Validity and Reliability of the Six-Minute Walking Test Compared to Cardiopulmonary Exercise Test in Individuals with Heart Failure Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Eligibility Criteria
2.3. Search Methods
2.4. Study Selection
2.5. Study Quality and Risk of Bias
2.6. Data Extraction
2.7. Data Analysis and Synthesis
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
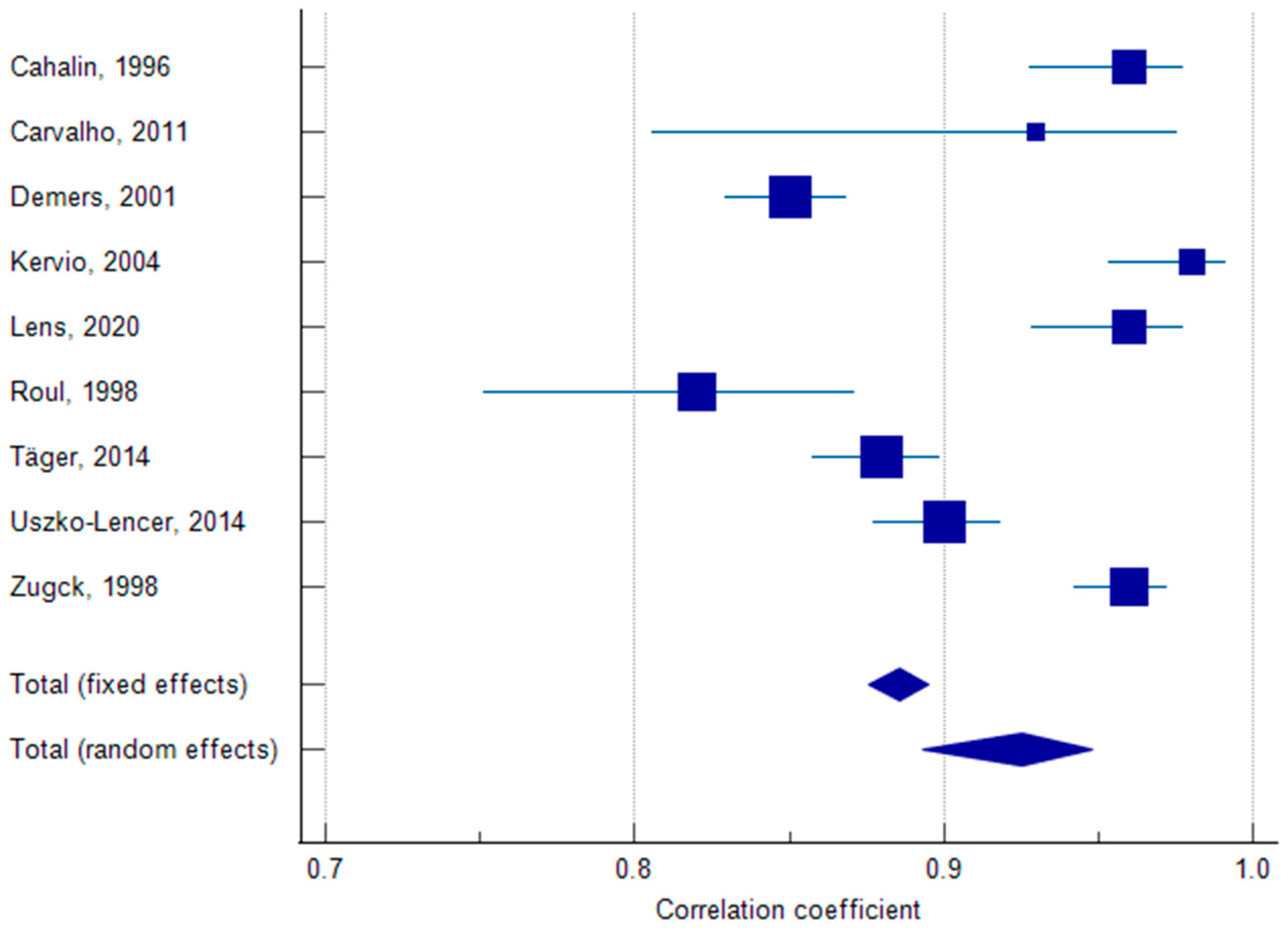
3.3. Reliability
3.4. Validity
3.5. Adverse Events and Symptoms Occurrence During 6MWT
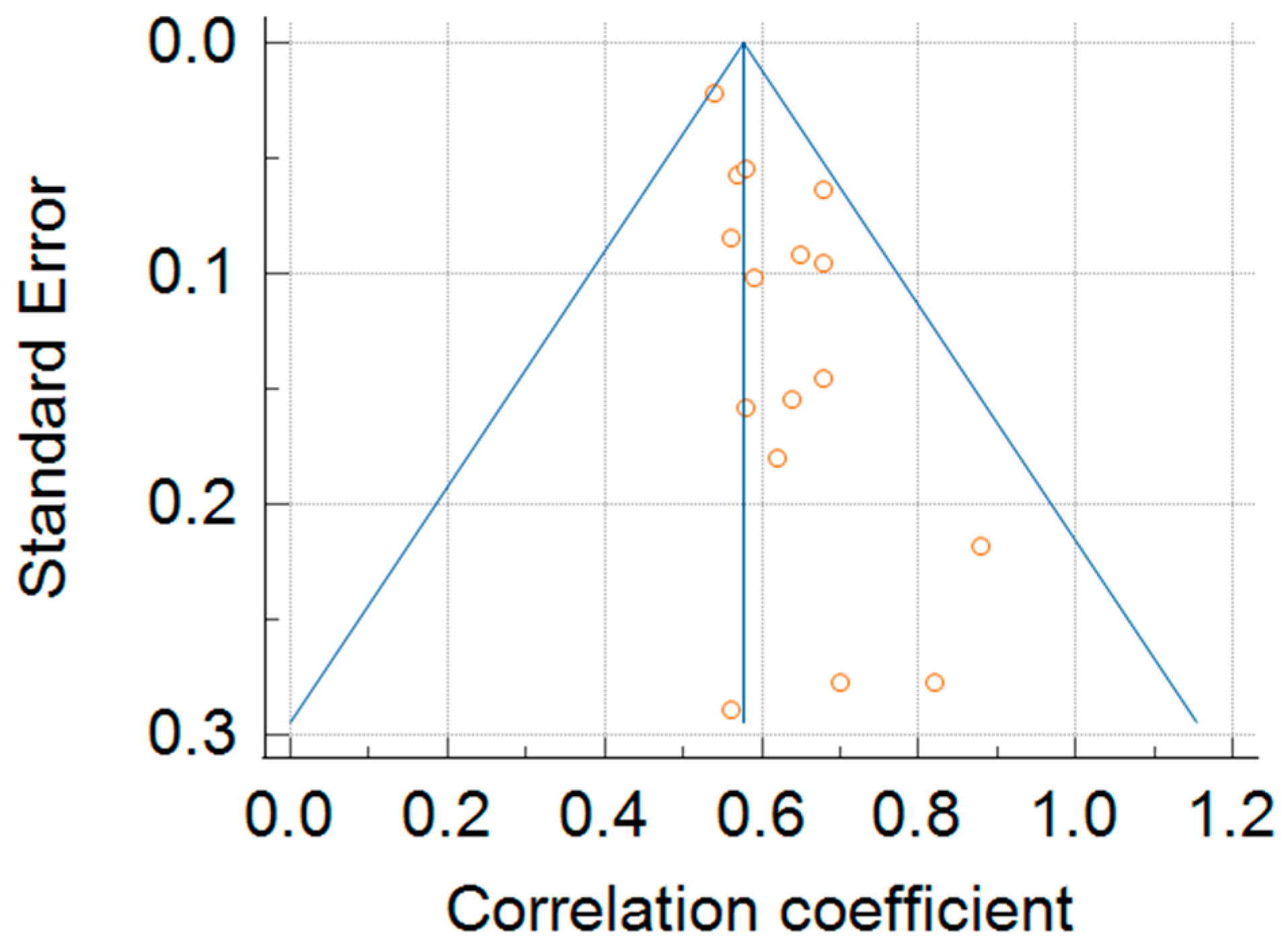
3.6. Meta-Analysis of Test–Retest Reliability of 6MWT
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPET | Cardiopulmonary Exercise Test |
| 6MWT | Six-Minute Walk Test |
| HF | Heart Failure |
| NYHA | New York Heart Association |
| MCID | Minimal Clinically Important Difference |
| VO2peak | Peak Oxygen Uptake |
| PICOS | Population, Intervention, Comparison, Outcomes, Study Design |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
References
- Butler, J.; Fonarow, G.C.; Zile, M.R.; Lam, C.S.; Roessig, L.; Schelbert, E.B.; Shah, S.J.; Ahmed, A.; Bonow, R.O.; Cleland, J.G.; et al. Developing therapies for heart failure with preserved ejection fraction: Current state and future directions. JACC Heart Fail. 2014, 2, 97–112. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Pepera, G.; Ingle, L.; Sandercock, G.R. Predictors of the six-minute walk test in patients with chronic heart failure. Br. J. Card. Nurs. 2015, 10, 454–549. [Google Scholar]
- Pepera, G.; Sandercock, G.R. Incremental shuttle walking test to assess functional capacity in cardiac rehabilitation: A narrative review. Int. J. Ther. Rehabil. 2022, 29, 1–10. [Google Scholar] [CrossRef]
- Gutekunst, D.J. Isokinetic torque timing parameters and ceramides as markers of muscle dysfunction in systolic heart failure. J. Card. Fail. 2016, 22, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Dickstein, K.; Vicenzi, M.; Arena, R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: A comparative analysis on clinical and prognostic insights. Circ. Heart Fail. 2009, 2, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Strong, B.M.; Weisenberg, J.; Kakade, A.; Gao, Q.; Cuddihy, P.; Delisle, S.; Kachnowski, S.; Maurer, M.S. Maximum daily six minutes of activity: An index of functional capacity derived from actigraphy and its application to older adults with heart failure. J. Am. Geriatr. Soc. 2010, 58, 931–936. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sullivan, M.J.; Thompson, P.J.; Fallen, E.L.; Pugsley, S.O.; Taylor, D.W.; Berman, L.B. The six-minute walk: A new measure of exercise capacity in patients with chronic heart failure. CMAJ 1985, 132, 919–923. [Google Scholar]
- Zielińska, D.; Bellwon, J.; Rynkiewicz, A.; Elkady, M.A. Prognostic value of the six-minute walk test in heart failure patients undergoing cardiac surgery: A literature review. Rehabil. Res. Pract. 2013, 2013, 965494. [Google Scholar] [CrossRef]
- Hamilton, D.M.; Haennel, R.G. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. J. Cardiopulm. Rehabil. 2000, 20, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bellet, R.N.; Adams, L.; Morris, N.R. The six-minute walk test in outpatient cardiac rehabilitation: Validity, reliability and responsiveness—A systematic review. Physiotherapy 2012, 98, 277–286. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Cahalin, L.P.; Mathier, M.A.; Semigran, M.J.; Dec, G.W.; DiSalvo, T.G. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996, 110, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.E.V.D.; Costa, D.C.; Crescêncio, J.C.; Santi, G.L.D.; Papa, V.; Marques, F.; Schmidt, A.; Marin-Neto, J.A.; Simões, M.V.; Gallo Junior, L. Heart failure: Comparison between six-minute walk test and cardiopulmonary test. Arq. Bras. Cardiol. 2011, 97, 59–64. [Google Scholar] [CrossRef]
- Deboeck, G.; Van Muylem, A.; Vachiéry, J.L.; Naeije, R. Physiological response to the six-minute walk test in chronic heart failure patients versus healthy control subjects. Eur. J. Prev. Cardiol. 2014, 21, 997–1003. [Google Scholar] [CrossRef]
- Demers, C.; McKelvie, R.S.; Negassa, A.; Yusuf, S. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am. Heart J. 2001, 142, 698–703. [Google Scholar] [CrossRef]
- Forman, D.E.; Fleg, J.L.; Kitzman, D.W.; Brawner, C.A.; Swank, A.M.; McKelvie, R.S.; Clare, R.M.; Ellis, S.J.; Dunlap, M.E.; Bittner, V. Six-minute walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J. Am. Coll. Cardiol. 2012, 60, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, C.; Taylor, R.; Burke, V.; O’Driscoll, G.; Green, D.J. The six-minute walk test does not reliably detect changes in functional capacity of patients awaiting cardiac transplantation. J. Heart Lung Transplant. 2005, 24, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Jehn, M.; Schmidt-Trucksäss, A.; Schuster, T.; Weis, M.; Hanssen, H.; Halle, M.; Koehler, F. Daily walking performance as an independent predictor of advanced heart failure: Prediction of exercise capacity in chronic heart failure. Am. Heart J. 2009, 157, 292–298. [Google Scholar] [CrossRef]
- Kervio, G.; Ville, N.S.; Leclercq, C.; Daubert, J.C.; Carré, F. Intensity and daily reliability of the six-minute walk test in moderate chronic heart failure patients. Arch. Phys. Med. Rehabil. 2004, 85, 1513–1518. [Google Scholar] [CrossRef]
- Uszko-Lencer, N.H.; Mesquita, R.; Janssen, E.; Werter, C.; Brunner-La Rocca, H.P.; Pitta, F.; Wouters, E.F.; Spruit, M.A. Reliability, construct validity and determinants of six-minute walk test performance in patients with chronic heart failure. Int. J. Cardiol. 2017, 240, 285–290. [Google Scholar] [CrossRef]
- Täger, T.; Hanholz, W.; Cebola, R.; Fröhlich, H.; Franke, J.; Doesch, A.; Katus, H.A.; Wians, F.H., Jr.; Frankenstein, L. Minimal important difference for six-minute walk test distances among patients with chronic heart failure. Int. J. Cardiol. 2014, 176, 94–98. [Google Scholar] [CrossRef]
- Yoshimura, K.; Urabe, Y.; Maeda, N.; Yuguchi, S.; Yoshida, T.M. Dynamics of cardiorespiratory response during and after the six-minute walk test in patients with heart failure. Physiother. Theory Pract. 2020, 36, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Zugck, C.; Krüger, C.; Dürr, S.; Gerber, S.; A Haunstetter, A.; Hornig, K.; Kübler, W.; Haass, M. Is the six-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur. Heart J. 2000, 21, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Lans, C.; Cider, Å.; Nylander, E.; Brudin, L. Test–retest reliability of six-minute walk tests over a one-year period in patients with chronic heart failure. Clin. Physiol. Funct. Imaging 2020, 40, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Roul, G.; Germain, P.; Bareiss, P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am. Heart J. 1998, 136, 449–457. [Google Scholar] [CrossRef]
- Lucas, C.; Stevenson, L.W.; Johnson, W.; Hartley, H.; Hamilton, M.A.; Walden, J.; Lem, V.; Eagen-Bengsten, E. The six-minute walk and peak oxygen consumption in advanced heart failure: Aerobic capacity and survival. Am. Heart J. 1999, 138, 618–624. [Google Scholar] [CrossRef]
- Opasich, C.; Pinna, G.; Mazza, A.; Febo, O.; Riccardi, R.; Riccardi, P.; Capomolla, S.; Forni, G.; Cobelli, F.; Tavazzi, L. Six-minute walking performance in patients with moderate-to-severe heart failure; Is it a useful indicator in clinical practice? Eur. Heart J. 2001, 22, 488–496. [Google Scholar] [CrossRef]
- Rostagno, C.; Galanti, G.; Comeglio, M.; Boddi, V.; Olivo, G.; Neri Serneri, G.G. Comparison of different methods of functional evaluation in patients with chronic heart failure. Eur. J. Heart Fail. 2000, 2, 273–280. [Google Scholar] [CrossRef]
- Omar, H.R.; Guglin, M. Longitudinal relationship between six-minute walk test and cardiopulmonary exercise testing, and association with symptoms in systolic heart failure: Analysis from the ESCAPE trial. Eur. J. Intern. Med. 2017, 40, e26–e28. [Google Scholar] [CrossRef]
- Clays, E.; Puddu, P.E.; Luštrek, M.; Pioggia, G.; Derboven, J.; Vrana, M.; De Sutter, J.; Le Donne, R.; Baert, A.; Bohanec, M.; et al. Proof-of-concept trial results of the HeartMan mobile personal health system for self-management in congestive heart failure. Sci. Rep. 2021, 11, 5663. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Dahlström, Ö.; Andersson, G.; Jaarsma, T.; Körner Höhler, A.; Johansson, P. Effect of guided web-based cognitive behavioral therapy on patients with depressive symptoms and heart failure: A pilot randomized controlled trial. J. Med. Internet Res. 2016, 18, e194. [Google Scholar] [CrossRef]
- Jehn, M.; Prescher, S.; Koehler, K.; von Haehling, S.; Winkler, S.; Deckwart, O.; Honold, M.; Sechtem, U.; Baumann, G.; Halle, M.; et al. Tele-accelerometry as a novel technique for assessing functional status in patients with heart failure: Feasibility, reliability and patient safety. Int. J. Cardiol. 2013, 168, 4723–4728. [Google Scholar] [CrossRef]
- Mezzani, A.; Agostoni, P.; Cohen-Solal, A.; Corrà, U.; Jegier, A.; Kouidi, E.; Mazic, S.; Meurin, P.; Piepoli, M.; Simon, A.; et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 249–267. [Google Scholar] [CrossRef]
- Corrà, U.; Piepoli, M.F.; Adamopoulos, S.; Agostoni, P.; Coats, A.J.; Conraads, V.; Lambrinou, E.; Pieske, B.; Piotrowicz, E.; Schmid, J.; et al. Cardiopulmonary exercise testing in systolic heart failure in 2014: The evolving prognostic role. Eur. J. Heart Fail. 2014, 16, 929–941. [Google Scholar] [CrossRef]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. Six-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719870084. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynowska-Teodorczyk, K.; Rudzinska, E.; Lazorczyk, M.; Nowakowska, K.; Banasiak, W.; Ponikowski, P.; Wozniewski, M.; Jankowska, E.A. Distance covered during a six-minute walk test predicts long-term cardiovascular mortality and hospitalisation in men with systolic heart failure. J. Physiother. 2013, 59, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Safley, D.M.; Parikh, S.; Shah, A.; Sullivan, R.; Garg, M. Correlation of six-minute walk test results with other measures of congestive heart failure. Chest 2003, 124, 185S. [Google Scholar] [CrossRef]
- Yap, J.; Lim, F.Y.; Gao, F.; Teo, L.L.; Lam, C.S.; Yeo, K.K. Correlation of the New York Heart Association classification and the six-minute walk distance: A systematic review. Clin. Cardiol. 2015, 38, 621–628. [Google Scholar] [CrossRef]
- Schulz, A.; Krabatsch, T.; Schmitto, J.D.; Hetzer, R.; Seidel, M.; Dohmen, P.M.; Hotz, H. Preliminary results from the C-Pulse OPTIONS HF European multicenter post-market study. Med. Sci. Monit. Basic Res. 2016, 22, 14–19. [Google Scholar] [CrossRef]
- Flynn, K.E.; Lin, L.; Moe, G.W.; Howlett, J.G.; Fine, L.J.; Spertus, J.A.; McConnell, T.R.; Piña, I.L.; Weinfurt, K.P. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am. Heart J. 2012, 163, 88–94.e3. [Google Scholar] [CrossRef]
- van der Stam, J.A.; Bouwmeester, S.; van Loon, S.L.; van Riel, N.A.; Dekker, L.R.; Boer, A.K.; Houthuizen, P.; Acharnhorst, V. Prognostic value of combined biomarkers in patients with heart failure: The Heartmarker Score. Ann. Lab. Med. 2023, 43, 253–262. [Google Scholar] [CrossRef]
- Meys, R.; Janssen, S.; Franssen, F.; Vaes, A.; Stoffels, A.; van Hees, H.; Borst, B.v.D.; Klijn, P.; Burtin, C.; Hul, A.V.; et al. Test–retest reliability, construct validity and determinants of six-minute walk test performance in adult patients with asthma. Pulmonology 2023, 29, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sanderson, B.; Bittner, V. The six-minute walk test: How important is the learning effect? Am. Heart J. 2003, 146, 129–132. [Google Scholar] [CrossRef]
- Halliday, S.J.; Wang, L.; Yu, C.; Vickers, B.P.; Newman, J.H.; Fremont, R.D.; Huerta, L.E.; Brittain, E.L.; Hemnes, A.R. Six-minute walk distance in healthy young adults. Respir. Med. 2020, 165, 105933. [Google Scholar] [CrossRef]
- Adsett, J.; Mullins, R.; Hwang, R.; Hogden, A.; Gibson, E.; Houlihan, K.; Tuppin, M.; Korczyk, D.; Mallitt, K.-A.; Mudge, A. Repeat six-minute walk tests in patients with chronic heart failure: Are they clinically necessary? Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Cavero-Redondo, I.; Saz-Lara, A.; Bizzozero-Peroni, B.; Núñez-Martínez, L.; Díaz-Goñi, V.; Calero-Paniagua, I.; Matínez-García, I.; Pascual-Morena, C. Accuracy of the 6-Minute Walk Test for Assessing Functional Capacity in Patients With Heart Failure With Preserved Ejection Fraction and Other Chronic Cardiac Pathologies: Results of the ExIC-FEp Trial and a Meta-Analysis. Sports Med. Open 2024, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Irlik, K.; Ispoglou, T.; Ferentinos, P.; Mitropoulos, A.; Schlögl, M.; Isanejad, M.; Kegler, K.; Nabrdalik, K.; Lip, G.Y.H. Exercise capacity in heart failure: A systematic review and meta-analysis of HFrEF and HFpEF disparities in VO2peak and 6-minute walking distance. Eur. Heart J. Open 2025, 5, oeaf055. [Google Scholar] [CrossRef]
- Pepera, G.K.; Sandercock, G.R.; Sloan, R.; Cleland, J.J.; Ingle, L.; Clark, A.L. Influence of step length on six-minute walk test performance in patients with chronic heart failure. Physiotherapy 2012, 98, 325–329. [Google Scholar] [CrossRef]
- Marinho, R.; Jürgensen, S.; Arcuri, J.; Goulart, C.; dos Santos, P.; Roscani, M.; Mendes, R.; de Oliveira, C.; Caruso, F.; Borghi-Silva, A. Reliability and validity of six-minute step test in patients with heart failure. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas Biol. 2021, 54, e10514. [Google Scholar] [CrossRef]
- Shoemaker, M.J.; Curtis, A.B.; Vangsnes, E.; Dickinson, M.G. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm. Phys. Ther. J. 2013, 24, 21–29. [Google Scholar] [CrossRef]
- Shoemaker, M.J.; Curtis, A.B.; Vangsnes, E.; Dickinson, M.G. Triangulating clinically meaningful change in the six-minute walk test in individuals with chronic heart failure: A systematic review. Cardiopulm. Phys. Ther. J. 2012, 23, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Anker, S.D.; Friede, T.; Jankowska, E.A.; Metra, M.; Piña, I.L.; Coats, A.J.; Rosano, G.; Roubert, B.; Goehring, U.-M.; et al. Minimal clinically important differences in six-minute walk test in patients with HFrEF and iron deficiency. J. Card. Fail. 2023, 29, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Pepera, G.B.; Paul, D.; Sandercock, G.R.H. A pilot study to investigate the safety of exercise training and testing in cardiac rehabilitation patients. Br. J. Cardiol. 2013, 20, 78. [Google Scholar]
- Nurhayati, N.; Wijaya, A.; Yustisia, N.; Anggraini, T. The six-minute walk as a safety exercise in improving the health-related quality of life among heart failure patients: A preliminary research. Health Technol. J. 2023, 1, 665–670. [Google Scholar] [CrossRef]
- Ferreira, P.; Menezes, A.; Camelier, F. Safety of the six-minute walk test in hospitalized cardiac patients. Int. J. Cardiovasc. Sci. 2015, 28, 70–76. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Reza, N.; Donald, E.; Givertz, M.M.; Lindenfeld, J.; Jessup, M. Considerations for heart failure care during the COVID-19 pandemic. JACC Heart Fail. 2020, 8, 681–691. [Google Scholar] [CrossRef]
- Pepera, G.; Karanasiou, E.; Blioumpa, C.; Antoniou, V.; Kalatzis, K.; Lanaras, L.; Batalik, L. Tele-assessment of functional capacity through the six-minute walk test in patients with diabetes mellitus type 2: Validity and reliability of repeated measurements. Sensors 2023, 23, 1045. [Google Scholar] [CrossRef]
- Nevelikova, M.; Dosbaba, F.; Pepera, G.; Felsoci, M.; Batalikova, K.; Su, J.J.; Batalik, L. Validity and reliability of automated treadmill six-minute walk test in patients entering exercise-based cardiac rehabilitation. Ann. Med. 2023, 55, 2304664. [Google Scholar] [CrossRef] [PubMed]

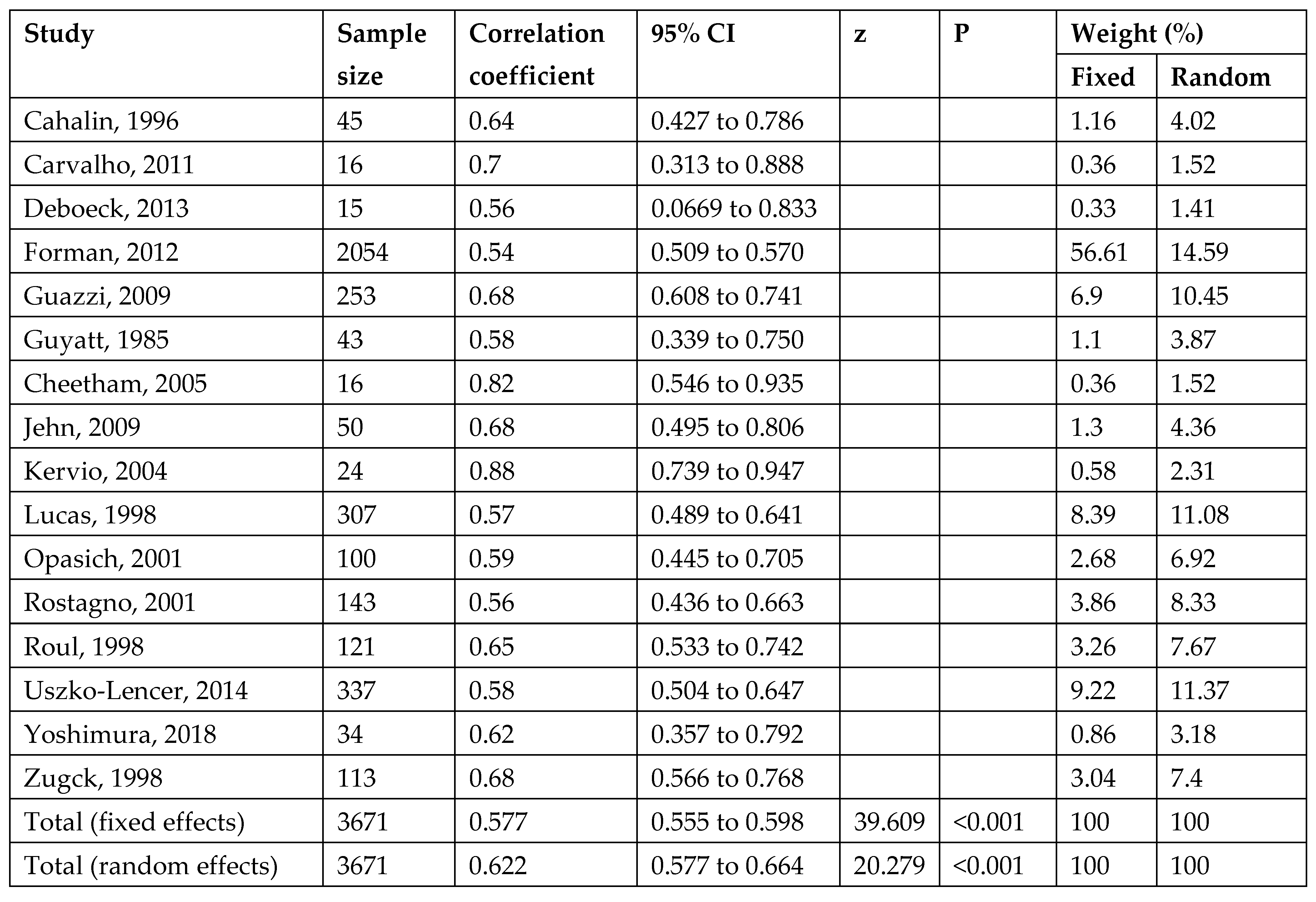
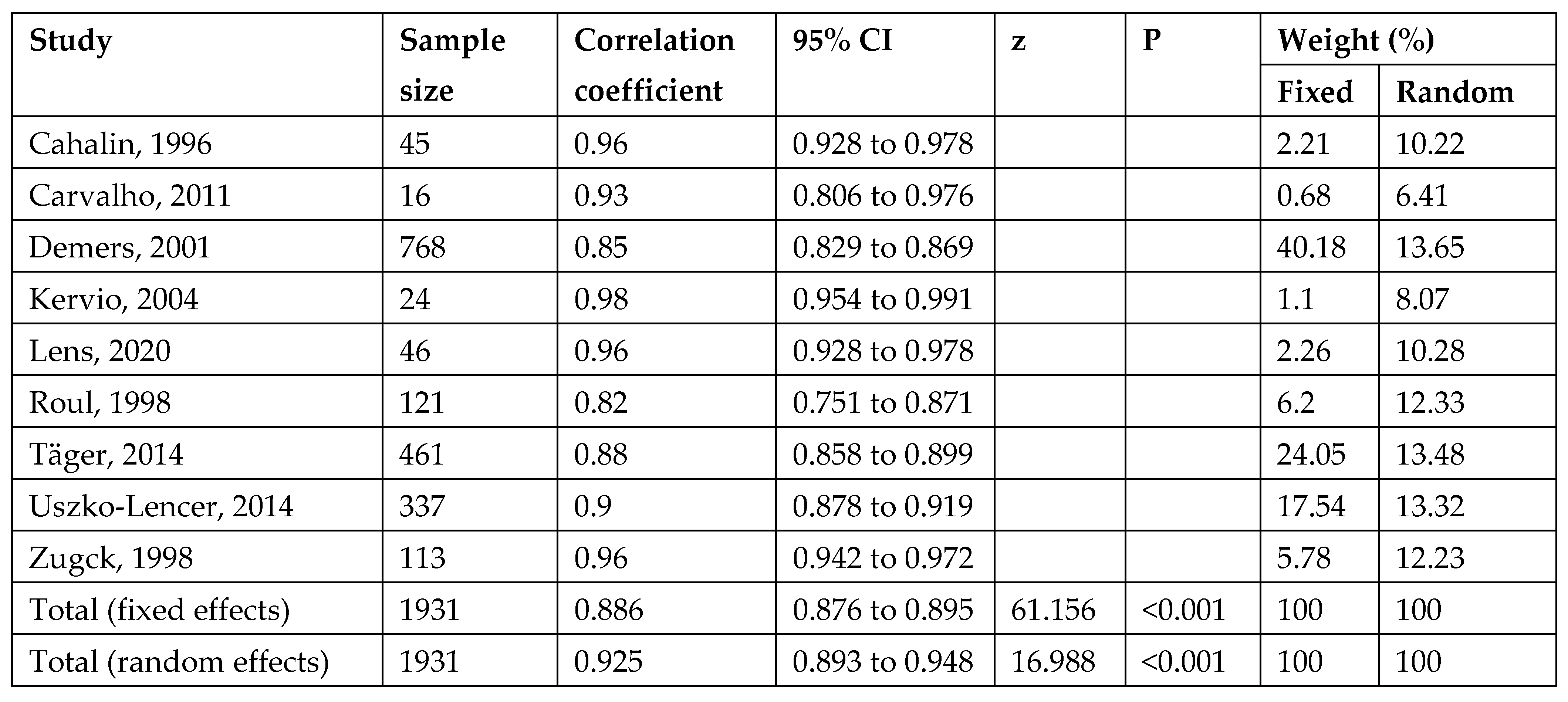
| Reference | NYHA | Diagnosis (%) | LVEF (%) | Sex (n) | Age | 6MWT Protocol | 6MWT Mean | CPET Protocol | mL/kg/min | Correlations | Reliability 6MWT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cahalin (1996) [14] USA | 3.3 (0.6) | DCM 73, Ischemic 22 Myocarditis 5 | 20.0 | 45 Men 40 Women 5 | 49.0 (8) | Guyatt 1985 [8] | 310 (100) | Bicycle ergometer (Ramp) | 12.2 (4) | r = 0.64 * DW-6MWT/pVO2-CPET | 0.96 |
| Carvalho (2011) [15] Brazil | I–II | DCM 29 Other 71 | 31.4 | 16 Men 12 Women 4 | 57.5 (10) | ATS | 550 (66) | Bicycle ergometer (Ramp) | 14.1 (4) | r = 0.70 * DW-6MWT/pVO2-CPET | 0.93 |
| Deboeck et al. (2013) [16] Belgium | I–III | DCM 20 Ischemic 60 Other 20 | 31 | 15 Men 13 Women 2 | 52 | ATS | 445 (95) | Bicycle ergometer (Ramp) | 13.9 (3) | r = 0.56 * DW-6MWT/pVO2-CPET | - |
| Demers et al. (2001) [17] Canada | I–IV | Ischemic 71 Other 29 | 27 | 768 Men 637 Women 131 | 63 (11) | Lipkin 1986 | 381 (84) | NR | NR | NR | 0.85 |
| Forman et al. (2012) [18] USA | II–IV | DCM 49 Ischemic 51 | 24.9 | 2054 Men 1459 Women 595 | 59 | ATS | 372 | Treadmill (modified Naughton) | 14.6 | r = 0.54 * DW-6MWT/pVO2-CPET | |
| Guazzi et al. (2009) [6] Italy | 2.2 (0.8) | DCM 39 Ischemic 61 | 36.3 | 253 Men 199 Women 54 | 62 (10) | Roul 1998 [27] | 351 | Bicycle ergometer (Ramp) | 15 (4.7) | r = 0.68 * DW-6MWT/pVO2-CPET | |
| Guyatt et al. (1985) [8] USA | II–IV | NR | NR | 43 Men 34 Women 9 | 65 (8) | Guyatt 1985 [27] | NR | Bicycle ergometer (Ramp) | NR | r = 0.58 * DW-6MWT/pVO2-CPET | NR |
| Cheetham (2005) [19] Australia | III–IV | DCM 31 Ischemic 63 Other 6 | 23 | 16 Men 14 Women 2 | 59 (3) | Guyatt 1985 [27] | 458 (21) | Treadmill (Bruce protocol) | 16.3 (1.1) | r = 0.82 * DW-6MWT/pVO2-CPET | |
| Jehn et al. (2009) [20] Germany | I–III | DCM 28 Ischemic 42 Other 30 | 39 | 50 Men 38 Women 12 | 61 (14) | NR | 510 (129) | Bicycle ergometer (Ramp) | 20.5 | r = 0.68 * DW-6MWT/pVO2-CPET | |
| Kervio et al. (2004) [21] France | II–III | DCM 79 Ischemic 21 | 27 | 24 Men 19 Women 5 | 65 | Guyatt 1985 [27] | 427 | Treadmill (Weber protocol) | 16.7 | r = 0.88 * DW-6MWT/pVO2-CPET | 0.98 |
| Lans et al. (2020) [26] Sweden | II–III | DCM 26 Ischemic 61 Other 13 | 29 | 46 Men 37 Women 9 | 68 (9) | ATS | 411 (96) | NR | NR | NR | 0.96 |
| Lucas et al. (1998) [28] USA | NR | NR | 23 | 307 Men 240 Women 67 | 52 (13) | Lipkin 1986 | 393 (104) | Bicycle ergometer (Ramp) | 14 (5) | r = 0.57 * DW-6MWT/pVO2-CPET | - |
| Omar et al. (2017) [31] USA | I–IV | NR | 30 | 433 Men 320 Women 113 | 56 | NR | NR | NR | NR | r = 0.4 * DW-6MWT/pVO2-CPET | NR |
| Opasich et al. (2001) [29] Italy | II–III | DCM 40 Ischemic 41 Other 19 | 26 | 100 Men 87 Women 13 | 53 (9) | NR | 396 (92) | Bicycle ergometer (Ramp) | 14.6 (4) | r = 0.59 * DW-6MWT/pVO2-CPET | - |
| Rostagno et al. (2001) [30] Italy | II–III | DCM 36 Ischemic 37 Other 27 | 33 | 143 Men 78 Women 65 | 57 | Guyatt 1985 [27] | NR | Bicycle ergometer (Ramp) | NR | r = 0.56 * DW-6MWT/pVO2-CPET | - |
| Roul et al. (1998) [27] France | II–III | DCM 47 Ischemic 29 Other 24 | 30 | 111 Men 99 Women 22 | 59(11) | Guyatt 1985 [27] | 433 (108) | Bicycle ergometer (Ramp) | 17 (4.5) | r = 0.65 * DW-6MWT/pVO2-CPET | 0.82 |
| Täger et al. (2014) [23] Germany | I–III | NR | 31.5 | 461 Men 360 Women 101 | 57 (12) | NR | 480 (106) | NR | NR | - | 0.88 |
| Uszko-Lencer (2017) [22] Netherlands | I–III | NR | 35 | 337 Men 235 Women 102 | 65 | ERS/ATS | 473 | Bicycle ergometer (Ramp) | 15.5 | r = 0.58 * DW-6MWT/pVO2-CPET | 0.90 |
| Yoshimura (2020) [24] Japan | II–III | DCM 56 Ischemic 3 Other 41 | 37 | 34 Men 13 Women 21 | 69 | ATS | 412 (10) | Bicycle ergometer (Ramp) | 15.8 (4) | r = 0.62 * DW-6MWT/pVO2-CPET | - |
| Zugck et al. (2000) [25] Germany | 2.2 (0.8) | DCM 100 | 19 | 113 Men 90 Women 23 | 54 (12) | Guyatt 1985 [27] | 466 (107) | Bicycle ergometer (Ramp) | 15.4 (5.4) | r = 0.68 * DW-6MWT/pVO2-CPET | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepera, G.; Antoniou, V.; Karagianni, E.; Batalik, L.; Su, J.J. Validity and Reliability of the Six-Minute Walking Test Compared to Cardiopulmonary Exercise Test in Individuals with Heart Failure Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 8303. https://doi.org/10.3390/jcm14238303
Pepera G, Antoniou V, Karagianni E, Batalik L, Su JJ. Validity and Reliability of the Six-Minute Walking Test Compared to Cardiopulmonary Exercise Test in Individuals with Heart Failure Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(23):8303. https://doi.org/10.3390/jcm14238303
Chicago/Turabian StylePepera, Garyfallia, Varsamo Antoniou, Eleni Karagianni, Ladislav Batalik, and Jing Jing Su. 2025. "Validity and Reliability of the Six-Minute Walking Test Compared to Cardiopulmonary Exercise Test in Individuals with Heart Failure Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 23: 8303. https://doi.org/10.3390/jcm14238303
APA StylePepera, G., Antoniou, V., Karagianni, E., Batalik, L., & Su, J. J. (2025). Validity and Reliability of the Six-Minute Walking Test Compared to Cardiopulmonary Exercise Test in Individuals with Heart Failure Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(23), 8303. https://doi.org/10.3390/jcm14238303








