Anatomical Considerations in the Twin Block Technique for the Treatment of Masticatory Myofascial Pain: An Anatomical Review
Abstract
1. Introduction
2. Trigeminal Nerve
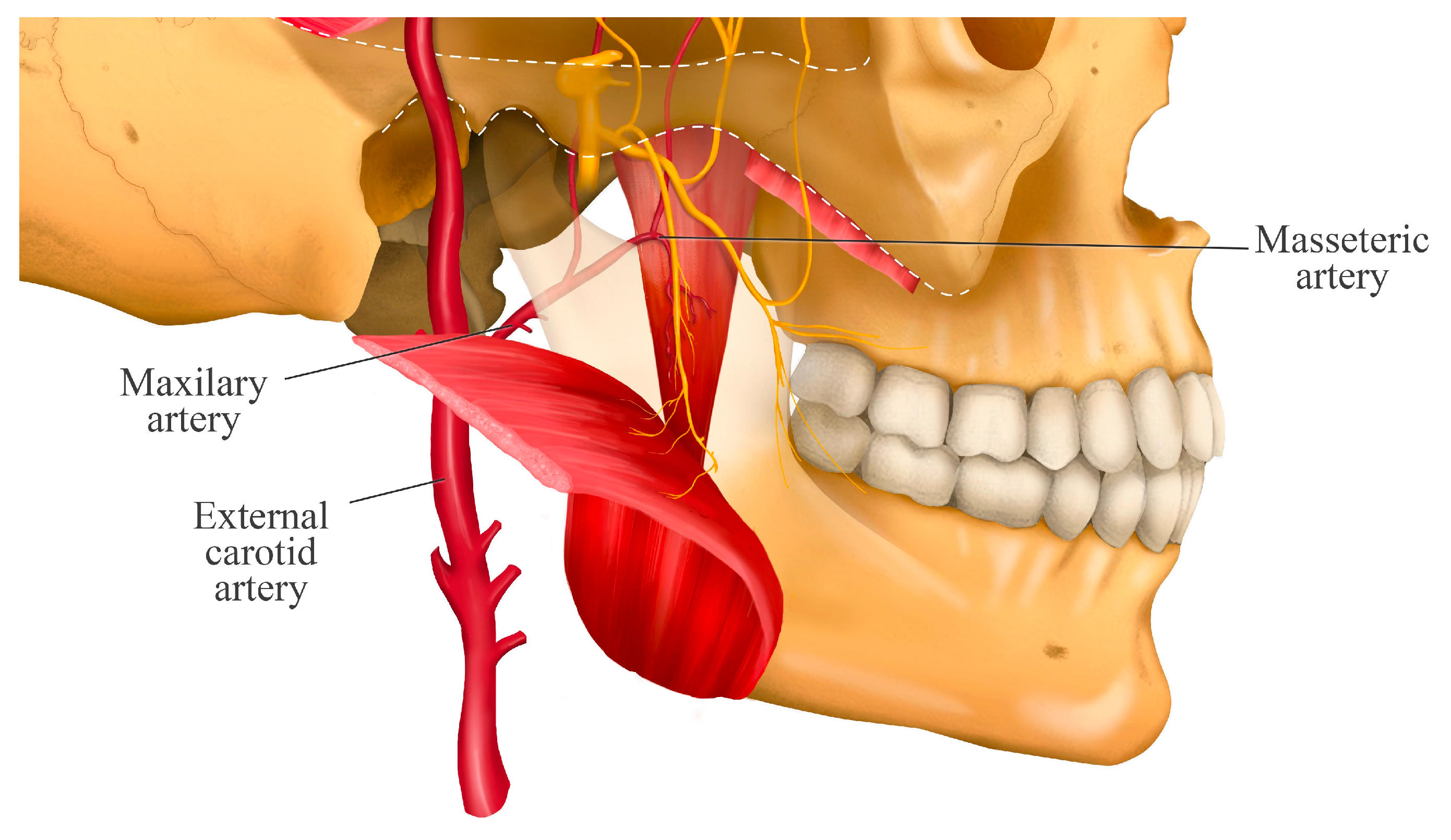
3. Course of the Masseteric Nerve
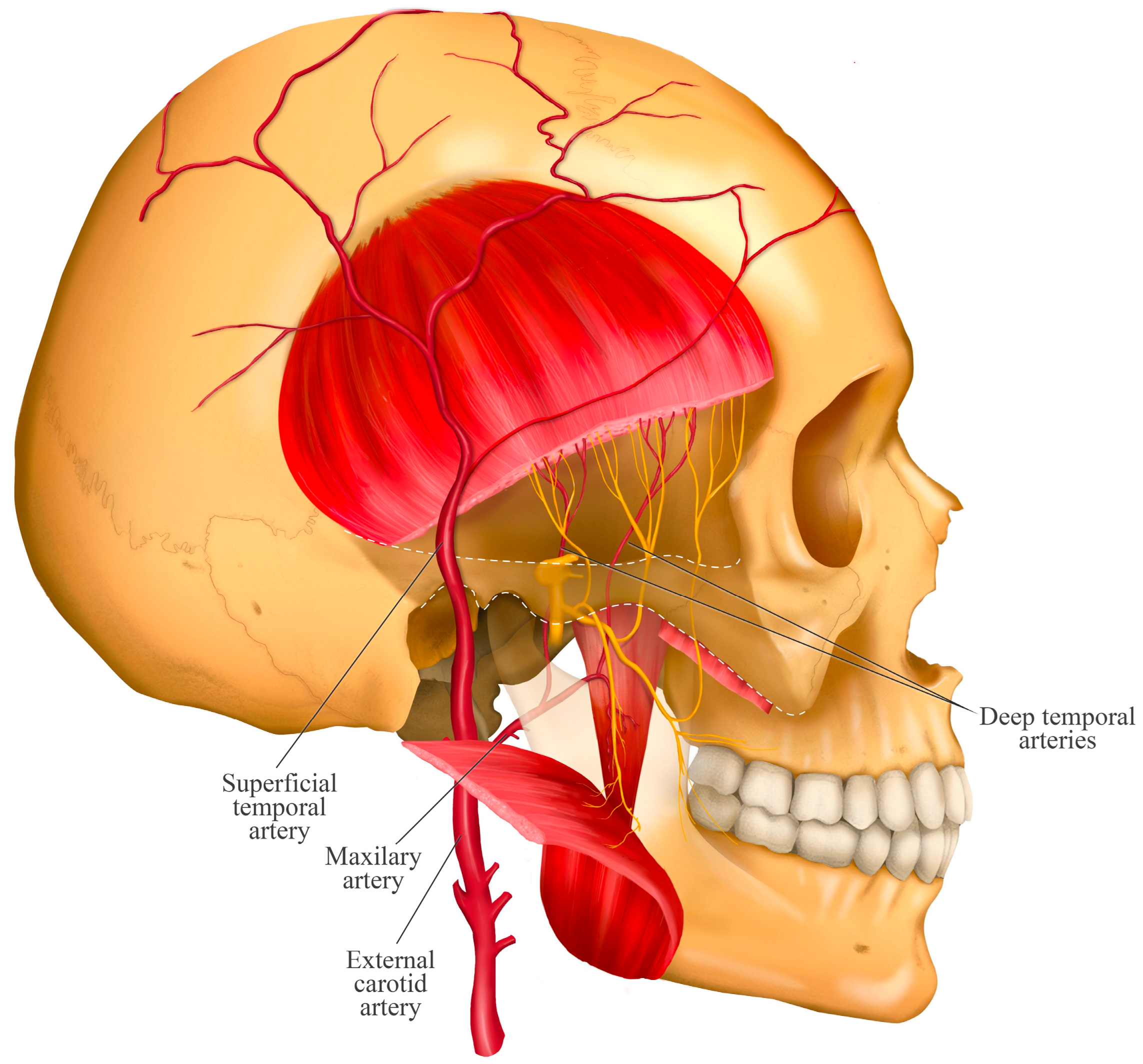
4. Course of the Deep Temporal Nerves
- The middle deep temporal nerve (MDTN) is a branch that is not always present [24]. When present, it branches off early from the main nerve (anterior division of the mandibular nerve). It typically travels along the superior surface of the lateral pterygoid muscle and often perforates its upper head to distribute into the middle portion of the temporalis muscle. The branching patterns are variable and have been classified into four types based on the number of branches: Type A (one branch), Type B (two branches), Type C (three branches), and Type D (four branches) [22].
- The posterior deep temporal nerve (PDTN) arises independently from the temporomasseteric nerve. It travels along the superior surface of the upper head of the lateral pterygoid muscle and distributes to the posterior portion of the temporalis muscle [22]. It has also been described as having two to five branches [23].
5. Relationship with the Deep Temporal Artery
6. Isolated Masseteric Nerve Block
7. Isolated Deep Temporal Nerve Block
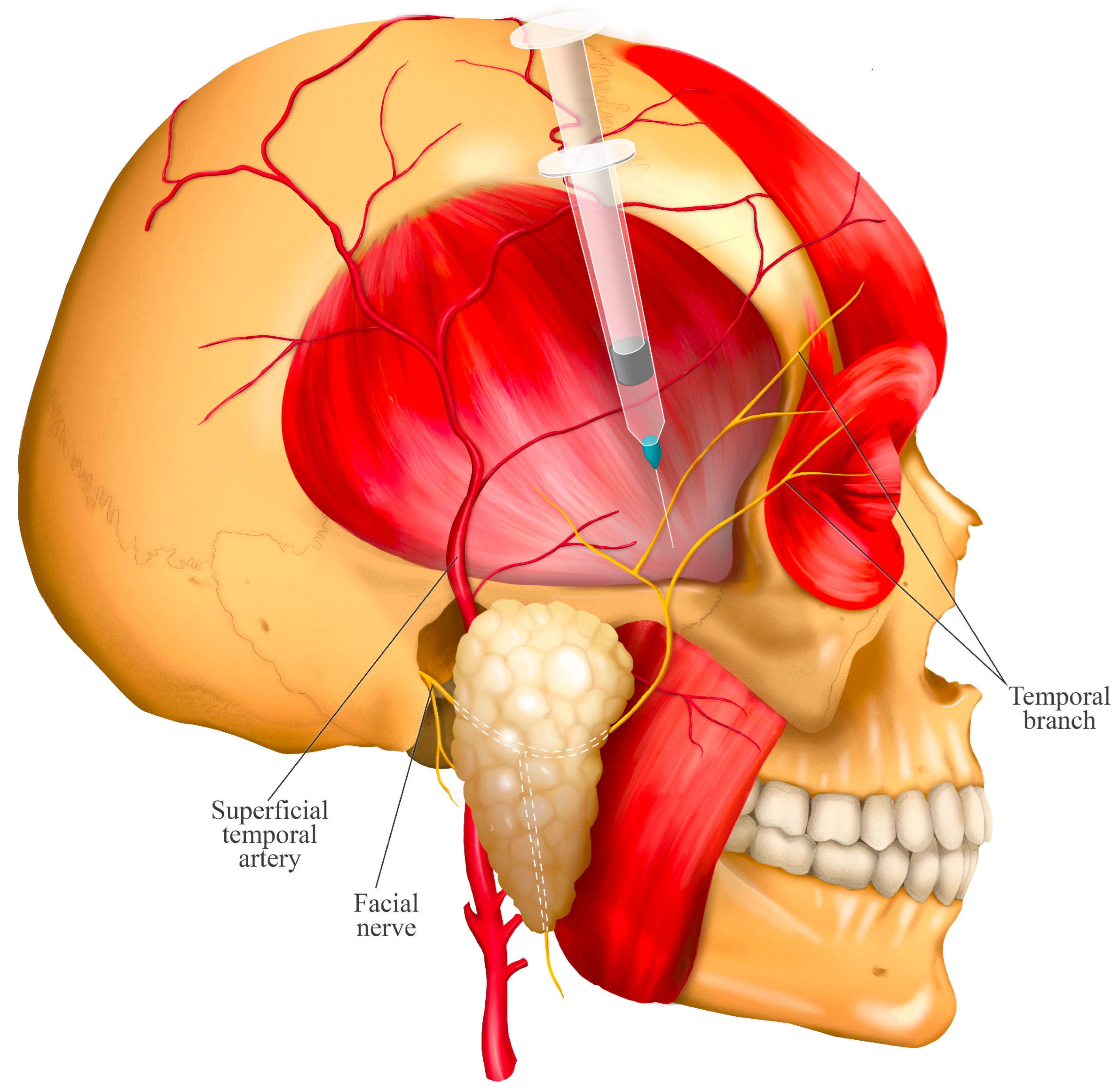
8. Twin Block Technique: Simultaneous Blockade of the Masseteric and Deep Temporal Nerves
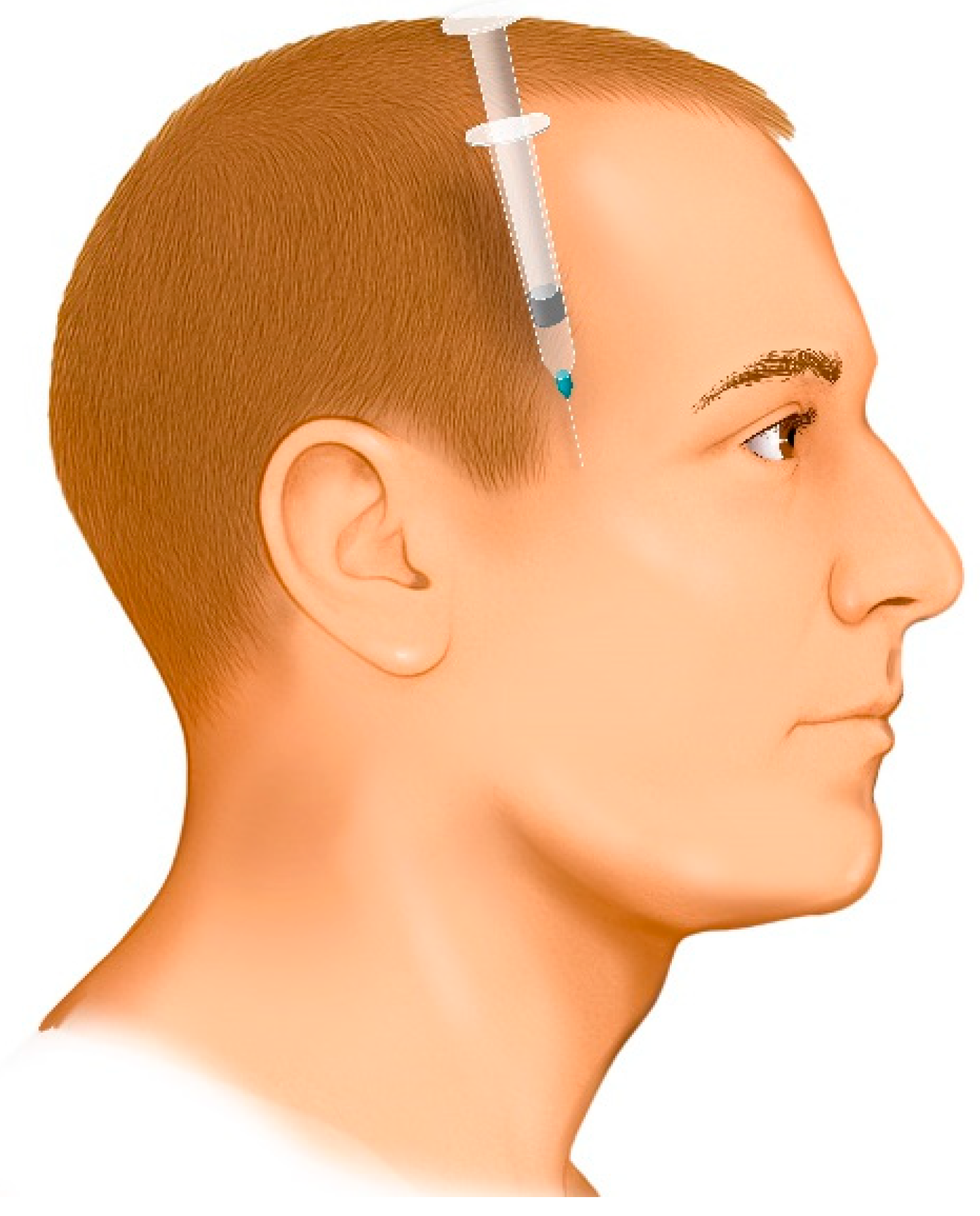
- The entry point is determined by palpating the superior margin of the zygomatic arch and locating the anatomical depression formed by the contact between the greater wing of the sphenoid bone and the superior border of the zygomatic process. This landmark is located approximately 10 mm posterior to the posterior border of the frontal process of the zygomatic bone (approximately the width of an index finger).
- Disinfect the injection site using 70% alcohol.
- The needle is directed downward between the zygomatic arch and the infratemporal fossa at an angle of 35° to 45° relative to the cranium (Figure 5). This angulation can be easily achieved by seating the patient in an upright position and aligning the needle to bisect the angle between the temporal bone (vertical plane) and the horizontal plane. If the needle is not angled properly, it may contact the coronoid process of the mandible, obstructing its advance. In such cases, the needle should be withdrawn slightly and redirected at a wider angle.
- Advance the needle fully to its entire length.
- Administer the anesthetic.
- If a second injection is needed, a new sterile needle must be used to avoid cross-contamination.
9. Discussion
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Miernik, M.; Wieckiewicz, M.; Paradowska, A.; Wieckiewicz, W. Massage Therapy in Myofascial TMD Pain Management. Adv. Clin. Exp. Med. 2012, 21, 681–685. [Google Scholar] [PubMed]
- Saxena, A.; Chansoria, M.; Tomar, G.; Kumar, A. Myofascial Pain Syndrome: An Overview. J. Pain Palliat. Care Pharmacother. 2015, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, R.D.; Dommerholt, J.; Shah, J.P. An Expansion of Simons’ Integrated Hypothesis of Trigger Point Formation. Curr. Sci. Inc. 2004, 8, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Nowak, Z.; Chęciński, M.; Nitecka-Buchta, A.; Bulanda, S.; Ilczuk-Rypuła, D.; Postek-Stefańska, L.; Baron, S. Intramuscular Injections and Dry Needling within Masticatory Muscles in Management of Myofascial Pain. Syst. Rev. Clin. Trials. IJERPH 2021, 18, 9552. [Google Scholar] [CrossRef]
- Cao, Q.-W.; Peng, B.-G.; Wang, L.; Huang, Y.-Q.; Jia, D.-L.; Jiang, H.; Lv, Y.; Liu, X.-G.; Liu, R.-G.; Li, Y.; et al. Expert Consensus on the Diagnosis and Treatment of Myofascial Pain Syndrome. WJCC 2021, 9, 2077–2089. [Google Scholar] [CrossRef]
- Taşkesen, F.; Cezairli, B. The Effectiveness of the Masseteric Nerve Block Compared with Trigger Point Injections and Dry Needling in Myofascial Pain. CRANIO® 2023, 41, 96–101. [Google Scholar] [CrossRef]
- Iturriaga, V.; Bornhardt, T.; Wen, S.; Avila, M.; Del Sol, M. Mandibular Condyle Depth Analysis in Magnetic Resonance of Patients with Temporomandibular Disorders. Int. J. Morphol. 2020, 38, 458–460. [Google Scholar] [CrossRef]
- Quek, S.Y.P.; Subramanian, G.; Patel, J.; Ananthan, S.; Zagury, J.G.; Khan, J. Efficacy of Regional Nerve Block in Management of Myofascial Pain of Masseteric Origin. CRANIO® 2015, 33, 286–291. [Google Scholar] [CrossRef]
- Young, A.L.; Khan, J.; Thomas, D.C.; Quek, S.Y.P. Use of Masseteric and Deep Temporal Nerve Blocks for Reduction of Mandibular Dislocation. Anesth. Prog. 2009, 56, 9–13. [Google Scholar] [CrossRef]
- Ananthan, S.; Kanti, V.; Zagury, J.G.; Quek, S.Y.P.; Benoliel, R. The Effect of the Twin Block Compared with Trigger Point Injections in Patients with Masticatory Myofascial Pain: A Pilot Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 222–228. [Google Scholar] [CrossRef]
- Quek, S.; Young, A.; Subramanian, G. The Twin Block: A Simple Technique to Block Both the Masseteric and the Anterior Deep Temporal Nerves with One Anesthetic Injection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, e65–e67. [Google Scholar] [CrossRef]
- Ali, A.; Lo, S.; Nduka, C.; Adds, P. Anatomy of the Infratemporal Crest: Implications for Cross-Facial Nerve Grafting in Temporal Myoplasty. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, e54–e59. [Google Scholar] [CrossRef]
- Quek, S.Y.P.; Gomes-Zagury, J.; Subramanian, G. Twin Block in Myogenous Orofacial Pain: Applied Anatomy, Technique Update, and Safety. Anesth. Prog. 2020, 67, 103–106. [Google Scholar] [CrossRef]
- Bathla, G.; Hegde, A.N. The Trigeminal Nerve: An Illustrated Review of Its Imaging Anatomy and Pathology. Clin. Radiol. 2013, 68, 203–213. [Google Scholar] [CrossRef]
- Moore, K.L.; Dalley, A.F.; Agur, A.M.R. Anatomía con Orientación Clínica, 8th ed.; Wolters Kluwer: Barcelona, Spain, 2018; ISBN 978-84-17033-63-7. [Google Scholar]
- Schünke, M.; Schulte, E.; Schumacher, U. Prometheus: Texto y Atlas de Anatomía, 5th ed.; Mejorada y Amp.; Médica Panamericana: Madrid, Spain; Buenos Aires, Argentina, 2021; ISBN 978-84-9110-972-3. [Google Scholar]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Gray, H., Standring, S., Anhand, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-7020-7705-0. [Google Scholar]
- Netter, F.H. Atlas de Anatomía Humana, 7th ed.; Elsevier: Barcelona, Spain, 2019; ISBN 978-84-9113-468-8. [Google Scholar]
- Collar, R.M.; Byrne, P.J.; Boahene, K.D.O. The Subzygomatic Triangle: Rapid, Minimally Invasive Identification of the Masseteric Nerve for Facial Reanimation. Plast. Reconstr. Surg. 2013, 132, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Apaydin, N.; Loukas, M.; Tubbs, R.S. The Topographic Anatomy of the Masseteric Nerve: A Cadaveric Study with an Emphasis on the Effective Zone of Botulinum Toxin a Injections in Masseter. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Hong, H.-S.; Won, S.-Y.; Kim, H.-J.; Hu, K.-S.; Choi, J.-H.; Kim, H.-J. Intramuscular Nerve Distribution of the Masseter Muscle as a Basis for Botulinum Toxin Injection. J. Craniofacial Surg. 2010, 21, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.H.; Ko, S.J.; Jung, H.S.; Park, H.D.; Chung, I.H.; Kim, H.J. Topographic Anatomy of the Deep Temporal Nerves, with References to the Superior Head of Lateral Pterygoid. Surg. Radiol. Anat. 2003, 25, 393–399. [Google Scholar] [CrossRef]
- De Jongh, F.W.; Pouwels, S.; Sanches, E.E.; Van Heerbeek, N.; Ingels, K.J.A.O. Deep Temporal Nerve Transfer: A Systematic Review of Anatomy, Anatomical Landmarks and Clinical Applications. JPRAS Open 2022, 33, 106–113. [Google Scholar] [CrossRef]
- Karagoz, H.; Ozturk, S.; Malkoc, I.; Diyarbakir, S.; Demirkan, F. Anatomy of the Anterior Deep Temporal Nerve: Implications for Neurotization in Blinking Restoration in Facial Paralysis. Ann. Plast. Surg. 2015, 75, 316–318. [Google Scholar] [CrossRef]
- Kalladka, M.; Shastry, S.P.; Thondebhavi, M.; Quek, S.Y.P.; Singh, S.; Khan, J. Nerve Blocks in the Management of Acute Temporomandibular Disorder Emergencies—A Narrative Review. J. Oral Maxillofac. Anesth. 2022, 1, 15. [Google Scholar] [CrossRef]
- Kanti, V.; Ananthan, S.; Subramanian, G.; Quek, S.Y.P. Efficacy of the Twin Block, a Peripheral Nerve Block for the Management of Chronic Masticatory Myofascial Pain: A Case Series. Quintessence Int. 2017, 48, 829–833. [Google Scholar] [CrossRef]
- Montes-Carmona, J.-F.; Gonzalez-Perez, L.-M.; Infante-Cossio, P. Treatment of Localized and Referred Masticatory Myofascial Pain with Botulinum Toxin Injection. Toxins 2020, 13, 6. [Google Scholar] [CrossRef]
- Yildiz, G.; Akkaya, O.T. A Comparison Between the Efficacy of Trigeminal Ganglion Radiofrequency Thermocoagulation and Ultrasound-Guided Maxillary-Mandibular Nerve Pulsed Radiofrequency in the Treatment of Trigeminal Neuralgia: A Randomized Clinical Trial. Cureus 2024, 16, e61565. [Google Scholar] [CrossRef]


| Nerves | Innervation |
|---|---|
| Sensory branches of the mandibular nerve | |
| Auriculotemporal nerve | Skin of the temporal region, the ear, and part of the external acoustic pore of the skull, and part of the temporomandibular joint. |
| Lingual nerve | Anterior two-thirds of the tongue. |
| Inferior alveolar nerve | Lower gum and teeth, the terminal branch of which corresponds to the mental nerve that innervates the skin of the chin and lower lip. |
| Buccal nerve | Skin of the cheek and mucosa of the buccal region, and the gingiva and buccal groove of the lower molars and second premolar. |
| Mixed branches of the mandibular nerve (motor and sensory) | |
| Masseteric nerve * | Masseter muscle. |
| Deep temporal nerves (anterior, middle and posterior) ** | Temporal muscle. |
| Pterygoid nerves (medial and lateral) | Medial and lateral pterygoid muscles. |
| Mylohyoid nerve | Mylohyoid muscle and anterior belly of the digastric muscle. This is a branch of the inferior alveolar nerve before it enters the mandibular canal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venegas-Ocampo, C.; Iturriaga, V.; Ottone, N.E.; Torres-Villar, C.; Marinelli, F.; Gelabert, R.; Fuentes, R. Anatomical Considerations in the Twin Block Technique for the Treatment of Masticatory Myofascial Pain: An Anatomical Review. J. Clin. Med. 2025, 14, 8299. https://doi.org/10.3390/jcm14238299
Venegas-Ocampo C, Iturriaga V, Ottone NE, Torres-Villar C, Marinelli F, Gelabert R, Fuentes R. Anatomical Considerations in the Twin Block Technique for the Treatment of Masticatory Myofascial Pain: An Anatomical Review. Journal of Clinical Medicine. 2025; 14(23):8299. https://doi.org/10.3390/jcm14238299
Chicago/Turabian StyleVenegas-Ocampo, Camila, Veronica Iturriaga, Nicolás E. Ottone, Carlos Torres-Villar, Franco Marinelli, Ramón Gelabert, and Ramón Fuentes. 2025. "Anatomical Considerations in the Twin Block Technique for the Treatment of Masticatory Myofascial Pain: An Anatomical Review" Journal of Clinical Medicine 14, no. 23: 8299. https://doi.org/10.3390/jcm14238299
APA StyleVenegas-Ocampo, C., Iturriaga, V., Ottone, N. E., Torres-Villar, C., Marinelli, F., Gelabert, R., & Fuentes, R. (2025). Anatomical Considerations in the Twin Block Technique for the Treatment of Masticatory Myofascial Pain: An Anatomical Review. Journal of Clinical Medicine, 14(23), 8299. https://doi.org/10.3390/jcm14238299






