Unveiling the Diagnostic and Prognostic Value of Inflammatory Cytokines in Preeclampsia: A Review of Ιnterleukins IL-15, IL-16, IL-17 and IL-35
Abstract
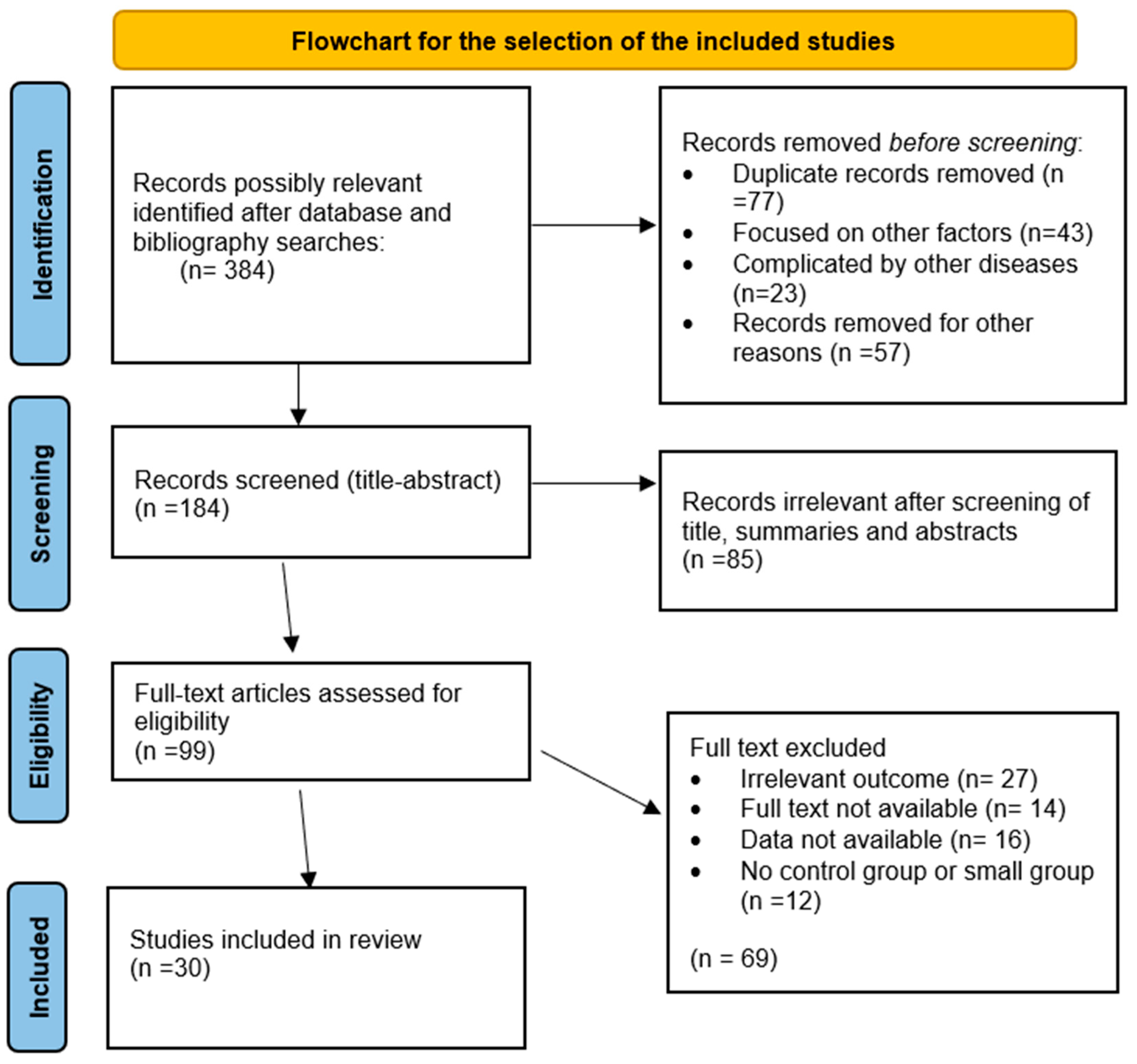
1. Introduction
2. Material Methods
3. Results
3.1. IL-15
3.2. IL-16
3.3. IL-35
3.4. IL-17
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Förger, F.; Villiger, P.M. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat. Rev. Rheumatol. 2020, 16, 113–122. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar] [CrossRef]
- Hernández-Díaz, S.; Toh, S.; Cnattingius, S. Risk of pre-eclampsia in first and subsequent pregnancies: Prospective cohort study. BMJ 2009, 338, b2255. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef]
- Orlic, N.K.; Joksić, I. Preeclampsia pathogenesis and prediction—Where are we now: The focus on the role of galectins and miRNAs. Hypertens. Pregnancy 2025, 44, 2470626. [Google Scholar] [CrossRef] [PubMed]
- Laresgoiti-Servitje, E.; Gómez-lópez, N.; Olson, D.M. An immunological insight into the origins of pre-eclampsia. Hum. Reprod. Update 2010, 16, 510–524. [Google Scholar] [CrossRef]
- Daher, S.; de Arruda, K.; Denardi, G.; Blotta, M.H.; Mamoni, R.L.; Monteiro Reck, A.P.; Camano, L.; Mattar, R. Cytokines in recurrent pregnancy loss. J. Reprod. Immunol. 2004, 62, 151–157. [Google Scholar] [CrossRef]
- Xiao, J.P.; Yin, Y.X.; Gao, Y.F.; Lau, S.; Shen, F.; Zhao, M.; Chen, Q. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine 2012, 60, 856–860. [Google Scholar] [CrossRef]
- Holder, B.S.; Tower, C.L.; Jones, C.J.P.; Aplin, J.D.; Abrahams, V.M. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol. Reprod. 2012, 86, 1–7. [Google Scholar] [CrossRef]
- Becknell, B.; Caligiuri, M.A. Interleukin-2, Interleukin-15, and Their Roles in Human Natural Killer Cells. Adv. Immunol. 2005, 86, 209–239. [Google Scholar] [CrossRef]
- Chaouat, G.; Zourbas, S.; Ostojic, S.; Lappree-Delage, G.; Dubanchet, S.; Ledee, N.; Martal, J. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J. Reprod. Immunol. 2002, 53, 241–256. [Google Scholar] [CrossRef]
- Lodolce, J.; Burkett, P.; Koka, R.; Boone, D.; Chien, M.; Chan, F.; Madonia, M.; Chai, S.; Ma, A. Interleukin-15 and the regulation of lymphoid homeostasis. Mol. Immunol. 2002, 39, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.L.; Keller, A.C.; Paget, C.; Fujio, M.; Trottein, F.; Savage, P.B.; Wong, C.H.; Schneider, E.; Dy, M.; Leite-de-Moraes, M.C. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007, 204, 995–1001. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, J.; Virtue, A.; Yin, Y.; Gong, R.; Sha, X.; Gutchigian, S.; Frisch, A.; Hodge, I.; Jiang, X.; et al. IL-35 Is a Novel Responsive Anti-inflammatory Cytokine—A New System of Categorizing Anti-inflammatory Cytokines. PLoS ONE 2012, 7, e33628. [Google Scholar] [CrossRef]
- Bardel, E.; Larousserie, F.; Charlot-Rabiega, P.; Coulomb-L’Herminé, A.; Devergne, O. Human CD4+CD25+Foxp3+ Regulatory T Cells Do Not Constitutively Express IL-35. J. Immunol. 2008, 181, 6898–6905. [Google Scholar] [CrossRef]
- Luo, Y.H.; Zhang, Y.Y.; Li, M.Q.; Zhang, X.Y.; Zheng, Z.M. Emerging Roles of IL-27 in Trophoblast Cells and Pregnancy Complications. Am. J. Reprod. Immunol. 2024, 92, e13942. [Google Scholar] [CrossRef]
- El-Baradie, S.M.Y.; Mahmoud, M.; Makhlouf, H.H. Elevated Serum Levels of Interleukin-15, Interleukin-16, and Human Chorionic Gonadotropin in Women With Preeclampsia. J. Obstet. Gynaecol. Can. 2009, 31, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, R.; Akbari, F.; Vodjgani, M.; Mahboudi, F.; Kalantar, F.; Mirahmadian, M. Serum Cytokines Profile in Iranian Patients with Preeclampsia. Iran. J. Immunol. 2007, 4, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, H.; Wang, Z.; Huang, H.; Dong, M. Elevated serum levels of interleukin-15 and interleukin-16 in preeclampsia. J. Reprod. Immunol. 2007, 73, 166–171. [Google Scholar] [CrossRef]
- Kalantar, F.; Rajaei, S.; Heidari, A.B.; Mansouri, R.; Rashidi, N.; Izad, M.H.; Mirahmadian, M. Serum levels of tumor necrosis factor-α, interleukin-15 and interleukin-10 in patients with pre-eclampsia in comparison with normotensive pregnant women. Iran. J. Nurs. Midwifery Res. 2013, 18, 463. [Google Scholar]
- Chaiworapongsa, T.; Romero, R.; Gomez-Lopez, N.; Suksai, M.; Gallo, D.M.; Jung, E.; Berry, S.M.; Awonuga, A.; Tarca, A.L.; Bryant, D.R. Preeclampsia at term: Evidence of disease heterogeneity based on the profile of circulating cytokines and angiogenic factors. Am. J. Obstet. Gynecol. 2024, 230, 450.e1–450.e18. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, H.; Shi, Y.; Sun, Y.; Huang, H.; Dong, M. Serum IL-16, not IL-15, was elevated in early second trimester of pregnancy in women who subsequently developed preeclampsia. Clin. Chim. Acta 2007, 383, 175–177. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Garza-Veloz, I.; Carrillo-Sanchez, K.; Martinez-Gaytan, V.; Cortes-Flores, R.; Ochoa-Torres, M.A.; Guerrero, G.G.; Rodriguez-Sanchez, I.P.; Cancela-Murrieta, C.O.; Zamudio-Osuna, M.; et al. Expression levels of seven candidate genes in human peripheral blood mononuclear cells and their association with preeclampsia. Hypertens. Pregnancy 2014, 33, 191–203. [Google Scholar] [CrossRef]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J. Reprod. Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef]
- RǍdulescu, C.; Bacârea, A.; Huanu, A.; Gabor, R.; Dobreanu, M. Placental Growth Factor, Soluble fms-Like Tyrosine Kinase 1, Soluble Endoglin, IL-6, and IL-16 as Biomarkers in Preeclampsia. Mediat. Inflamm. 2016, 2016, 3027363. [Google Scholar] [CrossRef]
- Gu, Y.; Lewis, D.F.; Deere, K.; Groome, L.J.; Wang, Y. Elevated maternal IL-16 levels, enhanced IL-16 expressions in endothelium and leukocytes, and increased IL-16 production by placental trophoblasts in women with preeclampsia. J. Immunol. 2008, 181, 4418–4422. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhen, G.; Jidong, H.U.; Xinyu, T.; Lili, W.; Chunhong, C.; Huanhuan, Z. Relationship between serum SOCS-3, TNF-α, IL-16, Th1/Th2 ratio and hypertensive disorder complicating pregnancy. Acad. J. Chin. PLA Med. Sch. 2017, 38, 426–429. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gu, Y.; Philibert, L.; Lewis, D. Maternal serum IL-16 levels were elevated in women with preeclampsia compared to that in women with normal pregnancies. Am. J. Obstet. Gynecol. 2006, 195, S125. [Google Scholar] [CrossRef]
- Molvarec, A.; Czegle, I.; Szijártó, J.; Rigó, J. Increased circulating interleukin-17 levels in preeclampsia. J. Reprod. Immunol. 2015, 112, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Peng, Q.; Chen, D.; Chen, X.; Jiang, M. Expression imbalance of IL-17/IL-35 in peripheral blood and placental tissue of pregnant women in preeclampsia. Taiwan. J. Obstet. Gynecol. 2020, 59, 409–414. [Google Scholar] [CrossRef]
- Cao, W.; Wang, X.; Chen, T.; Zhu, H.; Xu, W.; Zhao, S.; Cheng, X.; Xia, L. The Expression of Notch/Notch Ligand, IL-35, IL-17, and Th17/Treg in Preeclampsia. Dis. Markers 2015, 2015, 316182. [Google Scholar] [CrossRef]
- Cao, W.; Wang, X.; Chen, T.; Xu, W.; Feng, F.; Zhao, S.; Wang, Z.; Hu, Y.; Xie, B. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp. Ther. Med. 2018, 16, 427–435. [Google Scholar] [CrossRef]
- Ozkan, Z.S.; Simsek, M.; Ilhan, F.; Deveci, D.; Godekmerdan, A.; Sapmaz, E. Plasma IL-17, IL-35, interferon-γ, SOCS3 and TGF-β levels in pregnant women with preeclampsia, and their relation with severity of disease. J. Matern.-Fetal Neonatal Med. 2014, 27, 1513–1517. [Google Scholar] [CrossRef]
- El Shahaway, A.A.; Elhady, R.R.A.; Abdelrhman, A.A.; Yahia, S. Role of maternal serum interleukin 17 in preeclampsia: Diagnosis and prognosis. J. Inflamm. Res. 2019, 12, 175. [Google Scholar] [CrossRef]
- Batebi, A.; Namavar-Jahromi, B.; Ali-Hassanzadeh, M.; Ahmadi, M.; Hosseini, M.S.; Gharesi-Fard, B. Evaluation of IL-17 and IL-35 Serum Levels in Patients with Preeclampsia. J. Reprod. Infertil. 2019, 20, 237. [Google Scholar]
- Poordast, T.; Najib, F.S.; Baharlou, R.; Bijani, A.; Alamdarloo, S.M.; Poordast, A. Assessment of T helper 17-associated cytokines in third trimester of pregnancy. Iran. J. Immunol. 2017, 14, 172–179. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kolarz, B.; Kludka-Sternik, M.; Oleszczuk, J. The Role of Interleukin-17, Interleukin-23, and Transforming Growth Factor-β in Pregnancy Complicated by Placental Insufficiency. Biomed. Res. Int. 2017, 2017, 6904325. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Ding, Y. Association of microRNA-155, interleukin 17A, and proteinuria in preeclampsia. Medicine 2017, 96, e6509. [Google Scholar] [CrossRef] [PubMed]
- Barnie, P.A.; Lin, X.; Liu, Y.; Xu, H.; Su, Z. IL-17 producing innate lymphoid cells 3 (ILC3) but not Th17 cells might be the potential danger factor for preeclampsia and other pregnancy associated diseases. Int. J. Clin. Exp. Pathol. 2015, 8, 11100. [Google Scholar]
- Lang, X.; Liu, W.; Hou, Y.; Zhao, W.; Yang, X.; Chen, L.; Yan, Q.; Cheng, W. IL-17A polymorphism (rs2275913) and levels are associated with preeclampsia pathogenesis in Chinese patients. BMC Med. Genom. 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Perucci, L.O.; da Silva, S.P.G.; Bearzoti, E.; de Castro Pinto, K.M.; Alpoim, P.N.; de Barros Pinheiro, M.; Godoi, L.C.; de Moraes, L.Â.G.; de Sousa, L.P.; Sant Ana Dusse, L.M.; et al. Neuroserpin: A potential biomarker for early-onset severe preeclampsia. Immunobiology 2023, 228, 152339. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, L.; Wang, D.; Xu, Y.; Gao, H.; Tan, W.; Wang, C. Contribution of regulatory T cells to immune tolerance and association of microRNA-210 and Foxp3 in preeclampsia. Mol. Med. Rep. 2018, 19, 1150. [Google Scholar] [CrossRef]
- Toldi, G.; Rigó, J.; Stenczer, B.; Vásárhelyi, B.; Molvarec, A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am. J. Reprod. Immunol. 2011, 66, 223–229. [Google Scholar] [CrossRef]
- Agha, A.M.; Abdelrahman, A.M.; Thabet, A.M.; Sakr, B.E.; Ahmed, H.M. Study the Role of Interleukin-4, Interleukin-17 and Interleukin-35 in Diagnosis and Prognosis of Preeclampsia in Egyptian Patients. Benha J. Appl. Sci. 2020, 5, 183–188. [Google Scholar] [CrossRef]
- Li, M.; Qian, L.; Yu, J.; Zou, Y. Interleukin-35 inhibits human umbilical vein endothelial cell injury induced by sera from pre-eclampsia patients by up-regulating S100A8 protein expression. Hypertens. Pregnancy 2020, 39, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Gao, W.; Ma, C.; Sun, J.; Liu, J.; Shao, Q.; Song, B.; Qu, X. Human placental trophoblasts express the immunosuppressive cytokine IL-35. Hum. Immunol. 2013, 74, 872–877. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Cottrell, J.; Amaral, L.M.; LaMarca, B. Inflammatory mediators: A causal link to hypertension during preeclampsia. Br. J. Pharmacol. 2019, 176, 1914–1921. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular immune responses in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef]
- Black, K.D.; Horowitz, J.A. Inflammatory Markers and Preeclampsia: A Systematic Review. Nurs. Res. 2018, 67, 242–251. [Google Scholar] [CrossRef]
- Guan, X.; Fu, Y.; Liu, Y.; Cui, M.; Zhang, C.; Zhang, Q.; Li, C.; Zhao, J.; Wang, C.; Song, J.; et al. The role of inflammatory biomarkers in the development and progression of pre-eclampsia: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1156039. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am. J. Reprod. Immunol. 2007, 58, 21–30. [Google Scholar] [CrossRef]
- Xie, C.; Yao, M.Z.; Liu, J.B.; Xiong, L.K. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine 2011, 56, 550–559. [Google Scholar] [CrossRef]
- Laskowska, M.; Leszczyńska-Gorzelak, B.; Laskowska, K.; Oleszczuk, J. Evaluation of maternal and umbilical serum TNFalpha levels in preeclamptic pregnancies in the intrauterine normal and growth-restricted fetus. J. Matern.-Fetal Neonatal Med. 2006, 19, 347–351. [Google Scholar] [CrossRef]
- Saito, S.; Umekage, H.; Sakamoto, Y.; Sakai, M.; Tanebe, K.; Sasaki, Y.; Morikawa, H. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am. J. Reprod. Immunol. 1999, 41, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sones, J.L.; Lob, H.E.; Isroff, C.E.; Davisson, R.L. Role of decidual natural killer cells, interleukin-15, and interferon-γ in placental development and preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R490–R492. [Google Scholar] [CrossRef]
- Ozbey, G.; Tanriverdi, E.S.; Cakir, A.; Yilmaz, E. Investigation of the Relationship Between IL-17, IL-27, IL-2 Blood Levels in Spontaneous Abortion and Healthy Pregnant Women. Life 2025, 15, 326. [Google Scholar] [CrossRef]
- Agarwal, R.; Loganath, A.; Roy, A.C.; Wong, Y.C.; Ng, S.C. Expression profiles of interleukin-15 in early and late gestational human placenta and in pre-eclamptic placenta. Mol. Hum. Reprod. 2001, 7, 97–101. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Lin, H.; Lan, K.C.; Ou, C.Y.; Tsai, C.C.; Cheng, B.H.; Yang, K.D.; Wong, Y.H.; Hung, T.H.; Hsiao, P.Y.; et al. High interleukin-16 concentrations in the early second trimester amniotic fluid: An independent predictive marker for preterm birth. J. Matern.-Fetal Neonatal Med. 2013, 26, 285–289. [Google Scholar] [CrossRef]
- Athayde, N.; Romero, R.; Maymon, E.; Gomez, R.; Pacora, P.; Yoon, B.H.; Edwin, S.S. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am. J. Obstet. Gynecol. 2000, 182, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.M.; Romero, R.; Bosco, M.; Chaiworapongsa, T.; Gomez-Lopez, N.; Arenas-Hernandez, M.; Jung, E.; Suksai, M.; Gotsch, F.; Erez, O.; et al. Maternal plasma cytokines and the subsequent risk of uterine atony and postpartum hemorrhage. J. Perinat. Med. 2022, 51, 219–232. [Google Scholar] [CrossRef]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; Nanan, R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef]
- Plug, A.; Barenbrug, L.; Moerings, B.G.J.; de Jong, E.M.G.; van der Molen, R.G. Understanding the role of immune-mediated inflammatory disease related cytokines interleukin 17 and 23 in pregnancy: A systematic review. J. Transl. Autoimmun. 2025, 10, 100279. [Google Scholar] [CrossRef]
- Martínez-García, E.A.; Chávez-Robles, B.; Sánchez-Hernández, P.E.; Núñez-Atahualpa, L.; Martín-Máquez, B.T.; Muñoz-Gómez, A.; González-López, L.; Gámez-Nava, J.I.; Salazar-Páramo, M.; Dávalos-Rodríguez, I.; et al. IL-17 increased in the third trimester in healthy women with term labor. Am. J. Reprod. Immunol. 2011, 65, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hee, L.; Kirkegaard, I.; Vogel, I.; Thorsen, P.; Skogstrand, K.; Hougaard, D.M.; Uldbjerg, N.; Sandager, P. Low serum interleukin-17 is associated with preterm delivery. Acta Obstet. Gynecol. Scand. 2011, 90, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.E.; Roșca, I.; Jura, A.M.C.; Cioca, A.; Pop, O.; Lungu, N.; Popa, Z.L.; Rațiu, A.; Boia, M. Prompt Placental Histopathological and Immunohistochemical Assessment after SARS-CoV-2 Infection during Pregnancy-Our Perspective of a Small Group. Int. J. Mol. Sci. 2024, 25, 1836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coroleucă, C.A.; Coroleucă, C.B.; Coroleucă, R.; Brătilă, P.C.; Nodiți, A.R.; Roșca, I.; Brîndușe, L.A.; Brătilă, E.; Boț, M. Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis. Int. J. Mol. Sci. 2025, 26, 2983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coulomb-L’Herminé, A.; Larousserie, F.; Pflanz, S.; Bardel, E.; Kastelein, R.A.; Devergne, O. Expression of Interleukin-27 by Human Trophoblast Cells. Placenta 2007, 28, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Gong, Y.; Pu, Y.; Wang, Y.; Zhou, B.; Song, Y.; Wang, T.; Zhang, L. Association between polymorphisms in IL-27 gene and pre-eclampsia. Placenta 2016, 37, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Zhang, H.; Luo, X.; Ding, Y.; Xiao, X.; Liu, X.; Shan, N.; Zhang, X.; Deng, Q.; Zhuang, B.; et al. IL-27 Activates Human Trophoblasts to Express IP-10 and IL-6: Implications in the Immunopathophysiology of Preeclampsia. Mediat. Inflamm. 2014, 2014, 926875. [Google Scholar] [CrossRef]
- Gharesi-Fard, B.; Mobasher-Nejad, F.; Nasri, F. The Expression of T-Helper Associated Transcription Factors and Cytokine Genes in Pre-Eclampsia. Iran. J. Immunol. 2016, 13, 296–308. [Google Scholar] [PubMed]
- Forghani, F.; Ranjbar, N.; Jahantigh, D. The presence, severity, and onset of preeclampsia is associated with maternal interleukin-23 level: A case-control study. Int. J. Reprod. Biomed. 2023, 21, 295–302. [Google Scholar] [CrossRef]
| Selection | Compatibility | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non- Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Start of Study | Assessment of Outcome | Adequacy of Duration of Follow-Up | Adequacy of Completeness of Follow-Up | |||
| El-Barabie et al., 2009 [22] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Mansouri et. 2007 [23] | √ | - | √ | √ | - | √ | √ | √ | 6/9 |
| Hu et al., 2007 [24] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Kalantar et al., 2013 [25] | √ | - | √ | √ | - | √ | √ | √ | 6/9 |
| Chaiworapongsa et al., 2024 [26] | √ | √ | √ | √ | √√ | √ | √ | √ | 9/9 |
| Lu et al., 2007 [27] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Martinez-Fierro et al., 2014 [28] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Jonsson et al., 2006 [29] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Rădulescu et al., 2016 [30] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Gu et al., 2008 [31] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Li W et al., 2017 [32] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Wang et al., 2006 [33] | √ | √ | √ | √ | - | √ | √ | √ | 7/9 |
| Molvarec et al., 2015 [34] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Lu et al., 2020 [35] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Cao et al., 2015 [36] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Cao et al., 2018 [37] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Ozkan ZS et al., 2014 [38] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| El Shahaway AA et al., 2019 [39] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Batebi et al., 2019 [40] | √ | √ | - | √ | √ | √ | √ | √ | 7/9 |
| Poordast et al., 2017 [41] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Darmochwal-Kolarz D et al., 2012 [42] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Darmochwal-Kolarz D et al., 2017 [43] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Yang et al., 2017 [44] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Barnie et al., 2015 [45] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Lang et al., 2021 [46] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Perucci et al., 2023 [47] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Chen et al., 2018 [48] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Toldi et al., 2011 [49] | √ | √ | - | √ | - | √ | √ | √ | 6/9 |
| Agha et al., 2020 [50] | √ | √ | √ | √ | - | √ | √ | - | 6/9 |
| Li et al., 2020 [51] | √ | √ | √ | √ | - | √ | √ | - | 6/9 |
| Authors | Type of Study | Study Center | Recruitment Period | Ν Preeclampsia | N Control Group | Outcomes |
|---|---|---|---|---|---|---|
| El-Barabie et al., 2009 [22] | Observational | Egypt | 12/2006– 9/2007 | 32 | 35 | ↑ IL-15 and β-hCG in preeclampsia (PE) Correlation with the severity of PE and with β-hCG |
| Mansouri et al., 2007 [23] | Observational | Iran | 3–10/2006 | 30 (10 severe and 20 mild PE) | 30 | ↑ IL-15 levels in PE group other: ↑ IL-12p70, IL-18, IL-4, and IFN-γ in PE group |
| Hu et al., 2007 [24] | Observational | China | N/A | 37 (22 severe and 15 mild PE) | 36 | ↑ IL-15 levels in PE group, correlation with the severity of PE |
| Kalantar et al., 2013 [25] | Observational | Iran | N/A | 44 | 40 | ↑ IL-15 levels in PE group ↑ TNF-α in PE group |
| Chaiworapongsa et al., 2024 [26] | Case–control | USA | 2006– 2010 | 96 | 213 | ↑ IL-15 in PE group Other: ↑ IL-6, IL-8, IL-12/IL-23p40, IL-16 with abnormal angiogenic profile with worse clinical severity Women with normal angiogenic profiles had only mild inflammatory response with ↑ MCP-4 and ↓ IL-1a |
| Lu et al., 2007 [27] | Retrospective | China | N/A | 40 | 40 | No significant difference |
| Martinez-Fierro et al., 2014 [28] | Observational | Mexico | 2009– 2010 | 108 (48 severe and 60 mild PE) | 69 | IL-15 and IL-6 gene expression in PBMCs was undetectable or extremely low in both groups |
| Jonsson et al., 2006 [29] | Case–control | Sweden | N/A | 15 | 15 | No statistical difference |
| Total | 402 | 478 | ||||
| Authors | Type of Study | Study Center | Recruitment Period | Ν Preeclampsia | N Control Group | Outcomes |
|---|---|---|---|---|---|---|
| El-Barabie et al., 2009 [22] | Observational | Egypt | 12/2006- 9/2007 | 32 | 35 | ↑ IL-16 in PE group Correlation with the severity of PE and β-hCG |
| Hu et al., 2007 [24] | Observational | China | N/A | 37 (22 severe and 15 mild PE) | 36 | ↑ IL-16 in PE group, Correlation with the severity of PE |
| Lu et al., 2007 [27] | Retrospective | China | N/A | 40 | 40 | ↑ IL-16 in women who later developed PE |
| Radulescu et al., 2016 [30] | Prospective | Romania | 1/2014– 7/2015 | 47 | 21 | ↑ IL-16 in PE group Correlation with the severity of PE |
| Chaiworapongsa et al., 2024 [26] | Case–control | USA | 2006– 2010 | 96 | 213 | ↑ IL-16 Other: ↑ IL-6, IL-8, IL-12/IL-23p40, IL-15 with abnormal angiogenic profile with worse clinical severity Women with normal angiogenic profiles had only mild inflammatory response with ↑ MCP-4 and ↓ IL-1a |
| Gu et al., 2008 [31] | Observational | USA | N/A | 66 (51 severe and 11 mild P) | 63 | ↑ IL-16 in PE group Correlation with the severity of PE |
| Li W et al., 2017 [32] | Case–control | China | 1/2014– 6/2016 | 131 | 65 | Significantly higher |
| Wang et al., 2006 [33] | Observational | USA | N/A | 34 | 37 | Significantly higher |
| Total | 510 | 479 |
| Authors | Type of Study | Study Center | Recruitment Period | N Preeclampsia | N Control Group | Outcomes |
|---|---|---|---|---|---|---|
| Li et al., 2020 [51] | Prospective | China | 6/2016– 10/2017 | 24 | 24 | ↓ IL-35 levels in PE patients iTr35 cells significantly reduced in PE patients and positively correlated with IL-35 levels |
| Cao et al., 2015 [36] | Observational | China | 6/2013–10/2014 | 44 (22 mild and 20 severe PE) | 22 | Decreased |
| Cao et al., 2018 [37] | Observational | China | 1/2013–6/2016 | 33 | 33 | Decreased |
| Batebi et al., 2019 [40] | Observational | Iran | N/A | 100 | 100 | ↑ IL-35 in PE group Higher levels in severe PE vs. in mild PE and control |
| Lu et al., 2020 [35] | Observational | China | 7/2015–7/2017 | 90 | 45 | Significantly lower in PE patients than in healthy pregnant women |
| Ozkan ZS et al., 2014 [38] | Case–control | Turkey | 8/2011–8/2012 | 40 | 40 | Significantly lower |
| Agha et al., 2020 [50] | Case–control | Egypt | N/A | 25 | 15 | No difference |
| Total | 354 | 279 |
| Authors | Type of Study | Study Center | Recruitment Period | N Preeclampsia | N Control Group | Outcomes |
|---|---|---|---|---|---|---|
| Molvarec et al., 2015 [34] | Observational | Hungary | 2013– 2015 | 59 | 60 | ↑ IL-17A levels in PE women No correlation between il-17A and sFlt-1, PIGF, or sFlt-1/PIGF ratio IL-17A and sFlt-1/PIGF ratio had an additive effect on PE risk |
| Lu et al., 2020 [35] | Observational | China | 7/2015–7/2017 | 90 | 45 | ↑ IL-17 in PE group |
| Cao et al., 2015 [36] | Observational | China | 6/2013–10/2014 | 44 (22 mild and 20 severe PE) | 22 | ↑ IL-17A in PE groups IL-17 levels positively correlated with DLL4 expression and negatively with Jagged-2 |
| Cao et al., 2018 [37] | Observational | China | 1/2013–6/2016 | 33 | 33 | ↑ IL-17 in PE group |
| Chaiworapongsa et al., 2024 [26] | Nested-case–control | USA | 2006– 2010 | 96 | 213 | ↑ IL-17A in PE group |
| Ozkan ZS et al., 2014 [38] | Case–control | Turkey | 8/2011–8/2012 | 40 | 40 | Significantly lower in PE group |
| Jonsson et al., 2006 [29] | Case–control | Sweden | N/A | 15 | 15 | No statistical difference |
| El Shahaway et al., 2019 [39] | Prospective case–control | Egypt | N/A | 20 | 20 | Mean value of IL-17 in the PE group: 18.5 pg/mL, versus the control group: 4.3 pg/mL. |
| Batebi et al., 2019 [40] | Observational | Iran | N/A | 100 | 100 | No significant difference in IL-17A levels between PE and control group |
| Poordast et al., 2017 [41] | Observational case–control | Iran | N/A | 30 | 30 | Significantly higher in PE group |
| Darmochwal-Kolarz D et al., 2012 [42] | Case–control | Poland | N/A | 34 | 27 | IL-17A was significantly higher in PE group |
| Darmochwal-Kolarz D et al., 2017 [43] | Case–control | Poland | N/A | 34 | 35 | Significantly higher in PE group |
| Yang X et al., 2017 [44] | Case–control | China | N/A | 60 | 20 | Significantly higher in PE group |
| Barnie et al., 2015 [45] | Case–control | China | 8/2013– 11/2014 | 17 | 17 | Significantly higher in PE group |
| Lang et al., 2021 [46] | Case–control | China | 3/2017–12/2019 | 115 | 102 | IL-17A significantly higher in PE group |
| Perucci et al., 2023 [47] | Case–control | Brazil | NA | 24 | 34 | IL-17A significantly higher in PE group |
| Chen et al., 2018 [48] | Case–control | China | 7/2016–3/2017 | 29 | 27 | Significantly higher in PE group |
| Toldi et al., 2011 [49] | Case–control | Hungary | NA | 20 | 22 | Significantly higher in PE group |
| Agha et al., 2020 [50] | Case–control | Egypt | N/A | 25 | 15 | Significantly higher in PE group |
| Total | 883 | 879 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalil, A.B.; Andreou, E.; Perros, P.; Sapanztoglou, I.; Koutras, A.; Prokopakis, I.; Metaxas, D.; Chionis, A.; Tsakaldimis, G.; Koutlaki, N.; et al. Unveiling the Diagnostic and Prognostic Value of Inflammatory Cytokines in Preeclampsia: A Review of Ιnterleukins IL-15, IL-16, IL-17 and IL-35. J. Clin. Med. 2025, 14, 8322. https://doi.org/10.3390/jcm14238322
Chalil AB, Andreou E, Perros P, Sapanztoglou I, Koutras A, Prokopakis I, Metaxas D, Chionis A, Tsakaldimis G, Koutlaki N, et al. Unveiling the Diagnostic and Prognostic Value of Inflammatory Cytokines in Preeclampsia: A Review of Ιnterleukins IL-15, IL-16, IL-17 and IL-35. Journal of Clinical Medicine. 2025; 14(23):8322. https://doi.org/10.3390/jcm14238322
Chicago/Turabian StyleChalil, Arzou B., Emmanouil Andreou, Paraskevas Perros, Ioakeim Sapanztoglou, Antonios Koutras, Ioannis Prokopakis, Dionysios Metaxas, Athanasios Chionis, Georgios Tsakaldimis, Nikoletta Koutlaki, and et al. 2025. "Unveiling the Diagnostic and Prognostic Value of Inflammatory Cytokines in Preeclampsia: A Review of Ιnterleukins IL-15, IL-16, IL-17 and IL-35" Journal of Clinical Medicine 14, no. 23: 8322. https://doi.org/10.3390/jcm14238322
APA StyleChalil, A. B., Andreou, E., Perros, P., Sapanztoglou, I., Koutras, A., Prokopakis, I., Metaxas, D., Chionis, A., Tsakaldimis, G., Koutlaki, N., Tsigalou, C., & Kontomanolis, E. N. (2025). Unveiling the Diagnostic and Prognostic Value of Inflammatory Cytokines in Preeclampsia: A Review of Ιnterleukins IL-15, IL-16, IL-17 and IL-35. Journal of Clinical Medicine, 14(23), 8322. https://doi.org/10.3390/jcm14238322






