Single-Shot Subcutaneous Lidocaine Infiltration at Closure Is Associated with Reduced Early Pain and Opioid Requirement After Single-Incision Laparoscopic Totally Extraperitoneal Hernia Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Surgical Technique and Lidocaine Infiltration Protocol
2.3. Intraoperative Anesthesia and PACU Management
2.4. Postoperative Ward Management
2.5. Measurement of Postoperative Pain Intensity
2.6. Outcomes and Variable Definitions
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics Before and After Matching
3.2. Postoperative Outcomes
3.3. Post-Matching Regression Adjustment: Pain-Related Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA-PS | American Society of Anesthesiologists Physical Status |
| AUR | Acute Urinary Retention |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| EHS | European Hernia Society |
| ERAS | Enhanced Recovery After Surgery |
| HCl | Hydrochloride |
| IQR | Interquartile Range |
| IRB | Institutional Review Board |
| IV | Intravenous |
| LOS | Length of Stay |
| NPIS | Numeric Pain Intensity Scale |
| OR | Odds Ratio |
| PACU | Post-Anesthesia Care Unit |
| POD | Postoperative Day |
| PSM | Propensity Score Matching |
| QLB | Quadratus Lumborum Block |
| SD | Standard Deviation |
| SILTEP | Single-Incision Laparoscopic Totally Extraperitoneal |
| SMD | Standardized Mean Difference |
| SSI | Surgical Site Infection |
| TAP | Transversus Abdominis Plane |
| TEP | Totally Extraperitoneal |
References
- Huerta, S.; Garza, A.M. A systematic review of open, laparoscopic, and robotic inguinal hernia repair: Management of inguinal hernias in the 21st century. J. Clin. Med. 2025, 14, 990. [Google Scholar] [CrossRef] [PubMed]
- Bullen, N.L.; Massey, L.H.; Antoniou, S.A.; Smart, N.J.; Fortelny, R.H. Open versus laparoscopic mesh repair of primary unilateral uncomplicated inguinal hernia: A systematic review with meta-analysis and trial sequential analysis. Hernia 2019, 23, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Filipović-Cugura, J.; Kirac, I.; Kuliš, T.; Janković, J.; Bekavac-Bešlin, M. Single-incision laparoscopic surgery (SILS) for totally extraperitoneal (TEP) inguinal hernia repair: First case. Surg. Endosc. 2009, 23, 920–921. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Jeong, W.J.; Lee, I.K.; Lee, S.C. Single-port versus conventional three-port laparoscopic totally extraperitoneal inguinal hernia repair: A randomized controlled trial. Hernia 2016, 20, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawhney, M.; Goldstein, D.H.; Wei, X.; Pare, G.C.; Wang, L.; VanDenKerkhof, E.G. Pain and haemorrhage are the most common reasons for emergency department use and hospital admission in adults following ambulatory surgery: Results of a population-based cohort study. Perioper. Med. 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solodkyy, A.; Feretis, M.; Fedotovs, A.; Di Franco, F.; Gergely, S.; Harris, A.M. Elective “true day case” laparoscopic inguinal hernia repair in a district general hospital: Lessons learned from 1000 consecutive cases. Minim. Invasive Surg. 2018, 2018, 7123754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arora, S.; Chhabra, A.; Subramaniam, R.; Arora, M.K.; Misra, M.C.; Bansal, V.K. Transversus abdominis plane block for laparoscopic inguinal hernia repair: A randomized trial. J. Clin. Anesth. 2016, 33, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bhoi, D.; Chhabra, A.; Mohan, V.K.; Darlong, V.; Prasad, G. Quadratus lumborum block vs. transversus abdominis plane block in laparoscopic trans-abdominal pre-peritoneal repair of inguinal hernia in adults: A randomized controlled trial. Indian J. Anaesth. 2023, 67, 207–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, S.R.; Kang, D.B.; Lee, C.; Park, W.C.; Lee, J.K. Postoperative pain relief using wound infiltration with 0.5% bupivacaine in single-incision laparoscopic surgery for an appendectomy. Ann. Coloproctology 2013, 29, 238–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suragul, W.; Tantawanit, A.; Rungsakulkij, N.; Muangkaew, P.; Tangtawee, P.; Mingphrudhi, S.; Vassanasiri, W.; Lertsithichai, P.; Aeesoa, S.; Apinyachon, W. Effect of local anaesthetic infiltration on postoperative pain after laparoscopic cholecystectomy: Randomized clinical trial. BJS Open 2022, 6, zrac066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garmi, G.; Parasol, M.; Zafran, N.; Rudin, M.; Romano, S.; Salim, R. Efficacy of single wound infiltration with bupivacaine and adrenaline during cesarean delivery for reduction of postoperative pain: A randomized clinical trial. JAMA Netw. Open 2022, 5, e2242203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.; Kim, T.; Lee, J.M. Innovative articulating instruments in single incision laparoscopic totally extraperitoneal hernioplasty (SILTEP): Evaluating feasibility, safety, and learning curve. Asian J. Surg. 2025, 48, 2262–2267. [Google Scholar] [CrossRef]
- Kulasegaran, S.; Rohan, M.; Pearless, L.; Hulme-Moir, M. Pre-peritoneal local anaesthetic does not reduce post-operative pain in laparoscopic total extra-peritoneal inguinal hernia repair: Double-blinded randomized controlled trial. Hernia 2017, 21, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sharma, N.; Singh, S. Port-site infiltration and extraperitoneal instillation of ropivacaine in totally extraperitoneal hernia repair: A randomized controlled trial. World J. Laparosc. Surg. 2024, 17, 33–37. [Google Scholar] [CrossRef]
- European Society of Anaesthesiology and Intensive Care (ESAIC); European Society of Regional Anaesthesia & Pain Therapy (ESRA). ENCORE: Conversion Tables for IV and Oral Analgesics. Published 2023. Available online: https://esaic.org/wp-content/uploads/2023/12/conversion-tables-for-iv-and-oral-analgesics.pdf (accessed on 5 November 2025).
- Kendrick, D.B.; Strout, T.D. The minimum clinically significant difference in patient-assigned numeric scores for pain. Am. J. Emerg. Med. 2005, 23, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Afonso, J.; Carneiro, A.L.; Vila, I.; Cunha, C.; Roque, S.; Silva, C.; Mesquita, A.; Cotter, J.; Correia-Neves, M.; et al. Exploring the diversity of visceral, subcutaneous and perivascular adipose tissue in a vascular surgery population. J. Cardiovasc. Dev. Dis. 2023, 10, 271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mungroop, T.H.; Bond, M.J.; Lirk, P.; Busch, O.R.; Hollmann, M.W.; Veelo, D.P.; Besselink, M.G. Preperitoneal or subcutaneous wound catheters as alternative for epidural analgesia in abdominal surgery: A systematic review and meta-analysis. Ann. Surg. 2019, 269, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, D.M.; Bezmarevic, M.; Bojic, S.; Unic-Stojanovic, D.; Stojkovic, D.; Slavkovic, D.Z.; Bancevic, V.; Maric, N.; Karanikolas, M. Updates on wound infiltration use for postoperative pain management: A narrative review. J. Clin. Med. 2021, 10, 4659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, J.B.; Song, J.; Mahabir, R.C. Onset and duration of intradermal mixtures of bupivacaine and lidocaine with epinephrine. Can. J. Plast. Surg. 2013, 21, 51–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eledjam, J.J.; de la Coussaye, J.E.; Bassoul, B.; Brugada, J. Mécanismes de la toxicité cardiaque de la bupivacaïne [Mechanisms of the cardiac toxicity of bupivacaine]. Ann. Fr. Anesth. Reanim. 1988, 7, 204–210. [Google Scholar] [CrossRef] [PubMed]

| Overall Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| No Lidocaine (n = 117) | Lidocaine (n = 82) | SMD | No Lidocaine (n = 82) | Lidocaine (n = 82) | SMD | |
| Age | 62.9 ± 14.8 | 65.0 ± 17.2 | 0.13 | 64.0 ± 14.2 | 65.0 ± 17.2 | 0.06 |
| Male sex | 107 (91.5) | 80 (97.6) | 0.27 | 80 (97.6) | 80 (97.6) | <0.01 |
| BMI > 25 kg/m2 | 40 (34.2) | 21 (25.6) | 0.19 | 24 (29.3) | 21 (25.6) | 0.08 |
| ASA PS ≥ III | 36 (30.8) | 37 (45.1) | 0.30 * | 33 (40.2) | 37 (45.1) | 0.09 |
| Previous lower abdominal surgery | 25 (21.4) | 16 (19.5) | 0.05 | 17 (20.7) | 16 (19.5) | 0.03 |
| Bilateral hernia | 19 (16.2) | 5 (6.1) | 0.33 * | 6 (7.3) | 5 (6.1) | 0.05 |
| EHS size classification | 0.24 | 0.11 | ||||
| 1 | 39 (33.9) | 19 (23.2) | 23 (28.0) | 19 (23.2) | ||
| 2 | 68 (59.1) | 56 (68.3) | 53 (64.6) | 56 (68.3) | ||
| 3 | 8 (7.0) | 8 (8.5) | 6 (7.3) | 7 (8.5) | ||
| Treatment period | 2.35 | 2.18 | ||||
| 2022.11–2024.10 | 111 (94.9) | 16 (19.5) | 76 (92.7) | 16 (19.5) | ||
| 2024.11–2025.07 | 6 (5.1) | 66 (80.5) | 6 (7.3) | 66 (80.5) | ||
| Intraoperative IV analgesia use | ||||||
| Opioid | 31 (26.5) | 32 (39.0) | 0.27 | 26 (31.7) | 32 (39.0) | 0.15 |
| Non-opioid | 67 (57.3) | 47 (57.3) | <0.01 | 50 (61.0) | 47 (57.3) | 0.07 |
| Dexamethasone | 49 (41.9) | 36 (43.9) | 0.04 | 35 (42.7) | 36 (43.9) | 0.02 |
| Lidocaine | 108 (92.3) | 67 (81.7) | 0.32 * | 73 (89.0) | 67 (81.7) | 0.21 |
| Overall Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| No Lidocaine (n = 117) | Lidocaine (n = 82) | p | No Lidocaine (n = 82) | Lidocaine (n = 82) | p | |
| Any rescue analgesia | 68 (58.1) | 31 (37.8) | 0.007 | 42 (51.2) | 31 (37.8) | 0.116 |
| Rescue analgesia on POD 0 | 66 (56.4) | 28 (34.1) | 0.003 | 40 (48.8) | 28 (34.1) | 0.081 |
| Rescue analgesia on POD 1 | 9 (7.7) | 8 (9.8) | 0.799 | 6 (7.3) | 8 (9.8) | 0.780 |
| Number of rescue doses during hospitalization | 1.00 ± 1.36 | 0.46 ± 0.65 | 0.008 | 0.96 ± 1.52 | 0.46 ± 0.65 | 0.007 |
| Morphine equivalent dose (mg) | 5.0 (0.0–5.0) | 0.0 (0.0–5.0) | 0.002 | 5.0 (0.0–5.0) | 0.0 (0.0–5.0) | 0.028 |
| Additional analgesia at outpatient follow-up | 4 (3.4) | 1 (1.2) | 0.329 | 4 (4.9) | 1 (1.2) | 0.364 |
| LOS | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.900 | 1 (1–1) | 1 (1–1) | 0.855 |
| All complications | 8 (6.8) | 5 (6.1) | 0.835 | 5 (6.1) | 5 (6.1) | >0.999 |
| Seroma | 4 (3.4) | 6 (7.3) | 0.215 | 3 (3.7) | 6 (7.3) | 0.493 |
| SSI | 4 (3.4) | 1 (1.2) | 0.329 | 2 (2.4) | 1 (1.2) | >0.999 |
| AUR | 3 (2.6) | 0 (0.0) | 0.144 | 1 (1.2) | 0 (0.0) | >0.999 |
| Arrhythmia | 1 (0.9) | 0 (0.0) | >0.999 | 1 (1.2) | 0 (0.0) | >0.999 |
| No Lidocaine (n = 82) | Lidocaine (n = 82) | p | |
|---|---|---|---|
| Primary outcome | |||
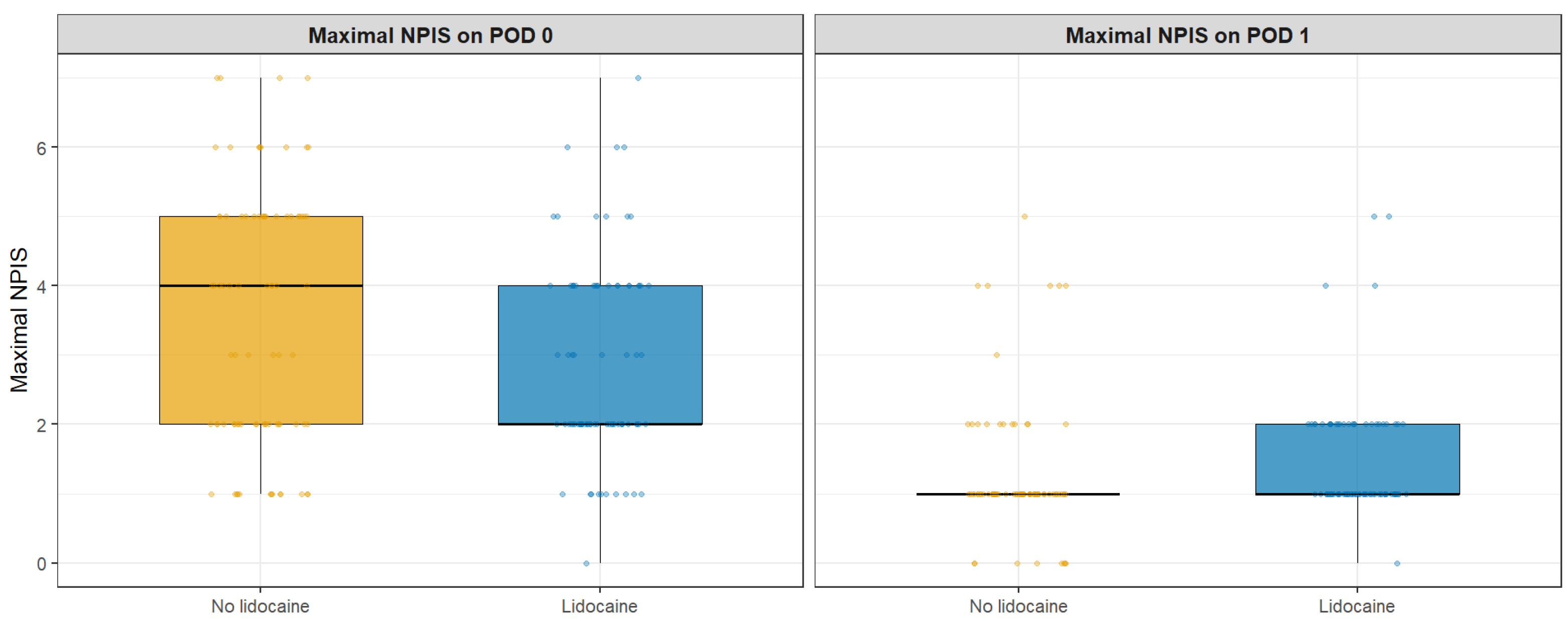
| Maximal NPIS on POD 0 | 4.0 (2.0–5.0) | 2.0 (2.0–4.0) | 0.023 |
| Secondary outcomes | |||
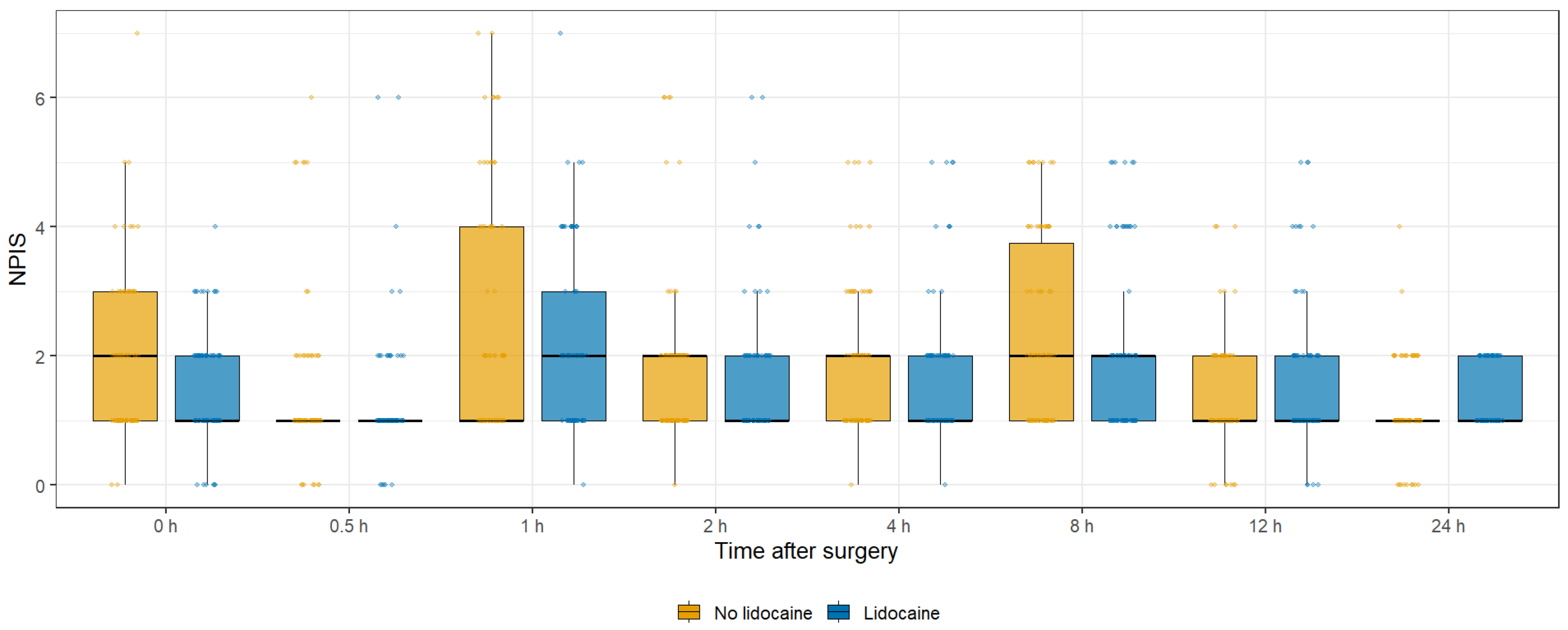
| NPIS at 0 h | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 0.092 |
| NPIS at 0.5 h | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.673 |
| NPIS at 1 h | 1.0 (1.0–4.0) | 2.0 (1.0–3.0) | 0.542 |
| NPIS at 2 h | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.164 |
| NPIS at 4 h | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.173 |
| NPIS at 8 h | 2.0 (1.0–3.8) | 2.0 (1.0–2.0) | 0.107 |
| NPIS at 12 h | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.123 |
| NPIS at 24 h | 1.0 (1.0–1.0) | 1.0 (1.0–2.0) | 0.010 |
| Maximal NPIS on POD 1 | 1.0 (1.0–1.0) | 1.0 (1.0–2.0) | 0.018 |
| Pain-Related Outcomes | OR (95% CI) | p | β (95% CI) | p |
|---|---|---|---|---|
| Primary outcome | ||||
| Maximal NPIS on POD 0 | −1.25 (−2.01–−0.50) | 0.001 | ||
| Secondary outcomes | ||||
| Rescue analgesia on POD 0 | 0. 12 (0.03–0.46) | 0.002 | ||
| Number of rescue doses during hospitalization | −1.11 (−1.62–−0.49) | <0.001 | ||
| Morphine equivalent dose | −5.14 (−7.79–−2.49) | <0.001 | ||
| NPIS at 0 h | −0.81 (−1.27–−0.35) | <0.001 | ||
| NPIS at 24 h | 0.26 (−0.01–0.53) | 0.062 | ||
| Maximal NPIS on POD 1 | 0.03 (−0.40–0.46) | 0.887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M. Single-Shot Subcutaneous Lidocaine Infiltration at Closure Is Associated with Reduced Early Pain and Opioid Requirement After Single-Incision Laparoscopic Totally Extraperitoneal Hernia Repair. J. Clin. Med. 2025, 14, 8324. https://doi.org/10.3390/jcm14238324
Lee JM. Single-Shot Subcutaneous Lidocaine Infiltration at Closure Is Associated with Reduced Early Pain and Opioid Requirement After Single-Incision Laparoscopic Totally Extraperitoneal Hernia Repair. Journal of Clinical Medicine. 2025; 14(23):8324. https://doi.org/10.3390/jcm14238324
Chicago/Turabian StyleLee, Jong Min. 2025. "Single-Shot Subcutaneous Lidocaine Infiltration at Closure Is Associated with Reduced Early Pain and Opioid Requirement After Single-Incision Laparoscopic Totally Extraperitoneal Hernia Repair" Journal of Clinical Medicine 14, no. 23: 8324. https://doi.org/10.3390/jcm14238324
APA StyleLee, J. M. (2025). Single-Shot Subcutaneous Lidocaine Infiltration at Closure Is Associated with Reduced Early Pain and Opioid Requirement After Single-Incision Laparoscopic Totally Extraperitoneal Hernia Repair. Journal of Clinical Medicine, 14(23), 8324. https://doi.org/10.3390/jcm14238324






