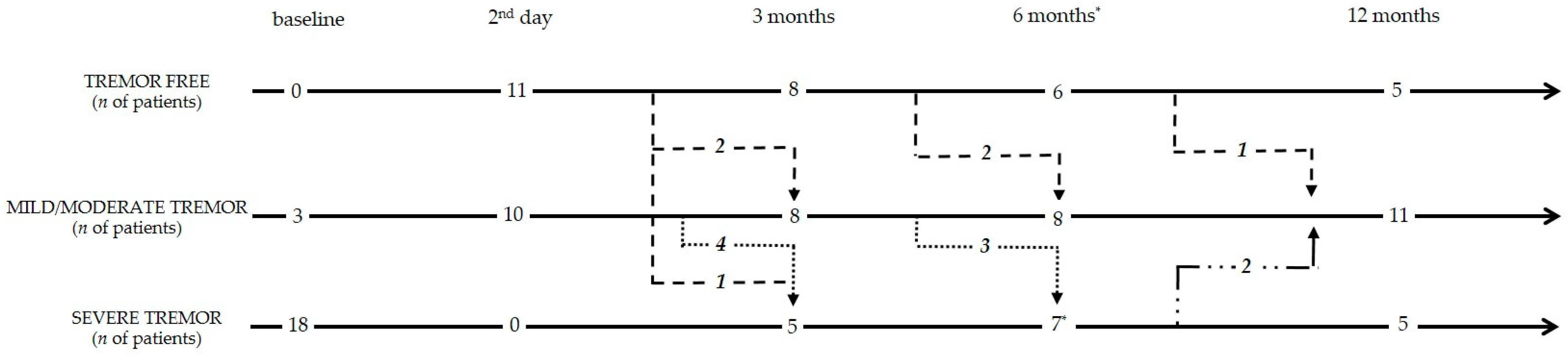
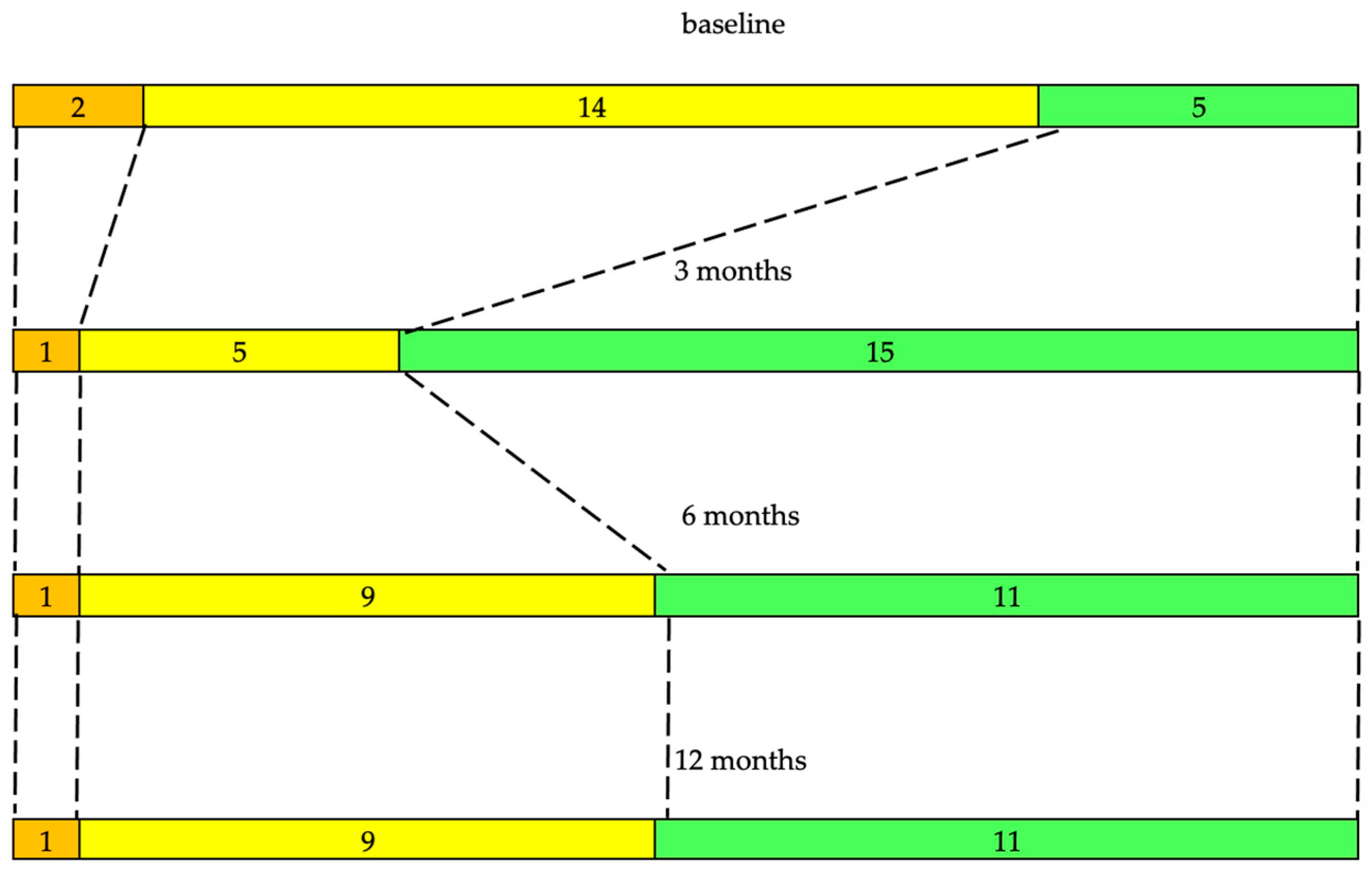
3.2. Follow-Up
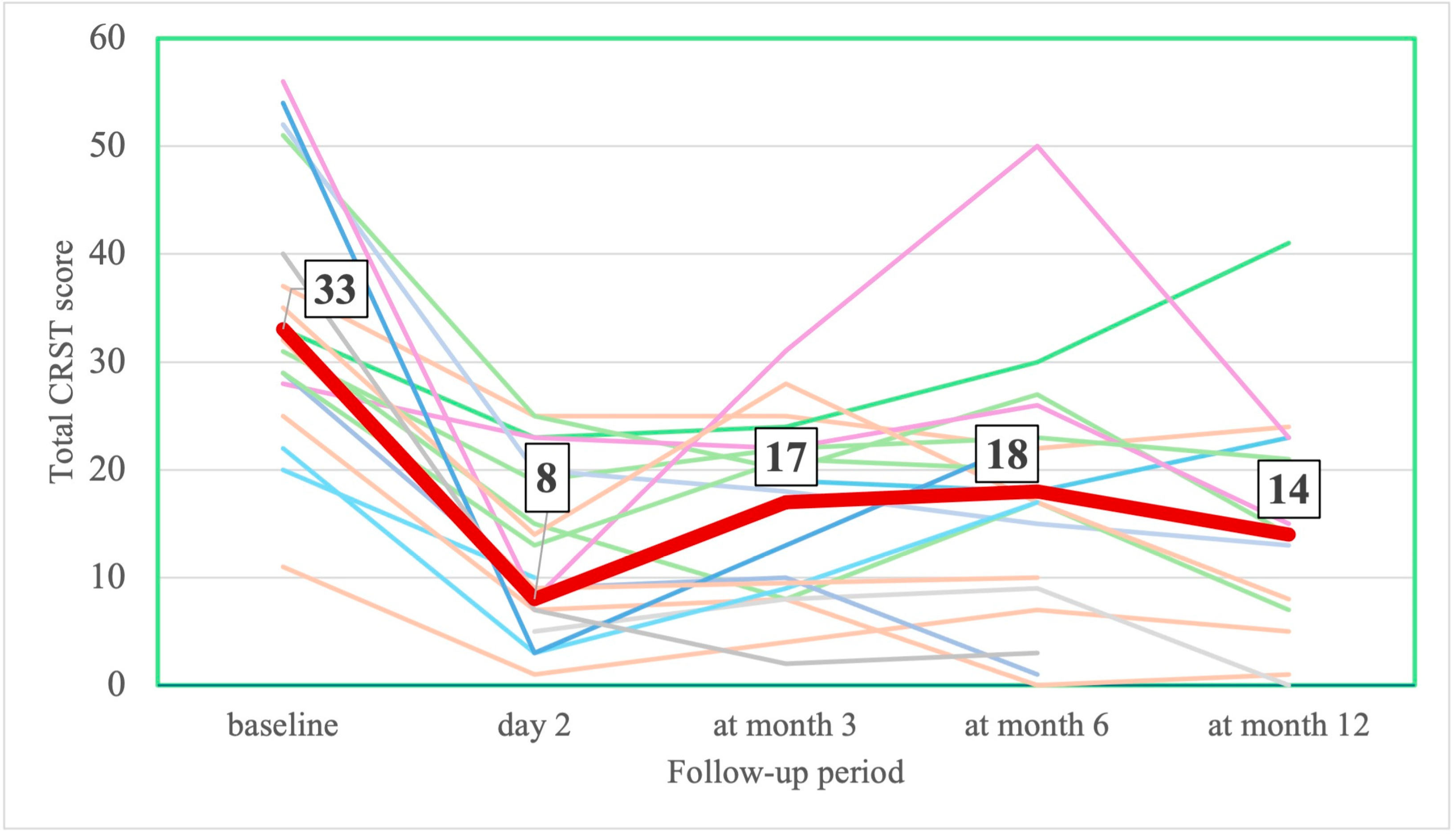
3.2.1. Tremor and Motor Assessments
Individual Patient Assessments
3.2.2. Non-Motor Assessment and Symptoms
3.2.3. LEDD
3.2.4. AE
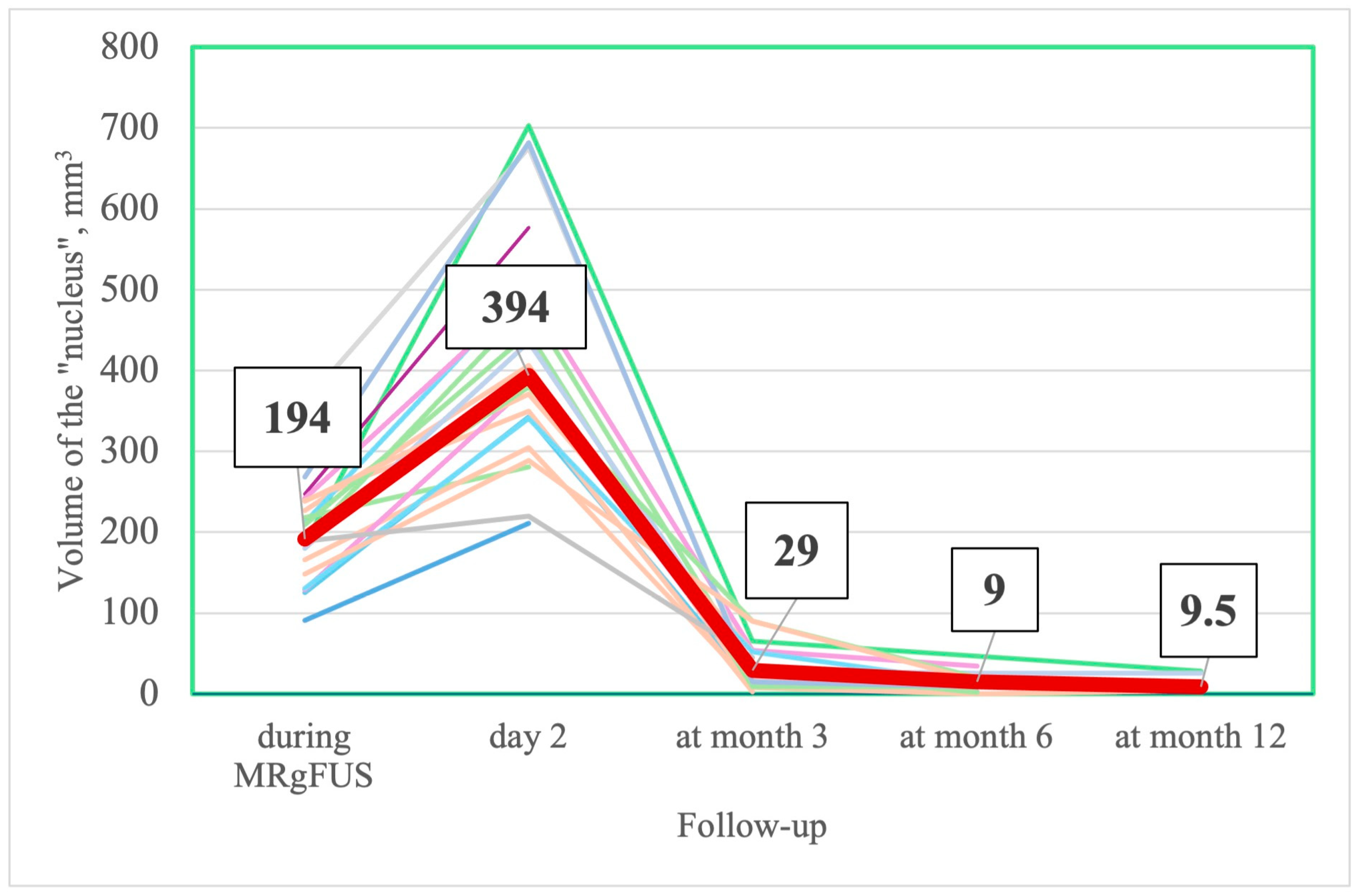
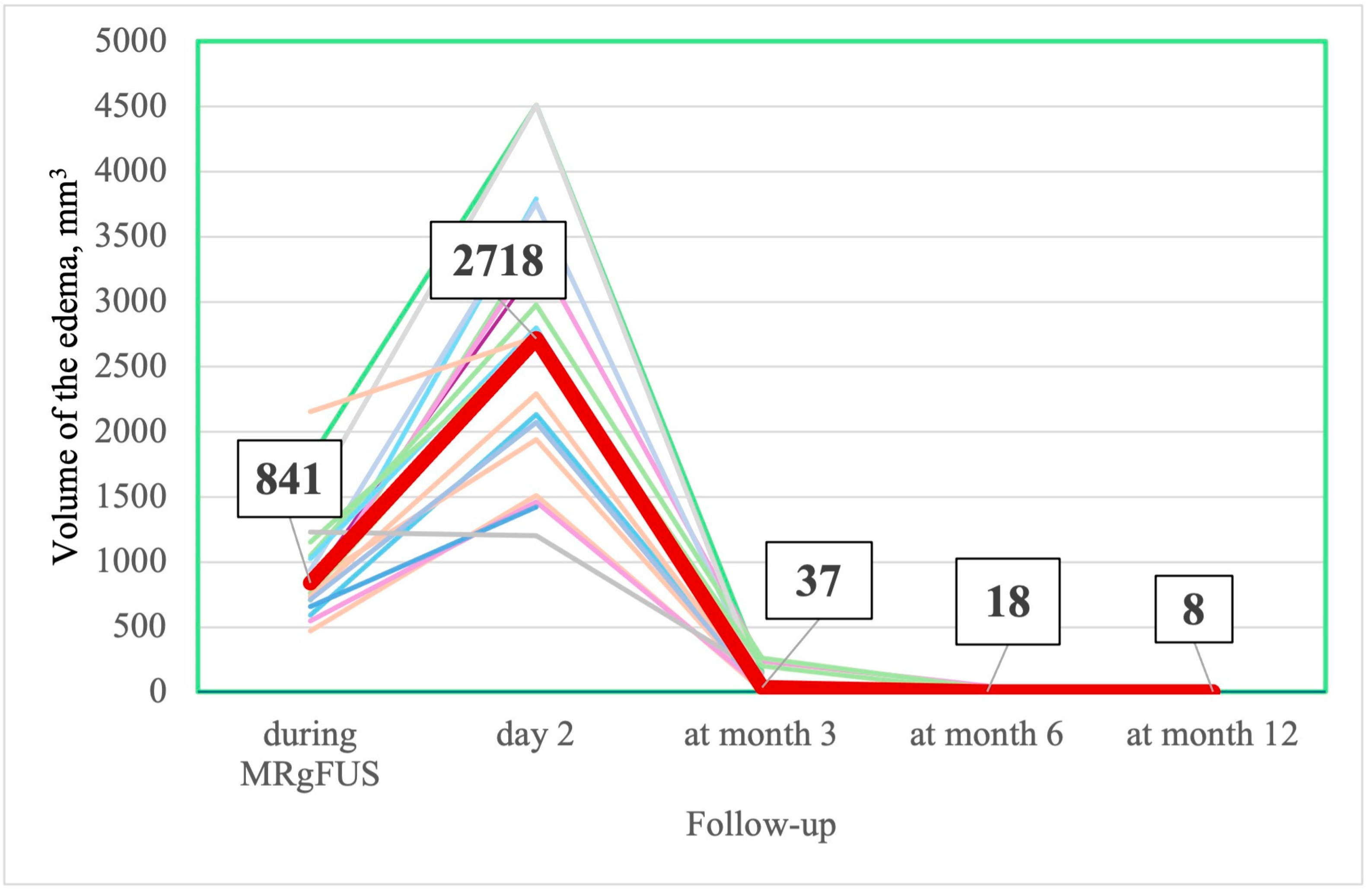
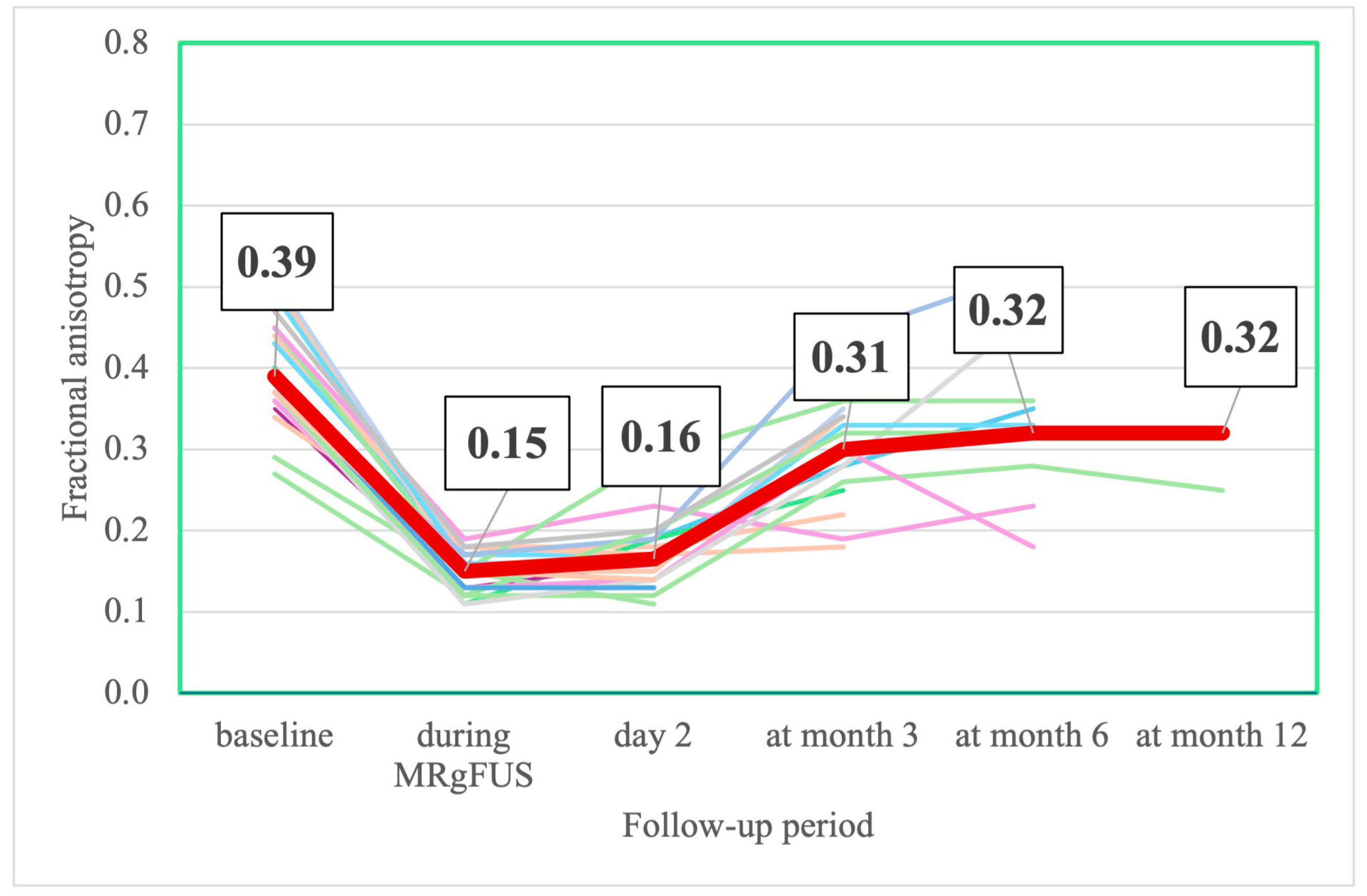
3.2.5. MRI Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| ET | Essential tremor |
| TDPD | Tremor-dominant Parkinson disease |
| MRgFUS | Magnetic resonance guided focused ultrasound |
| FUS | Focused ultrasound |
| MRI | Magnetic resonance imaging |
| DBS | Deep brain stimulation |
| AE | Adverse events |
| SDR | Scull density ratio |
| DDS | Dopamine dysregulation syndrome |
| BBB | Blood–brain barrier |
| LEDD | Levodopa equivalent daily dose |
| FA | Fractional anisotropy |
| VL | Ventral lateral nucleus |
| VIM | Ventral intermediate nucleus |
| VOP | Ventral oral posterior nucleus |
| VOA | Ventral oral anterior nucleus |
| GPi | Globus pallidus internus |
| MDC | Minimal detectable change |
| MCID | Minimal clinically important difference |
| S&E ADL | Schwab and England ADL Scale |
| ADL | Activities of daily living |
| PDQ-39 | Parkinson’s Disease Quality of Life Questionnaire-39 |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| NMSS | Non-Motor Symptoms Scale for Parkinson’s Disease |
| QUIP | Questionnaire for Impulsive–compulsive Disorders in Parkinson’s Disease Rating Scale |
| CRST | Clinical Rating Scale for Tremor |
| MoCA | Montreal Cognitive Assessment |
| QoL | Quality of life |
References
- Thenganatt, M.A.; Jankovic, J. Parkinson disease subtypes. JAMA Neurol. 2014, 71, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 2013, 28, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; McDermott, M.; Carter, J.; Gauthier, S.; Goetz, C.; Golbe, L.; Huber, S.; Koller, W.; Olanow, C.; Shoulson, I.; et al. Variable expression of Parkinson’s disease: A base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990, 40, 1529–1534. [Google Scholar] [CrossRef]
- Zaidel, A.; Arkadir, D.; Israel, Z.; Bergman, H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr. Opin. Neurol. 2009, 22, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Aleksovski, D.; Miljkovic, D.; Bravi, D.; Antonini, A. Disease progression in Parkinson subtypes: The PPMI dataset. Neurol. Sci. 2018, 39, 1971–1976. [Google Scholar] [CrossRef]
- Zach, H.; Dirkx, M.F.; Roth, D.; Pasman, J.W.; Bloem, B.R.; Helmich, R.C. Dopamine-responsive and dopamine-resistant resting tremor in Parkinson disease. Neurology 2020, 95, e1461–e1470. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Day, G.S.; Smith, D.B.; Rae-Grant, A.; Licking, N.; Armstrong, M.J.; de Bie, R.M.A.; Roze, E.; Miyasaki, J.M.; Hauser, R.A.; et al. Guideline Subcommittee of the AAN. Dopaminergic Therapy for Motor Symptoms in Early Parkinson Disease Practice Guideline Summary: A Report of the AAN Guideline Subcommittee. Neurology 2021, 97, 942–957. [Google Scholar] [CrossRef]
- Koller, W.; Pahwa, R.; Busenbark, K.; Hubble, J.; Wilkinson, S.; Lang, A.; Tuite, P.; Sime, E.; Lazano, A.; Hauser, R.; et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann. Neurol. 1997, 42, 292–299. [Google Scholar] [CrossRef]
- Zirh, A.; Reich, S.G.; Dougherty, P.M.; Lenz, F.A. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: Reassessment including a blinded measure of outcome. J. Neurol. Neurosurg. Psychiatry 1999, 66, 772–775. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Jung, N.Y.; Park, C.K.; Chang, W.S.; Jung, H.H.; Chang, J.W. Effects on cognition and quality of life with unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Neurosurg. Focus 2018, 44, E8. [Google Scholar] [CrossRef]
- Halpern, C.H.; Santini, V.; Lipsman, N.; Lozano, A.M.; Schwartz, M.L.; Shah, B.B.; Elias, W.J.; Cosgrove, G.R.; Hayes, M.T.; McDannold, N.; et al. Three-year follow-up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology 2019, 93, e2284–e2293. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Fukutake, S.; Yamamoto, K.; Yamaguchi, T.; Taira, T.; Kamei, T. Magnetic Resonance Imaging-guided Focused Ultrasound Thalamotomy for Parkinson’s Disease. Intern. Med. 2018, 57, 1027–1031. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Rodríguez-Rojas, R.; Del Álamo, M.; Hernández-Fernández, F.; Pineda-Pardo, J.A.; Dileone, M.; Alonso-Frech, F.; Foffani, G.; Obeso, I.; Gasca-Salas, C.; et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: A pilot study. Lancet Neurol. 2018, 17, 54–63. [Google Scholar] [CrossRef]
- Sinai, A.; Nassar, M.; Sprecher, E.; Constantinescu, M.; Zaaroor, M.; Schlesinger, I. Focused Ultrasound Thalamotomy in Tremor Dominant Parkinson’s Disease: Long-Term Results. J. Park. Dis. 2022, 12, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Fishman, P.S.; Eisenberg, H.M.; Kaplitt, M.; Baltuch, G.; Chang, J.W.; Chang, W.C.; Martinez Fernandez, R.; Del Alamo, M.; Halpern, C.H.; et al. Trial of Globus Pallidus Focused Ultrasound Ablation in Parkinson’s Disease. N. Engl. J. Med. 2023, 388, 683–693. [Google Scholar] [CrossRef]
- Jameel, A.; Akgun, S.; Yousif, N.; Smith, J.; Jones, B.; Nandi, D.; Bain, P.; Gedroyc, W. The evolution of ventral intermediate nucleus targeting in MRI-guided focused ultrasound thalamotomy for essential tremor: An international multi-center evaluation. Front. Neurol. 2024, 15, 1345873. [Google Scholar] [CrossRef]
- Segar, D.J.; Lak, A.M.; Lee, S.; Harary, M.; Chavakula, V.; Lauro, P.; McDannold, N.; White, J.; Cosgrove, G.R. Lesion location and lesion creation affect outcomes after focused ultrasound thalamotomy. Brain 2021, 144, 3089–3100. [Google Scholar] [CrossRef]
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.Q.; Gwinn, R.; Witt, J.; et al. Safety and Efficacy of Focused Ultrasound Thalamotomy for Patients with Medication-Refractory, Tremor-Dominant Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Sperling, S.A.; Shah, B.B.; Barrett, M.J.; Bond, A.E.; Huss, D.S.; Gonzalez Mejia, J.A.; Elias, W.J. Focused ultrasound thalamotomy in Parkinson disease: Nonmotor outcomes and quality of life. Neurology 2018, 91, e1275–e1284. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ito, H.; Fukutake, S.; Odo, T.; Kamei, T.; Yamaguchi, T.; Taira, T. Focused Ultrasound Thalamotomy for Tremor-dominant Parkinson’s Disease: A Prospective 1-year Follow-up Study. Neurol. Med. Chir. 2021, 61, 414–421. [Google Scholar] [CrossRef]
- Saporito, G.; Sucapane, P.; Ornello, R.; Cerone, D.; Bruno, F.; Splendiani, A.; Masciocchi, C.; Ricci, A.; Marini, C.; Sacco, S.; et al. Cognitive outcomes after focused ultrasound thalamotomy for tremor: Results from the COGNIFUS (COGNitive in Focused UltraSound) study. Park. Relat. Disord. 2023, 106, 105230. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Fahn, S.; Tolosa, E.; Marin, C. Clinical rating scale for tremor. In Parkinson’s Disease and Movement Disorder; Jankovic, J., Tolosa, E., Eds.; Urban and Schwarzenberg: Baltimore, MD, USA, 1988; pp. 225–234. [Google Scholar]
- Fahn, S.; Elton, R.L.; UPDRS program members. Unified Parkinsons Disease Rating Scale. In Recent Developments in Parkinsons Disease; Fahn, S., Marsden, C.D., Goldstein, M., Calne, D.B., Eds.; Macmillan Healthcare Information: Florham Park, NJ, USA, 1987; Volume 2, pp. 153–163. [Google Scholar]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P. Quantitation of non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 2–7. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Mayberg, H.S.; Preziosi, T.J.; Andrezejewski, P.; Leiguarda, R.; Robinson, R.G. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 134–139. [Google Scholar] [CrossRef]
- Weintraub, D.; Hoops, S.; Shea, J.A.; Lyons, K.E.; Pahwa, R.; Driver-Dunckley, E.D.; Adler, C.H.; Potenza, M.N.; Miyasaki, J.; Siderowf, A.D.; et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov. Disord. 2009, 24, 1461–1467. [Google Scholar] [CrossRef]
- Schwab, R.; England, A. Projection technique for evaluating surgery in Parkinson’s disease. In Third Symposium on Parkinson’s Disease; Gillingham, J.F., Donaldson, I.M.L., Eds.; Livingstone: Edinburgh, Scotland, 1969; pp. 152–157. [Google Scholar]
- Peto, V.; Jenkinson, C.; Fitzpatrick, R. PDQ-39: A review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J. Neurol. 1998, 245, S10–S14. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Gumin, I.S.; Malykhina, E.A.; Dzhafarov, V.M.; Katunina, E.A.; Senko, I.V.; Dolgushin, M.B. First experience of thalamotomy by focused ultrasound under MR-guided navigation in the treatment of tremor. Neuroimaging follow-up. Case report and literature review. Burdenko’s J. Neurosurg. 2022, 86, 81–88, (In English, In Russian). [Google Scholar] [CrossRef]
- Benabid, A.L.; Pollak, P.; Gervason, C.; Hoffmann, D.; Gao, D.M.; Hommel, M.; Perret, J.E.; de Rougemont, J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991, 337, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Zaaroor, M.; Sinai, A.; Goldsher, D.; Eran, A.; Nassar, M.; Schlesinger, I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: A report of 30 Parkinson’s disease and essential tremor cases. J. Neurosurg. 2018, 128, 202–210. [Google Scholar] [CrossRef]
- Dolgushin, M.B.; Prishchepina, K.A.; Martynov, M.Y.; Gumin, I.S.; Katunina, E.A.; Senko, I.V.; Tairova, R.T.; Dvoryanchikov, A.V. Dynamics of permeability of the blood-brain barrier after FUS thalamotomy according to contrast-enhanced MRI. Burdenko’s J. Neurosurg. 2025, 89, 51–60, (In Russian/In English). [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Hung, S.J.; Lin, K.C.; Chen, K.H.; Chen, P.; Tsay, P.K. Responsiveness, Minimal Clinically Important Difference, and Validity of the MoCA in Stroke Rehabilitation. Occup. Ther. Int. 2019, 2019, 2517658. [Google Scholar] [CrossRef]
- Lindvall, E.; Abzhandadze, T.; Quinn, T.J.; Sunnerhagen, K.S.; Lundström, E. Is the difference real, is the difference relevant: The minimal detectable and clinically important changes in the Montreal Cognitive Assessment. Cereb. Circ. Cogn. Behav. 2024, 6, 100222. [Google Scholar] [CrossRef]
- Lin, F.; Wu, D.; Yu, J.; Weng, H.; Chen, L.; Meng, F.; Chen, Y.; Ye, Q.; Cai, G. Comparison of efficacy of deep brain stimulation and focused ultrasound in parkinsonian tremor: A systematic review and network meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 434–443. [Google Scholar] [CrossRef]
- Liang, M.; Hou, L.; Liang, J.; Bao, S. Ameliorating motor performance and quality of life in Parkinson’s disease: A comparison of deep brain stimulation and focused ultrasound surgery. Front. Neurol. 2025, 16, 1449973. [Google Scholar] [CrossRef]
- Cesarano, S.; Saporito, G.; Sucapane, P.; Bruno, F.; Catalucci, A.; Pistoia, M.L.; Splendiani, A.; Ricci, A.; Di Cesare, E.; Totaro, R.; et al. Staged magnetic resonance-guided focused ultrasound thalamotomy for the treatment of bilateral essential tremor and Parkinson’s disease related tremor: A systematic review and critical appraisal of current knowledge. Front. Neurol. 2024, 15, 1409727. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Natera-Villalba, E.; Rodríguez-Rojas, R.; Del Álamo, M.; Pineda-Pardo, J.A.; Obeso, I.; Guida, P.; Jiménez-Castellanos, T.; Pérez-Bueno, D.; Duque, A.; et al. Staged Bilateral MRI-Guided Focused Ultrasound Subthalamotomy for Parkinson Disease. JAMA Neurol. 2024, 81, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Gagliardi, A.; Campanozzi, E.; Corridore, A.; Tommasino, E.; Pagliei, V.; Pertici, L.; Palumbo, P.; et al. Comprehensive Evaluation of Factors Affecting Tremor Relapse after MRgFUS Thalamotomy: A Case-Control Study. Brain Sci. 2021, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Braccia, A.; Golfrè Andreasi, N.; Ghielmetti, F.; Aquino, D.; Savoldi, A.P.; Cilia, R.; Telese, R.; Colucci, F.; Gaudiano, G.; Romito, L.M.; et al. Magnetic Resonance-Guided Focused Ultrasound Thalamotomy in a Prospective Cohort of 52 Patients with Parkinson’s Disease: A Possible Critical Role of Age and Lesion Volume for Predicting Tremor Relapse. Mov. Disord. 2025, 40, 478–489. [Google Scholar] [CrossRef]
- Jang, C.; Park, H.J.; Chang, W.S.; Pae, C.; Chang, J.W. Immediate and Longitudinal Alterations of Functional Networks after Thalamotomy in Essential Tremor. Front. Neurol. 2016, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C.; Janssen, M.J.; Oyen, W.J.; Bloem, B.R.; Toni, I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann. Neurol. 2011, 69, 269–281. [Google Scholar] [CrossRef]
- Qamhawi, Z.; Towey, D.; Shah, B.; Pagano, G.; Seibyl, J.; Marek, K.; Borghammer, P.; Brooks, D.J.; Pavese, N. Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain 2015, 138 Pt 10, 2964–2973. [Google Scholar] [CrossRef]
- Underwood, C.F.; Parr-Brownlie, L.C. Primary motor cortex in Parkinson’s disease: Functional changes and opportunities for neurostimulation. Neurobiol. Dis. 2021, 147, 105159. [Google Scholar] [CrossRef]
- Golfrè Andreasi, N.; Cilia, R.; Romito, L.M.; Bonvegna, S.; Straccia, G.; Elia, A.E.; Novelli, A.; Messina, G.; Tringali, G.; Levi, V.; et al. Magnetic Resonance-Guided Focused Ultrasound Thalamotomy May Spare Dopaminergic Therapy in Early-Stage Tremor-Dominant Parkinson’s Disease: A Pilot Study. Mov. Disord. 2022, 37, 2289–2295. [Google Scholar] [CrossRef]
- Chua, M.M.J.; Blitz, S.E.; Ng, P.R.; Segar, D.J.; McDannold, N.J.; White, P.J.; Christie, S.; Hayes, M.T.; Rolston, J.D.; Cosgrove, G.R. Focused Ultrasound Thalamotomy for Tremor in Parkinson’s Disease: Outcomes in a Large, Prospective Cohort. Mov. Disord. 2023, 38, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Purrer, V.; Pohl, E.; Borger, V.; Weiland, H.; Boecker, H.; Schmeel, F.C.; Wüllner, U. Motor and non-motor outcome in tremor dominant Parkinson’s disease after MR-guided focused ultrasound thalamotomy. J. Neurol. 2024, 271, 3731–3742. [Google Scholar] [CrossRef]
- Sammartino, F.; Krishna, V.; King, N.K.; Lozano, A.M.; Schwartz, M.L.; Huang, Y.; Hodaie, M. Tractography-Based Ventral Intermediate Nucleus Targeting: Novel Methodology and Intraoperative Validation. Mov. Disord. 2016, 31, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Zhuo, J.; Eisenberg, H.M.; Fishman, P.S.; Melhem, E.R.; Gullapalli, R.; Gandhi, D. Targeting of the dentato-rubro-thalamic tract for MR-guided focused ultrasound treatment of essential tremor. Neuroradiol. J. 2019, 32, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Hassler, R.; Reichert, T. Indikationen und Lokalisationsmethode der gezielten Hirnoperationen [Indications and localization of stereotactic brain operations]. Nervenarzt 1954, 25, 441–447. (In German) [Google Scholar]
- Macchi, G.; Jones, E.G. Toward an agreement on terminology of nuclear and subnuclear divisions of the motor thalamus. J. Neurosurg. 1997, 86, 77–92, Corrected and Republished in J. Neurosurg. 1997, 86, 670–685. [Google Scholar] [CrossRef]
- Owen, R.L.; Grewal, S.S.; Thompson, J.M.; Hassan, A.; Lee, K.H.; Klassen, B.T. Effectiveness of Thalamic Ventralis Oralis Anterior and Posterior Nuclei Deep Brain Stimulation for Posttraumatic Dystonia. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 137–142. [Google Scholar] [CrossRef]
- Hyam, J.A.; Owen, S.L.; Kringelbach, M.L.; Jenkinson, N.; Stein, J.F.; Green, A.L.; Aziz, T.Z. Contrasting connectivity of the ventralis intermedius and ventralis oralis posterior nuclei of the motor thalamus demonstrated by probabilistic tractography. Neurosurgery 2012, 70, 162–169, discussion 169. [Google Scholar] [CrossRef]
- Parras, O.; Domínguez, P.; Tomás-Biosca, A.; Guridi, J. The role of tractography in the localisation of the Vim nucleus of the thalamus and the dentatorubrothalamic tract for the treatment of tremor. Neurologia 2022, 37, 691–699. [Google Scholar] [CrossRef]
- Ohye, C.; Shibazaki, T.; Hirato, M.; Kawashima, Y.; Matsumura, M. Strategy of selective VIM thalamotomy guided by microrecording. Stereotact. Funct. Neurosurg. 1990, 54–55, 186–191. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Máñez-Miró, J.U.; Rodríguez-Rojas, R.; Del Álamo, M.; Shah, B.B.; Hernández-Fernández, F.; Pineda-Pardo, J.; Monje, M.H.G.; Fernández-Rodríguez, B.; Sperling, S.A.; et al. Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease. N. Engl. J. Med. 2020, 383, 2501–2513. [Google Scholar] [CrossRef]
- Ito, H.; Yamamoto, K.; Fukutake, S.; Kamei, T.; Yamaguchi, T.; Taira, T. Two-year follow-up results of magnetic resonance imaging-guided focused ultrasound unilateral pallidotomy for Parkinson’s disease. Neurol. Clin. Neurosci. 2021, 9, 73–76. [Google Scholar] [CrossRef]
- Horváth, K.; Aschermann, Z.; Kovács, M.; Makkos, A.; Harmat, M.; Janszky, J.; Komoly, S.; Karádi, K.; Kovács, N. Changes in Quality of Life in Parkinson’s Disease: How Large Must They Be to Be Relevant? Neuroepidemiology 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Monteiro, J.D.S.; Silva, B.B.E.; de Oliveira, R.R.; Borges, P.G.L.B.; Pereira, M.A.O.M.; Costa, K.A.; Nunes, A.L.S.; Telles, J.P.M.; Valença, M.M. Magnetic resonance-guided focused ultrasound ventral intermediate thalamotomy for Tremor-Dominant Parkinson’s disease: A systematic review and meta-analysis. Neurosurg. Rev. 2024, 47, 701. [Google Scholar] [CrossRef]
- Shi, Y.; Dobkin, R.; Weintraub, D.; Cho, H.R.; Caspell-Garcia, C.; Bock, M.; Brown, E.; Aarsland, D.; Dahodwala, N. Association of Baseline Depression and Anxiety with Longitudinal Health Outcomes in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2024, 11, 1103–1112. [Google Scholar] [CrossRef]
- Bouça-Machado, R.; Fernandes, A.; Ranzato, C.; Beneby, D.; Nzwalo, H.; Ferreira, J.J. Measurement tools to assess activities of daily living in patients with Parkinson’s disease: A systematic review. Front. Neurosci. 2022, 16, 945398. [Google Scholar] [CrossRef]
- Hariz, G.M.; Forsgren, L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol. Scand. 2011, 123, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Saporito, G.; Sucapane, P.; Bruno, F.; Catalucci, A.; Masciocchi, C.; Pistoia, M.L.; Splendiani, A.; Ricci, A.; Di Cesare, E.; Marini, C.; et al. Cognitive safety of focused ultrasound thalamotomy for tremor: 1-year follow-up results of the COGNIFUS part 2 study. Front. Neurol. 2024, 15, 1395282. [Google Scholar] [CrossRef] [PubMed]
- De Micco, R.; Satolli, S.; Siciliano, M.; Di Nardo, F.; Caiazzo, G.; Russo, A.; Giordano, A.; Esposito, F.; Tedeschi, G.; Tessitore, A. Connectivity Correlates of Anxiety Symptoms in Drug-Naive Parkinson’s Disease Patients. Mov. Disord. 2021, 36, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Boccalini, C.; Carli, G.; Pilotto, A.; Padovani, A.; Perani, D. Gender differences in dopaminergic system dysfunction in de novo Parkinson’s disease clinical subtypes. Neurobiol. Dis. 2022, 167, 105668. [Google Scholar] [CrossRef]
- Carey, G.; Viard, R.; Lopes, R.; Kuchcinski, G.; Defebvre, L.; Leentjens, A.F.; Dujardin, K. Anxiety in Parkinson’s Disease Is Associated with Changes in Brain Structural Connectivity. J. Park. Dis. 2023, 13, 989–998. [Google Scholar] [CrossRef]
- Dan, R.; Růžička, F.; Bezdicek, O.; Růžička, E.; Roth, J.; Vymazal, J.; Goelman, G.; Jech, R. Separate neural representations of depression, anxiety and apathy in Parkinson’s disease. Sci. Rep. 2017, 7, 12164. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, T.; Tachibana, H.; Oguru, M.; Matsui, K.; Toda, K.; Okuda, B.; Oka, N. Anxiety and depression in patients with Parkinson’s disease. Intern. Med. 2013, 52, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Cai, X.; Zhang, D.; Liu, J.; Na, P.; Li, W. Functional connectivity markers of depression in advanced Parkinson’s disease. Neuroimage Clin. 2020, 25, 102130. [Google Scholar] [CrossRef]
- Rohringer, C.R.; Sewell, I.J.; Gandhi, S.; Isen, J.; Davidson, B.; McSweeney, M.; Swardfager, W.; Scantlebury, N.; Swartz, R.H.; Hamani, C.; et al. Cognitive effects of unilateral thalamotomy for tremor: A meta-analysis. Brain Commun. 2022, 4, fcac287. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; McGough, J.; Gopinath, G.; Scantlebury, N.; Tripathi, R.; Brandmeir, C.; Boshmaf, S.Z.; Brandmeir, N.J.; Sewell, I.J.; Konrad, P.E.; et al. Cognitive outcomes following unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: Findings from two cohorts. Brain Commun. 2024, 6, fcae293. [Google Scholar] [CrossRef]
- Wang, Y. Thalamus and its functional connections with cortical regions contribute to complexity-dependent cognitive reasoning. Neuroscience 2024, 562, 125–134. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, J.; Li, J.; Dai, J.; Lin, F.; Wu, G. Altered Activation in Cerebellum Contralateral to Unilateral Thalamotomy May Mediate Tremor Suppression in Parkinson’s Disease: A Short-Term Regional Homogeneity fMRI Study. PLoS ONE 2016, 11, e0157562. [Google Scholar] [CrossRef]
- Dahmani, L.; Bai, Y.; Li, M.; Ren, J.; Shen, L.; Ma, J.; Li, H.; Wei, W.; Li, P.; Wang, D.; et al. Focused ultrasound thalamotomy for tremor treatment impacts the cerebello-thalamo-cortical network. npj Park. Dis. 2023, 9, 90. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Biundo, R.; Cecchin, D.; Frigo, A.C.; Kim, J.; Weis, L.; Strafella, A.P.; Antonini, A. Brain Amyloid Contribution to Cognitive Dysfunction in Early-Stage Parkinson’s Disease: The PPMI Dataset. J. Alzheimers Dis. 2018, 66, 229–237. [Google Scholar] [CrossRef]
- Park, S.H.; Baik, K.; Jeon, S.; Chang, W.S.; Ye, B.S.; Chang, J.W. Extensive frontal focused ultrasound mediated blood-brain barrier opening for the treatment of Alzheimer’s disease: A proof-of-concept study. Transl. Neurodegener. 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; D’Haese, P.F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Vieira Ligo Teixeira, C.; et al. Ultrasound Blood-Brain Barrier Opening and Aducanumab in Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Fernández-Rodríguez, B.; Pineda-Pardo, J.A.; Rodríguez-Rojas, R.; Obeso, I.; Hernández-Fernández, F.; Del Álamo, M.; Mata, D.; Guida, P.; Ordás-Bandera, C.; et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef]
- Pineda-Pardo, J.A.; Gasca-Salas, C.; Fernández-Rodríguez, B.; Rodríguez-Rojas, R.; Del Álamo, M.; Obeso, I.; Hernández-Fernández, F.; Trompeta, C.; Martínez-Fernández, R.; Matarazzo, M.; et al. Striatal Blood-Brain Barrier Opening in Parkinson’s Disease Dementia: A Pilot Exploratory Study. Mov. Disord. 2022, 37, 2057–2065. [Google Scholar] [CrossRef]
- Jeong, H.; Im, J.J.; Park, J.S.; Na, S.H.; Lee, W.; Yoo, S.S.; Song, I.U.; Chung, Y.A. A pilot clinical study of low-intensity transcranial focused ultrasound in Alzheimer’s disease. Ultrasonography 2021, 40, 512–519. [Google Scholar] [CrossRef]
- Khan, A.; Ezeugwa, J.; Ezeugwu, V.E. A systematic review of the associations between sedentary behavior, physical inactivity, and non-motor symptoms of Parkinson’s disease. PLoS ONE 2024, 19, e0293382. [Google Scholar] [CrossRef]
- Kim, R.; Lee, T.L.; Lee, H.; Ko, D.K.; Lee, J.H.; Shin, H.; Lim, D.; Jun, J.S.; Byun, K.; Park, K.; et al. Effects of physical exercise interventions on cognitive function in Parkinson’s disease: An updated systematic review and meta-analysis of randomized controlled trials. Park. Relat. Disord. 2023, 117, 105908. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Galvan, P.; Franco-Rosado, P.; Silva-Rodriguez, J.; Castro-Labrador, S.; Labrador-Espinosa, M.A.; Muñoz-Delgado, L.; Grothe, M.J.; Mir, P. Association of Physical Exercise with Structural Brain Changes and Cognitive Decline in Patients with Early Parkinson Disease. Neurology 2025, 105, e213932. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Res. Rev. 2020, 60, 101044. [Google Scholar] [CrossRef]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kim, H.S. Suppression of neuroinflammation and α-synuclein oligomerization by rotarod walking exercise in subacute MPTP model of Parkinson’s disease. Neurochem. Int. 2023, 165, 105519. [Google Scholar] [CrossRef]
- Maass, A.; Düzel, S.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövden, M.; Lindenberger, U.; Bäckman, L.; Braun-Dullaeus, R.; et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry 2015, 20, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; He, Y.X.; Wen, Q.; Liu, J.Y.; Dai, Y.; Fei, Y.Z.; Li, H.; Li, C.Q.; Zhou, H. Evaluation of the efficacy of Tai Chi on the cognitive function of patients with mild cognitive dysfunction and research on its mechanism. Front. Aging Neurosci. 2025, 17, 1435996. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Han, K.D.; Baek, M.S.; Cho, H.; Lee, E.J.; Lyoo, C.H. Association between physical activity and conversion from mild cognitive impairment to dementia. Alzheimers Res. Ther. 2020, 12, 136. [Google Scholar] [CrossRef]
- Demurtas, J.; Schoene, D.; Torbahn, G.; Marengoni, A.; Grande, G.; Zou, L.; Petrovic, M.; Maggi, S.; Cesari, M.; Lamb, S.; et al. Physical Activity and Exercise in Mild Cognitive Impairment and Dementia: An Umbrella Review of Intervention and Observational Studies. J. Am. Med. Dir. Assoc. 2020, 21, 1415–1422.e6. [Google Scholar] [CrossRef]
- Zur, G.; Lesman-Segev, O.H.; Schlesinger, I.; Goldsher, D.; Sinai, A.; Zaaroor, M.; Assaf, Y.; Eran, A.; Kahn, I. Tremor Relief and Structural Integrity after MRI-guided Focused US Thalamotomy in Tremor Disorders. Radiology 2020, 294, 676–685. [Google Scholar] [CrossRef]
- Boecker, H.; Schild, H.H.; Kindler, C.; Schmitt, A.; Solymosi, L.; Wüllner, U.; Pieper, C.C. MRI follow-up after magnetic resonance-guided focused ultrasound for non-invasive thalamotomy: The neuroradiologist’s perspective. Neuroradiology 2020, 62, 1111–1122. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Gumin, I.S.; Nikitin, D.V.; Shipilova, N.N.; Katunina, E.A.; Senko, I.V.; Dolgushin, M.B. Fractional anisotropy within zone of destruction, tremor evaluation and MRI manifestation follow up after focused ultrasound thalamotomy for patients with Parkinson’s disease. Med. Vis. 2024, 28, 11–20. (In Russian) [Google Scholar] [CrossRef]
- França, C.; Cury, R.G. The best of both worlds: Deep brain stimulation or high-frequency focused ultrasound for tremor refractory syndromes. Arq. Neuropsiquiatr. 2025, 83, 1–3. [Google Scholar] [CrossRef]
- Thomas, B.; Bellini, G.; Lee, W.Y.; Shi, Y.; Mogilner, A.; Pourfar, M.H. High Intensity Focused Ultrasound—Longitudinal Data on Efficacy and Safety. Tremor Other Hyperkinetic Mov. 2025, 15, 18. [Google Scholar] [CrossRef]
- Massruhá, K.S.; Cardoso, E.F. High-intensity focused ultrasound (HIFU) versus deep brain stimulation (DBS) for refractory tremor: Team HIFU. Arq. Neuropsiquiatr. 2025, 83, 1–4. [Google Scholar] [CrossRef] [PubMed]





| Study Parameter | Value |
|---|---|
| Age, median [IQR a], y | 61 [54; 67] |
| Men/women, No. (%) | 14 (66)/7 (34) |
| Disease duration, median [IQR], y | 7 [4.5; 8.5] |
| Duration of antiparkinsonian medications, median [IQR], y | 6 [4.5; 8.5] |
| Operated hemisphere: right/left, No. (%) | 12 (57)/9 (43) |
| H & Y b stage (1/1.5/2/2.5/3), No. | 1/2/11/5/2 |
| NMSS c (<10 points/10–20 points/21–30 points/>30 points); No. (%) | 11 (52.4%)/4 (19%)/5 (23.8%)/1 (4.8%) |
| Cognitive function, MoCA d (normal/MCI e), No. (%) | 11 (52.4)/10 (47.6) |
| Impulsive–compulsive disorder (QUIP f) (absent/present), No. (%) | 4 (19)/17 (81) |
| Apathy (absent/present), No. (%) | 4 (19)/17 (81) |
| Depression (absent/mild), No. (%) | 10 (47.6)/11 (52.4) |
| Anxiety (low/mild), No. (%) | 20 (95.2)/1 (4.8) |
| Quality of life (score >50/score 25–50/score < 25), No. (%) | 7 (33.3)/6 (28.6)/8 (38.1) |
| Activity of daily living mostly dependent (≤50)/partially dependent (60–80)/independent (90–100), No. (%) | 2 (9.6)/14 (66.6)/5 (23.8) |
| Variables | Before MRgFUS | Day 2 Post-MRgFUS | 3 Months Post-MRgFUS | 6 Months Post-MRgFUS | 12 Months Post-MRgFUS |
|---|---|---|---|---|---|
| UPDRS a, median [IQR b] | 37.0 [24.5; 47.0] | 18.0 [12.0; 31.0] # | 25.0 [17.5; 37.0] # | 26.0 [17.5; 32.5] * | 26.0 [19.0; 32.0] † |
| UPDRS, % reduction from baseline | 51% [95% CI 34–67%] # | 33% [95% CI 14–49%] # | 30% [95% CI 16–46%] # | 30% [95% CI 15–48%] # | |
| UPDRS III, median [IQR] | 23.5 [14.0; 35.75] | 13.0 [7.0; 18.0] # | 14.5 [11.25; 21.5] # | 14.0 [10.5; 22.5] # | 17.0 [13.0; 25.0] # |
| UPDRS III, % reduction from baseline | 45% [95% CI 28–63%] # | 37% [95% CI 15–46%] # | 32% [95% CI 7–56%] # | 33% [95% CI 11–55%] # | |
| Hemi-UPDRS c, median [IQR] | 16.5 [12.0–19.75] | 4.5 [2.0–8.25] # | 6.5 [6.0–13.0] # | 7.0 [5.0–10.5] # | 7.0 [5.5–11.5] # |
| Hemi-UPDRS, % reduction from baseline | 70% [95% CI 60–78%] # | 57% [95% CI 37–74%] # | 60% [95% CI 39–84%] # | 52% [95% CI 29–74%] # | |
| CRST d, median [IQR] | 33.0 [25.0–47.0] | 8.0 [3.0–20.0] # | 17.0 [6.0–23.0] # | 18.0 [12.0–28.5] # | 14.0 [6.0–22.5] # |
| CRST, % reduction from baseline | 62.5% [95% CI 48–76%] # | 48% [95% CI 34–61%] # | 39% [95% CI 17–55%] # | 44% [95% CI 31–65%] # | |
| Hemi-CRST e, median [IQR] | 16.5 [13.0–25.0] | 3.0 [2.0–5.0] # | 8.0 [4.0–12.0] # | 9.5 [3.5–12.75] # | 10.0 [4.0–13.0] # |
| Hemi-CRST, % reduction from baseline | 70% [95% CI 54–85%] # | 50% [95% CI 23–76%] # | 44% [95% CI 13–75%] # | 41% [95% CI 17–71%] # | |
| Rest tremor + action tremor on UPDRS in the operated side (item 20–21), median [IQR] | 7.0 [5.0–9.0] | 1.0 [0.0–1.0] # | 3.0 [1.0–6.0] # | 4.0 [0.0–5.0] # | 3.0 [1.0–4.0] # |
| Rest tremor + action tremor in the operated side (item 20–21), % reduction from baseline | 85% [95% CI 63–96%] # | 62% [95% CI 39–88%] # | 53% [95% CI 26–81%] # | 59% [95% CI 32–77%] # | |
| Rigidity on UPDRS (item 22), median [IQR] | 3.0 [2.0–4.0] | 1.0 [0.0–2.0] # | 1.0 [0.0–3.5] # | 1.0 [0.0–2.0] # | 2.0 [1.0–3.0] # |
| Rigidity on UPDRS (item 22), % reduction from baseline | 62% [95% CI 28–96%] # | 63% [95% CI 16–98%] # | 66% [95% CI 22–98%] # | 34% [95% CI 16–57%] # | |
| Hypokinesia on UPDRS (items 23–26), median [IQR] | 5.5 [2.25–9.0] | 4.0 [1.0–4.5] # | 4.0 [2.0–6.75] # | 3.0 [1.0–4.0] # | 4.0 [3.0–5.0] |
| Hypokinesia on UPDRS (items 23–26), % reduction from baseline | 24% [95% CI 11–38%] | 23% [95% CI 8–40%] # | 36% [95% CI 17–54%] | 24% [95% CI 10–36%] |
| Scale | Before MRgFUS | 3 Months Post-MRgFUS | 6 Months Post-MRgFUS | 12 Months Post-MRgFUS |
|---|---|---|---|---|
| NMSS a | 7 [4.5; 23] | 9 [5.5; 18.5] | 11 [4.5; 20.0] | 11 [8.5; 22.0] |
| PDQ-39 b | 43.0 [21.0; 57.0] | 33.0 [16.5; 51.5] | 34.0 [16.0; 53.5] | 32.0 [13.0; 52.5] |
| Schwab and England Activities of Daily Living Scale | 80 [75; 85] | 90 [80; 90] | 90 [70; 90] | 90 [75; 90] |
| Beck Anxiety Inventory | 6.0 [4.0; 10.5] | 12.5 [7.25; 19.0] * | 10.5 [6.25; 20.0] * | 10.0 [6.5; 19.5] * |
| Beck Depression Inventory | 10.0 [5.0; 15.5] | 11.0 [7.0; 15.0] | 14.0 [7.0; 16.0] | 11.0 [8.0; 15.0] |
| Apathy Scale | 9.0 [7.5; 12.0] | 10.0 [7.0; 13.5] | 10.0 [8.0; 13.0] | 9.0 [6.5; 15.0] |
| MoCA c total | 26.0 ± 2.9 | 26.6 ± 2.8 | 27.0 ± 2.0 | 26.7 ± 2.7 |
| MoCA < 26 (n = 10) | 23.1 ± 1.2 | 25.3 ± 2.2 ˟ | 26.0 ± 2.1 ˟ | 26.1 ± 2.7 ˟ |
| MoCA ≥ 26 (n = 11) | 28.1 ± 1.5 | 27.6 ± 2.9 | 27.8 ± 1.5 | 27.2 ± 2.7 |
| Independent Variables | B | 95% CI | T | p |
|---|---|---|---|---|
| Baseline | ||||
| Depression | 1.5589 | 0.2784; 2.8395 | 2.569 | 0.0199 |
| UPDRS | 0.6696 | 0.2398; 1.0994 | 3.287 | 0.0043 |
| 12 months | ||||
| Depression | 3.4349 | 1.8033; 5.0666 | 4.463 | 0.0004 |
| ADL | 0.7900 | 0.0329; 1.5471 | 2.212 | 0.0419 |
| Variable | Baseline | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| “increased” LEDD group (n = 12) | ||||
| LEDD | 461.5 ± 312.3 | 512.7 ± 295.0 | 565.6 ± 252.6 * | 682.3 ± 279.3 * |
| “combined” LEDD group (n = 9) | ||||
| LEDD | 565.3 ± 292.9 | 500.8 ± 257.4 | 508.3 ± 266.3 | 502.7 ± 275.1 |
| Time Interval | Paired Difference | 95% CI | T | p |
|---|---|---|---|---|
| baseline vs. month 3 | 51.2 | −24.2; 126.3 | 1.49 | 0.163 |
| baseline vs. month 6 | 104.2 | 14.8; 193.6 | 2.56 | 0.026 |
| baseline vs. month 12 | 220.8 | 165.3; 276.4 | 8.76 | 0.000 |
| month 3 vs. month 6 | 52.9 | −32.8; 138.6 | 1.36 | 0.201 |
| month 3 vs. month 12 | 169.6 | 103.4; 235.9 | 5.64 | 0.000 |
| month 6 vs. month 12 | 116.7 | 56.8; 176.5 | 4.29 | 0.001 |
| Time Interval | Paired Difference | 95% CI | T | p |
|---|---|---|---|---|
| baseline vs. month 3 | −65.3 | −145.6; 15.1 | 1.87 | 0.098 |
| baseline vs. month 6 | −56.9 | −128.1; 14.2 | 1.85 | 0.102 |
| baseline vs. month 12 | −62.5 | −131.8; 6.8 | 2.08 | 0.071 |
| month 3 vs. month 6 | 8.3 | −5.3; 21.9 | 1.41 | 0.195 |
| month 3 vs. month 12 | 2.8 | −23.0; 17.5 | 0.32 | 0.760 |
| month 6 vs. month 12 | 5.5 | −7.3; 18.4 | 1.00 | 0.347 |
| Symptom | Me (IQR) | Z | p | |
|---|---|---|---|---|
| Increased LEDD | Combined LEDD | |||
| Motor symptoms | ||||
| Tremor- + hypokinesia- + rigidity-treated side | 14.25 (17.5; 21.5) | 12.0 (10.0; 18.5) | −1.4597 | 0.1444 |
| Tremor-treated side | 25.25 (32.0; 48.75) | 30.0 (20.5; 39.25) | −0.5789 | 0.5627 |
| UPDRS | 27.75 (37.5; 41.0) | 27.0 (18.0; 55.0) | −0.7467 | 0.4553 |
| Hemi-CRST-untreated side | 5.5 (2.0; 9.5) | 7.0 (3.5; 12.0) | −0.9638 | 0.3351 |
| Hemi-CRST-treated side | 20.0 (14.0; 23.0) | 16.0 (13.0; 25.5) | −0.6417 | 0.5211 |
| Non-motor symptoms | ||||
| ADL | 80.0 (72.5; 85.25) | 80.0 (75.0; 95.0) | −1.1168 | 0.2641 |
| Anxiety | 6.5 (4.25; 14.5) | 5.0 (3.5; 10.5) | −1.1468 | 0.2515 |
| Apathy | 8.0 (6.25; 10.75) | 9.0 (8.0; 14.0) | −0.8609 | 0.3893 |
| Depression | 12.0 (6.25; 16.75) | 7.0 (2.5; 14.0) | −1.0332 | 0.3015 |
| LEDD | 310.0 (152.5; 806.25) | 550.0 (325.0; 862.5) | −0.7845 | 0.4327 |
| MoCA | 25.5 (24.25; 29.25) | 24.0 (23.0; 27.5) | −1.4674 | 0.1423 |
| NMSS | 10.5 (5.0; 23.5) | 7.0 (2.5; 20.0) | −1.1051 | 0.2691 |
| QoL | 44.5 (19.5; 62.5) | 43.0 (15.5; 53.5) | −0.7825 | 0.4339 |
| Symptom | Me (IQR) | Z | p | |
|---|---|---|---|---|
| Increased LEDD | Combined LEDD | |||
| Motor symptoms | ||||
| Tremor- + hypokinesia- + rigidity-treated side | 11.5 (6.75; 14.0) | 6.0 (4.0; 7.5) | −2.4630 | 0.0138 |
| Tremor-treated side | 19.5 (7.25; 23.0) | 11.0 (6.0; 15.0) | −1.1030 | 0.2700 |
| UPDRS | 30.5 (22.75; 36.75) | 25.0 (16.5; 27.0) | −1.8175 | 0.0691 |
| Hemi-CRST-untreated side | 6.5 (2.5; 9.0) | 8.0 (4.5; 12.0) | 0.8561 | 0.391 |
| Hemi-CRST-treated side | 11.5 (5.75; 15.0) | 3.0 (0.00; 10.0) | −2.3225 | 0.0202 |
| Non-motor symptoms | ||||
| ADL | 85.0 (70.0; 97.5) | 91.5 (88.0; 96.0) | −0.7720 | 0.4401 |
| Anxiety | 10.5 (7.25; 18.75) | 10.0 (4.5; 23.5) | −0.0711 | 0.9433 |
| Apathy | 9.0 (7.25; 16.0) | 12.0 (6.0; 16.0) | −0.0357 | 0.9715 |
| Depression | 12.0 (10.0; 16.0) | 9.0 (5.0; 15.0) | −1.0763 | 0.2818 |
| LEDD | 551.25 (431.25; 956.25) | 500.0 (275.0; 750.0) | −1.3525 | 0.1762 |
| MoCA | 26.5 (24.25; 28.75) | 28.0 (25.0; 29.5) | −0.8621 | 0.3886 |
| NMSS | 15.0 (9.0; 22.0) | 9.0 (7.0; 22.5) | −0.7139 | 0.4753 |
| QoL | 36.5 (14.0; 54.75) | 16.0 (12.0; 39.0) | −0.9600 | 0.3370 |
| Variable | B | 95% CI | T | p |
|---|---|---|---|---|
| progression of tremor + rigidity + hypokinesia between 2nd day and end of the 6th month (treated side) | 0.073 | 0.024; 0.122 | 3.25 | 0.0070 |
| Adverse Event | № (%) of Patients | |||
|---|---|---|---|---|
| 2nd Day | 3 Months | 6 Months | 12 Months | |
| Hemi- or monoparesis | 5 (23.8) | 4 (19.0) | 3 (14.3) | 1 (4.8) |
| Dysarthria | 4 (19.0) | 2 (9.5) | 1 (4.8) | 1 (4.8) |
| Hemiataxia | 4 (19.0) | 3 (14.3) | 1 (4.8) | 1 (4.8) |
| Parasthesia/numbness orofacial | 4 (19.0) | 2 (9.5) | 2 (9.5) | 2 (9.5) |
| Parasthesia/numbness finger | 1 (4.8) | - | - | - |
| Before MRgFUS | 3 hrs Post-MRgFUS | Day 2 Post-MRgFUS | 3 Months Post-MRgFUS | 6 Months Post-MRgFUS | 12 Months Post-MRgFUS |
|---|---|---|---|---|---|
| NUCLEUS | |||||
| - | 194 [157; 238] | 394 [314; 504.5] | 29 [12; 52] | 9 [2; 20] | 9.5 [4; 24.75] |
| EDEMA | |||||
| - | 841 [709–1101] | 2718.5 [1974.5; 3426.25] | 37 [13.5–50.0] | 18 [0.5; 44] | 8 [0; 16] |
| FRACTIONAL ANISOTROPY | |||||
| 0.39 [0.36; 0.45] | 0.15 [0.13; 0.17] z = −4.014; p = 0.0001 * | 0.16 [0.14; 0.19] z = −3.823; p = 0.0001 * | 0.31 [0.25; 0.34] z = −2.897; p = 0.0038 * | 0.32 [0.26; 0.36] z = −1.334; p = 0.182 * | 0.32 [0.27; 0.57] z = −0.405; p = 0.687 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katunina, E.A.; Martynov, M.Y.; Belousov, V.V.; Titova, N.V.; Dolgushin, M.B.; Tairova, R.T.; Shipilova, N.N.; Ivanova, M.Z.; Senko, I.V.; Gumin, I.S.; et al. The Results of a 12-Month Open-Label Follow-Up Study with MRI Monitoring of Patients with Parkinson’s Disease After MRI-Guided FUS. J. Clin. Med. 2025, 14, 8329. https://doi.org/10.3390/jcm14238329
Katunina EA, Martynov MY, Belousov VV, Titova NV, Dolgushin MB, Tairova RT, Shipilova NN, Ivanova MZ, Senko IV, Gumin IS, et al. The Results of a 12-Month Open-Label Follow-Up Study with MRI Monitoring of Patients with Parkinson’s Disease After MRI-Guided FUS. Journal of Clinical Medicine. 2025; 14(23):8329. https://doi.org/10.3390/jcm14238329
Chicago/Turabian StyleKatunina, Elena Anatolievna, Mikhail Yurievich Martynov, Vsevolod Vadimovich Belousov, Nataliya Vladimirovna Titova, Mikhail Borisovich Dolgushin, Raisa Tairovna Tairova, Natalia Nikolaevna Shipilova, Madina Zamirovna Ivanova, Ilya Vladimirovich Senko, Ivan Sergeevich Gumin, and et al. 2025. "The Results of a 12-Month Open-Label Follow-Up Study with MRI Monitoring of Patients with Parkinson’s Disease After MRI-Guided FUS" Journal of Clinical Medicine 14, no. 23: 8329. https://doi.org/10.3390/jcm14238329
APA StyleKatunina, E. A., Martynov, M. Y., Belousov, V. V., Titova, N. V., Dolgushin, M. B., Tairova, R. T., Shipilova, N. N., Ivanova, M. Z., Senko, I. V., Gumin, I. S., & Dzhafarov, V. M.-o. (2025). The Results of a 12-Month Open-Label Follow-Up Study with MRI Monitoring of Patients with Parkinson’s Disease After MRI-Guided FUS. Journal of Clinical Medicine, 14(23), 8329. https://doi.org/10.3390/jcm14238329







