Neurocognitive Recovery Following Continuous Positive Airway Pressure Therapy in Patients with Moderate to Severe Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Baseline Assessments
2.3. Polysomnography
2.4. CPAP Therapy and Compliance
2.5. Event-Related Potentials (ERPs)
2.6. Audiometry and Additional Measurements
2.7. Follow-Up
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea-hypopnea index |
| BAER | Brainstem auditory evoked response |
| CPAP | Continuous positive airway pressure |
| ERP | Event-related potentials |
| OSA | Obstructive sleep apnea |
References
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Bucks, R.S.; Olaithe, M.; Eastwood, P. Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology 2013, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kotterba, S.; Rasche, K.; Widdig, W.; Duscha, C.; Blombach, S.; Schultze-Werninghaus, G.; Malin, J.-P. Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J. Neurol. Sci. 1998, 159, 45–50. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Marelli, S.; Galbiati, A.; Castronovo, C. Effects of continuous positive airway pressure on cognitition and neuroimaging data in sleep apnea. Int. J. Psychophysiol. 2013, 89, 203–212. [Google Scholar] [CrossRef]
- Landry, S.; Anderson, C.; Andrewartha, P.; Sasse, A.; Conduit, R. The Impact of Obstructive Sleep Apnea on Motor Skill Acquisition and Consolidation. J. Clin. Sleep Med. 2014, 10, 491–496. [Google Scholar] [CrossRef]
- Lusic Kalcina, L.; Pavlinac Dodig, I.; Pecotic, R.; Valic, M.; Dogas, Z. Psychomotor Performance in Patients with Obstructive Sleep Apnea Syndrome. Nat. Sci. Sleep 2020, 12, 183–195. [Google Scholar] [CrossRef]
- Lima, N.C.; Kirov, R.; de Almondes, K.M. Impairment of executive functions due to sleep alterations: An integrative review on the use of P300. Front. Neurosci. 2022, 16, 906492. [Google Scholar] [CrossRef]
- Kushida, C.A.; Nichols, D.A.; Holmes, T.H.; Quan, S.F.; Walsh, J.K.; Gottlieb, D.J.; Simon, R.D.; Guilleminault, C.; White, D.P.; Goodwin, J.L.; et al. Effects of Continuous Positive Airway Pressure on Neurocognitive Function in Obstructive Sleep Apnea Patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012, 35, 1593–1602. [Google Scholar] [CrossRef]
- Akcali, A.; Sahin, E.; Ergenoglu, T.; Neyal, M. Latency of auditory P300 response is related with cognitive deficits in Obstructive Sleep Apnea Syndrome. Sleep Biol. Rhythm. 2015, 13, 49–56. [Google Scholar] [CrossRef]
- Chou, P.-S.; Chen, S.C.-J.; Hsu, C.-Y.; Liou, L.-M.; Wu, M.-N.; Liu, C.-K.; Lai, C.-L. Compensatory Neural Recruitment for Error-Related Cerebral Activity in Patients with Moderate-To-Severe Obstructive Sleep Apnea. J. Clin. Med. 2019, 8, 1077. [Google Scholar] [CrossRef]
- Yerlikaya, D.; Emek-Savaş, D.D.; Bircan Kurşun, B.; Öztura, İ.; Yener, G.G. Electrophysiological and neuropsychological outcomes of severe obstructive sleep apnea: Effects of hypoxemia on cognitive performance. Cogn. Neurodyn. 2018, 12, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gelir, E.; Başaran, C.; Bayrak, S.; Yağcıoğlu, S.; Budak, M.T.; Fırat, H.; Ungan, P. Electrophysiological Assessment of the Effects of Obstructive Sleep Apnea on Cognition. PLoS ONE 2014, 9, e90647. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.H.; de Castro, N.; Costa Filho, O.A.; de Souza Neto, O.M. Obstructive Sleep Apnea and P300 Evoked Auditory Potential. Braz. J. Otorhinolaryngol. 2011, 77, 700–705. [Google Scholar] [CrossRef]
- Sangal, R.B.; Sangal, J.M. Obstructive sleep apnea and abnormal P300 latency topography. Clin. Electroencephalogr. 1997, 28, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Afifi, L.; Guilleminault, C.; Colrain, I.M. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir. Physiol. Neurobiol. 2003, 136, 221–234. [Google Scholar] [CrossRef]
- Li, T.-H.; Shen, Y.-C.; Wang, H.-M.; Chang, E.-T.; Jan, H. Improvements in Cognitive Function after Continuous Positive Airway Pressure Treatment for Obstructive Sleep Apnea Syndrome. Neuropsychiatry 2017, 7, 781–787. [Google Scholar]
- Crawford-Achour, E.; Dauphinot, V.; Saint Martin, M.; Tardy, M.; Gonthier, R.; Barthelemy, J.C.; Roche, F. Protective Effect of Long-Term CPAP Therapy on Cognitive Performance in Elderly Patients with Severe OSA: The PROOF Study. J. Clin. Sleep Med. 2015, 11, 519–524. [Google Scholar] [CrossRef]
- Hobzova, M.; Hubackova, L.; Vanek, J.; Genzor, S.; Ociskova, M.; Grambal, A.; Prasko, J. Cognitive function and depressivity before and after cpap treatment in obstructive sleep apnea patients. Neuroendocrinol. Lett. 2017, 38, 145–153. [Google Scholar]
- Dostálová, V.; Kolečkárová, S.; Kuška, M.; Pretl, M.; Bezdicek, O. Effects of continuous positive airway pressure on neurocognitive and neuropsychiatric function in obstructive sleep apnea. J. Sleep Res. 2018, 28, e12761. [Google Scholar] [CrossRef]
- Davidescu, D.A.; Goman, A.; Voita-Mekeres, F.; Bradacs, A.I.; Sabina Florina, S.F.; Csep, A.N.; Szilagyi, G.; Motofelea, A.C.; Davidescu, L. Assessing Cognitive Impairments in Obstructive Sleep Apnea Patients Using Montreal Cognitive Assessment (MoCA) Scores. Cureus 2024, 16, e70085. [Google Scholar] [CrossRef]
- Su, K.; Feng, Z.; Wang, L.; Zhao, G.; Li, J. Prevalence of cognitive impairment among adults with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2025, 29, 323. [Google Scholar] [CrossRef]
- Gagnon, K.; Baril, A.-A.; Montplaisir, J.; Carrier, J.; Chami, S.; Gauthier, S.; Lafond, C.; Gagnon, J.-F.; Gosselin, N. Detection of mild cognitive impairment in middle-aged and older adults with obstructive sleep apnoea. Eur. Respir. J. 2018, 52, 1801137. [Google Scholar] [CrossRef]
- NCA—Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093N)—Decision Memo. Available online: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=19&fromdb=true (accessed on 19 October 2025).
- Jing, M.; Guo, M.; Wang, X.; Zhang, P.; Gu, C.; Zhang, N.; Ding, Z.; Su, K. Changes of regional brain activity in frontal areas associated with cognitive impairment in obstructive sleep apnea-hypopnea syndrome patients: A resting-state fMRI study. Front. Neurosci. 2025, 19, 1587180. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, J.; Zhao, D.; Mo, H.; Zeng, Z.; Xiong, M.; Dong, M.; Hu, K. Effects of the excitation or inhibition of basal forebrain cholinergic neurons on cognitive ability in mice exposed to chronic intermittent hypoxia. Brain Res. Bull. 2020, 164, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Gad, D.M.; Fathy, H.A.; Shehata, S.M. Effects of continuous positive airway pressure treatment on mood, cognition, and quality of life in patients with obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 2020, 69, 688–697. [Google Scholar] [CrossRef]
- Gemici, Y.İ.; Ozturk, L.; Celebi, C. Evaluation of cognitive functions in patients with obstructive sleep apnea before and after continuous positive airway pressure treatment. Neurol. Asia 2018, 23, 253–258. [Google Scholar]
- Tamilarasan, V.; Mohan, M.; Ramanjaneya, R.; Sadana, D.; Malapaka, R.C.; Annapandian, V.M.; Tousheed, S.Z.; Manjunath, P.H.; Sagar, C.; Kumar, H. Prevalence of cognitive impairment in patients with obstructive sleep apnea. ERJ Open Res. 2019, 5 (Suppl. S3), P115. [Google Scholar]
- Pierobon, A.; Giardini, A.; Fanfulla, F.; Callegari, S.; Majani, G. A multidimensional assessment of obese patients with obstructive sleep apnoea syndrome (OSAS): A study of psychological, neuropsychological and clinical relationships in a disabling multifaceted disease. Sleep Med. 2008, 9, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, T.; Abrahamyan, L.; Niazi, A.; Piyasena, D.; Arif, A.A.; Wong, J.; Osorio, R.S.; Ryan, C.M.; Chung, F. The prevalence of obstructive sleep apnea in mild cognitive impairment: A systematic review. BMC Neurol. 2019, 19, 195. [Google Scholar] [CrossRef]
- Ak, A.K.; Sarı, Ü.S.; Oktan, B.; Korkmaz, T.; Dinç Horasan, G.; Selçuki, D.; Yılmaz, H. Evaluation of Cognitive Function Using Objective and Subjective Tests in the Obstructive Sleep Apnea Syndrome. Turk. J. Sleep Med. 2017, 4, 76–83. [Google Scholar] [CrossRef]
- EL-Gharib, A.M.; Sharsher, R.S. Effect of nasal continuous positive airway pressure (CPAP) on auditory P300 in obstructive sleep apnea syndrome (OSAS). Egypt. J. Ear Nose Throat Allied Sci. 2017, 18, 55–59. [Google Scholar] [CrossRef]
- Vakulin, A.; Catcheside, P.G.; Baulk, S.D.; Antic, N.A.; van den Heuvel, C.J.; Banks, S.; McEvoy, R.D. Auditory evoked potentials remain abnormal after CPAP treatment in patients with severe obstructive sleep apnoea. Clin. Neurophysiol. 2012, 123, 310–317. [Google Scholar] [CrossRef] [PubMed]
| Anthropometric Characteristic | Median (IQR) | Minimum–Maximum |
|---|---|---|
| Body Weight (kg) | 105 (92–135) | 75–175 |
| Body Height (cm) | 176 (170–183) | 160–196 |
| BMI (kg/m2) | 33.60 (30.73–42.08) | 26.20–63.50 |
| Heart Rate (bpm) | 69 (62.6–75.4) | 46–96 |
| Systolic Blood Pressure (mmHg) | 130 (122–135) | 110–150 |
| Diastolic Blood Pressure (mmHg) | 80 (75–80) | 70–95 |
| Blood Glucose Level (mmol/L) | 5.5 (5.1–5.9) | 4.5–7.6 |
| Sleep Parameters | Median (IQR) | Minimum–Maximum |
|---|---|---|
| Time in bed (min) | 466.35 (443.70–501.45) | 369–776.30 |
| Total Sleep Time (min) | 405.45 (367–446.88) | 300.50–570 |
| Stage N1 (%) | 12.20 (8.05–15.3) | 1–34.60 |
| Stage N2 (%) | 57.65 (51.78–64.2) | 25–82.80 |
| Stage N3 (%) | 17.50 (12.73–25.5) | 2–33 |
| NREM sleep (%) | 88.70 (83.73–91.38) | 26.50–99.10 |
| REM sleep (%) | 10.90 (7.88–16.13) | 0.90–24.60 |
| Sleep Onset Latency (min) | 11.05 (5.93–24.35) | 0.40–109.50 |
| REM Latency (min) | 121.25 (94.25–195.3) | 53–426 |
| WASO (%) | 31.45 (10.20–62.70) | 0.10–241.40 |
| Number of Sleep Cycles | 4 (3–4) | 1–6 |
| Sleep Efficiency (%) | 86.30 (81.90–92.65) | 23.50–99.60 |
| Mean Saturation (%) | 91 (89–93) | 74–97 |
| Lowest Saturation (%) | 74.50 (64.25–80) | 35–90 |
| ODI (events/hour) | 59.10 (42.90–75.82) | 9.51–133.5 |
| Median (IQR) | Hodges-Lehmann Median Difference | 95% Confidence Interval | * p-Value | ||
|---|---|---|---|---|---|
| Before CPAP Therapy | After CPAP Therapy | ||||
| AHI (events/h) | 56.4 (23.5–71.7) | 3.0 (2.0–4.8) | −53.2 | −58.6 to −48.2 | <0.001 |
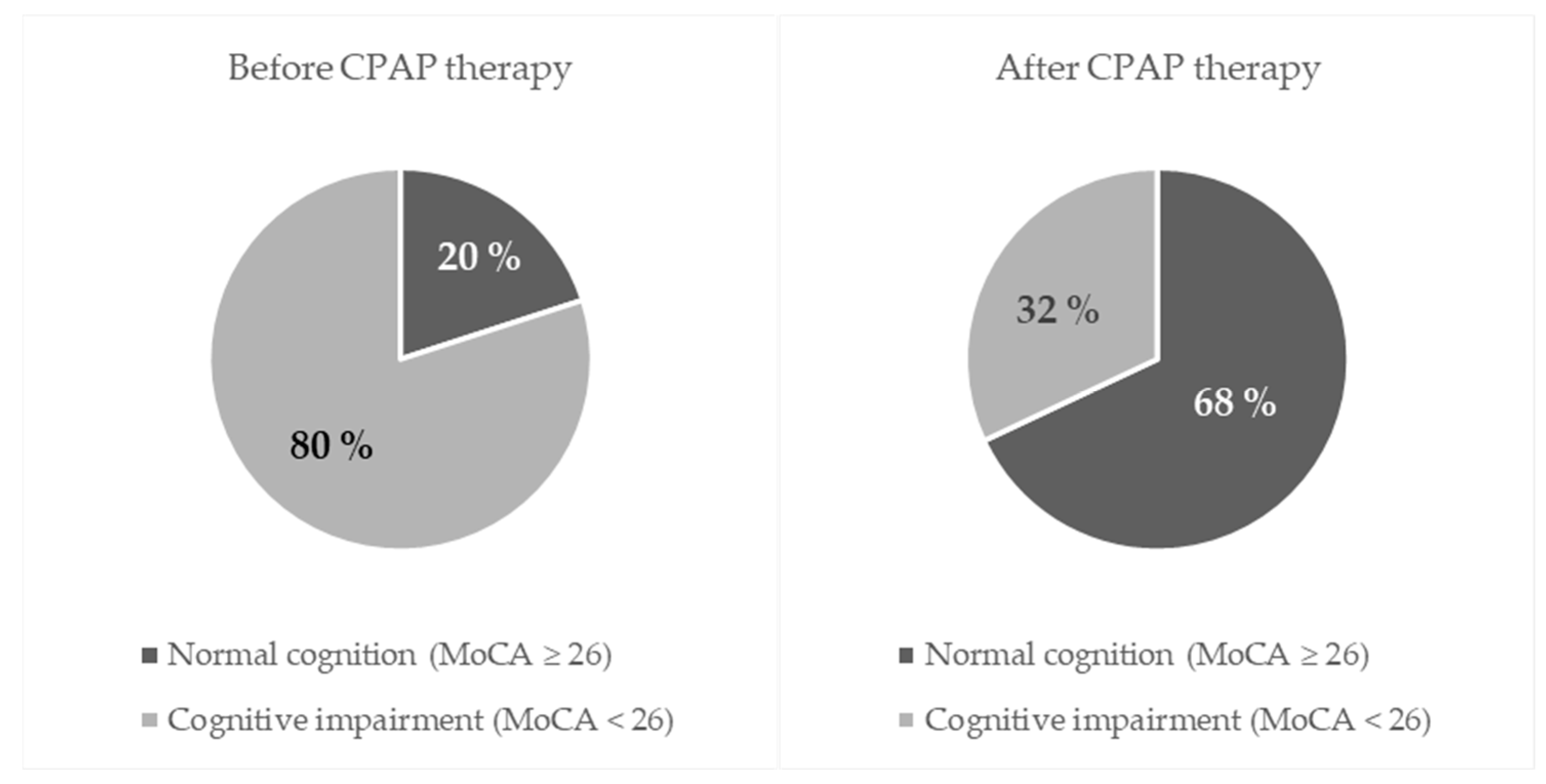
| Number (%) of Participants Before CPAP Therapy | * p-Value | ||||
|---|---|---|---|---|---|
| Normal Cognition (MoCA ≥ 26) | Cognitive Impairment (MoCA < 26) | Total | |||
| After CPAP therapy | Normal cognition (MoCA ≥ 26) | 12 | 29 | 41 (68) | <0.001 |
| Cognitive impairment (MoCA < 26) | 0 | 19 | 19 (32) | ||
| Total | 12 (20) | 48 (80) | 60 (100) | ||
| Median (IQR) | Hodges-Lehmann Median Difference | 95% Confidence Interval | * p-Value | ||
|---|---|---|---|---|---|
| Before CPAP Therapy | After CPAP Therapy | ||||
| Visuospatial/Executive Function | 3 (2–4) | 5 (3–5) | 1.5 | 1 to 1.5 | <0.001 |
| Naming | 3 (3–3) | 3 (3–3) | 0 | 0 to 0 | 0.06 |
| Attention | 5 (4–6) | 6 (5–6) | 0.5 | 0.5 to 1 | <0.001 |
| Language | 3 (2–3) | 3 (3–3) | 0 | 0 to 0 | 0.06 |
| Abstraction | 2 (2–2) | 2 (2–2) | 0 | 0 to 0 | 0.06 |
| Delayed Recall | 4 (2–5) | 5 (4–6) | 1.5 | 1 to 2 | <0.001 |
| Orientation | 6 (6–6) | 6 (6–6) | 0 | 0 to 0 | - |
| Overall MoCA score | 23 (20–25) | 27 (25–28) | 4 | 3 to 5 | <0.001 |
| Spearman’s Rho (p Value) | |
|---|---|
| Correlation Between Baseline AHI and Δ(Post–Pre) MoCA Scores | |
| ΔVisuospatial/Executive Function | 0.305 (0.02) |
| ΔNaming | 0.008 (0.95) |
| ΔAttention | 0.116 (0.38) |
| ΔLanguage | −0.333 (0.009) |
| ΔAbstraction | 0.145 (0.27) |
| ΔDelayed Recall | −0.053 (0.69) |
| ΔOrientation | - |
| ΔOverall MoCA score | 0.105 (0.42) |
| Median (IQR) | Hodges-Lehmann Median Difference | 95% Confidence Interval | * p-Value | ||
|---|---|---|---|---|---|
| Before CPAP Therapy | After CPAP Therapy | ||||
| P1 wave latency (ms) | 39 (25.5–48.5) | 31 (26–40) | −5.5 | −10.5 to −9.0 | 0.01 |
| N1 wave latency (ms) | 95 (90–108) | 94 (85.5–103.5) | −1.5 | −5 to 2.5 | 0.42 |
| P2 wave latency (ms) | 181 (171–196.5) | 180 (165–195) | 0 | −5.5 to 5.5 | 0.99 |
| N2 wave latency (ms) | 235.5 (225–262.5) | 236.5 (220.5–265) | −2 | −9.5 to 4.5 | 0.47 |
| P300 wave latency (ms) | 339.5 (325.5–359.0) | 313.5 (304–325.5) | −22 | −27.5 to −17.5 | <0.001 |
| P300 wave amplitude (μV) | 9.75 (6.25–14.1) | 10.3 (7.25–14.65) | 0.55 | −0.5 to 1.5 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarić Jurić, J.; Grebenar Čerkez, M.; Zubčić, Ž.; Bašić, S.; Bašić, I.; Jandrić, S.; Kralik, K.; Jurić, S. Neurocognitive Recovery Following Continuous Positive Airway Pressure Therapy in Patients with Moderate to Severe Obstructive Sleep Apnea. J. Clin. Med. 2025, 14, 8319. https://doi.org/10.3390/jcm14238319
Šarić Jurić J, Grebenar Čerkez M, Zubčić Ž, Bašić S, Bašić I, Jandrić S, Kralik K, Jurić S. Neurocognitive Recovery Following Continuous Positive Airway Pressure Therapy in Patients with Moderate to Severe Obstructive Sleep Apnea. Journal of Clinical Medicine. 2025; 14(23):8319. https://doi.org/10.3390/jcm14238319
Chicago/Turabian StyleŠarić Jurić, Jelena, Mirjana Grebenar Čerkez, Željko Zubčić, Silvio Bašić, Ivana Bašić, Sanja Jandrić, Kristina Kralik, and Stjepan Jurić. 2025. "Neurocognitive Recovery Following Continuous Positive Airway Pressure Therapy in Patients with Moderate to Severe Obstructive Sleep Apnea" Journal of Clinical Medicine 14, no. 23: 8319. https://doi.org/10.3390/jcm14238319
APA StyleŠarić Jurić, J., Grebenar Čerkez, M., Zubčić, Ž., Bašić, S., Bašić, I., Jandrić, S., Kralik, K., & Jurić, S. (2025). Neurocognitive Recovery Following Continuous Positive Airway Pressure Therapy in Patients with Moderate to Severe Obstructive Sleep Apnea. Journal of Clinical Medicine, 14(23), 8319. https://doi.org/10.3390/jcm14238319






