Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Symptomatic Individuals with Primary Mitral Regurgitation? A Narrative Review of Traditional and Innovative Prognostic Indicators
Abstract
1. Introduction
2. Literature Search and Evidence Selection
3. Traditional Prognostic Indicators Assessed Using Transthoracic Echocardiography
3.1. Echocardiographic Criteria of PMR Severity Recommended by Current Guidelines
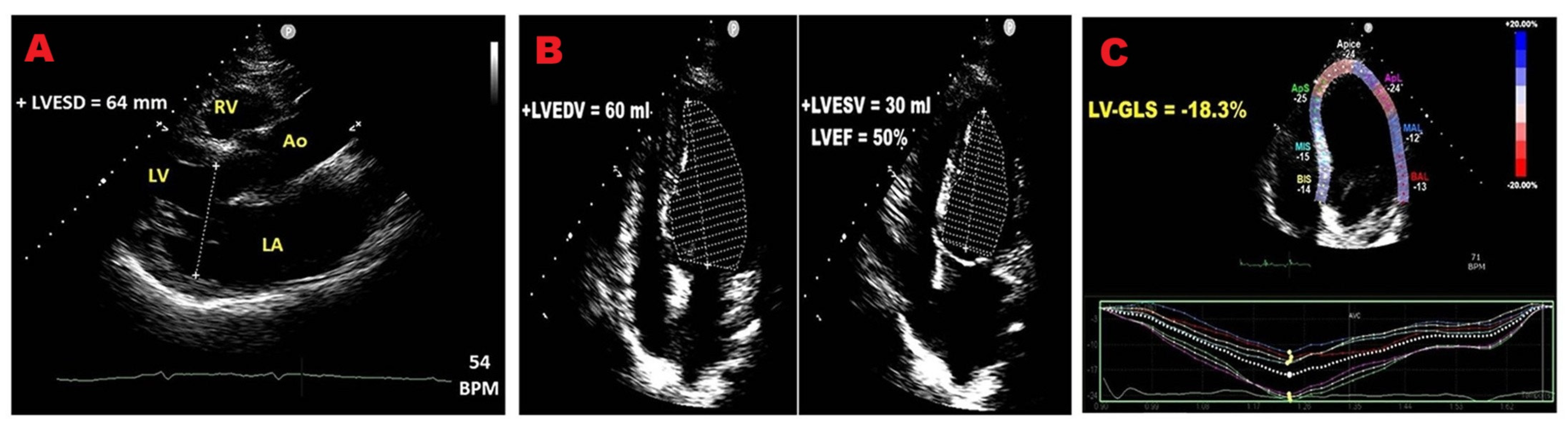
3.2. Left Ventricular Size
3.3. Left Ventricular Function
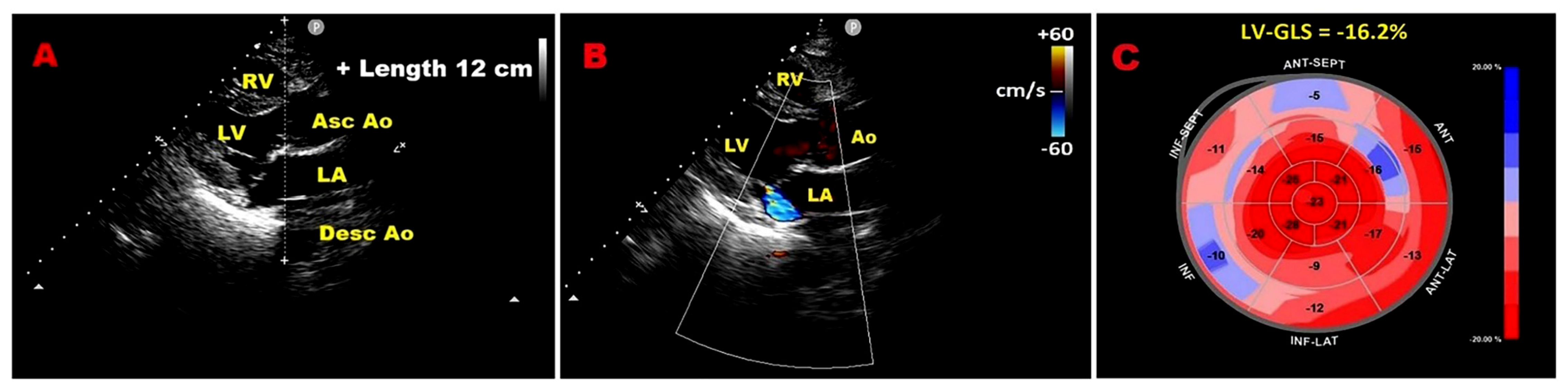
3.4. Left Ventricular Global Longitudinal Strain
3.5. Left Atrial Size
3.6. Left Atrial Reservoir Strain
3.7. Pulmonary Hypertension
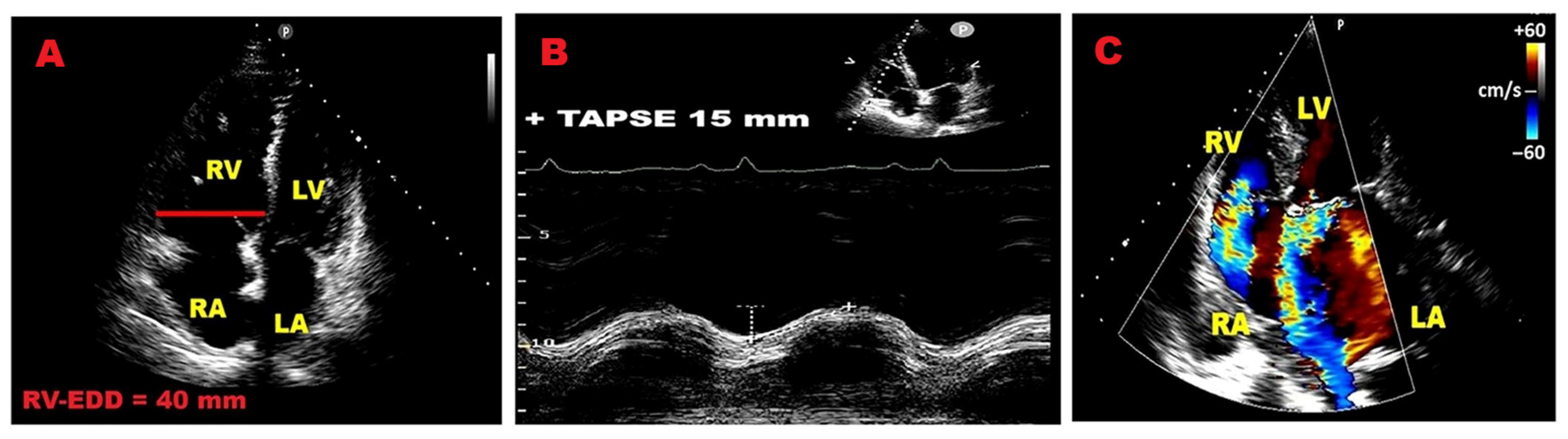
3.8. Right Ventricular Size and Function
3.9. Right Ventricular Strain
3.10. Functional Tricuspid Regurgitation
4. Prognostic Indicators Assessed Using Exercise Stress Echocardiography
5. Critical Appraisal of Established Echocardiographic Prognostic Markers in Primary Mitral Regurgitation
6. Contributive Role of Cardiac MRI and PET/MR in Prognostic Stratification of Primary Mitral Regurgitation
7. Laboratory Prognostic Indicators
8. Surgical or Percutaneous Treatment of PMR
9. Innovative Anthropometric Prognostic Indicators of PMR Severity
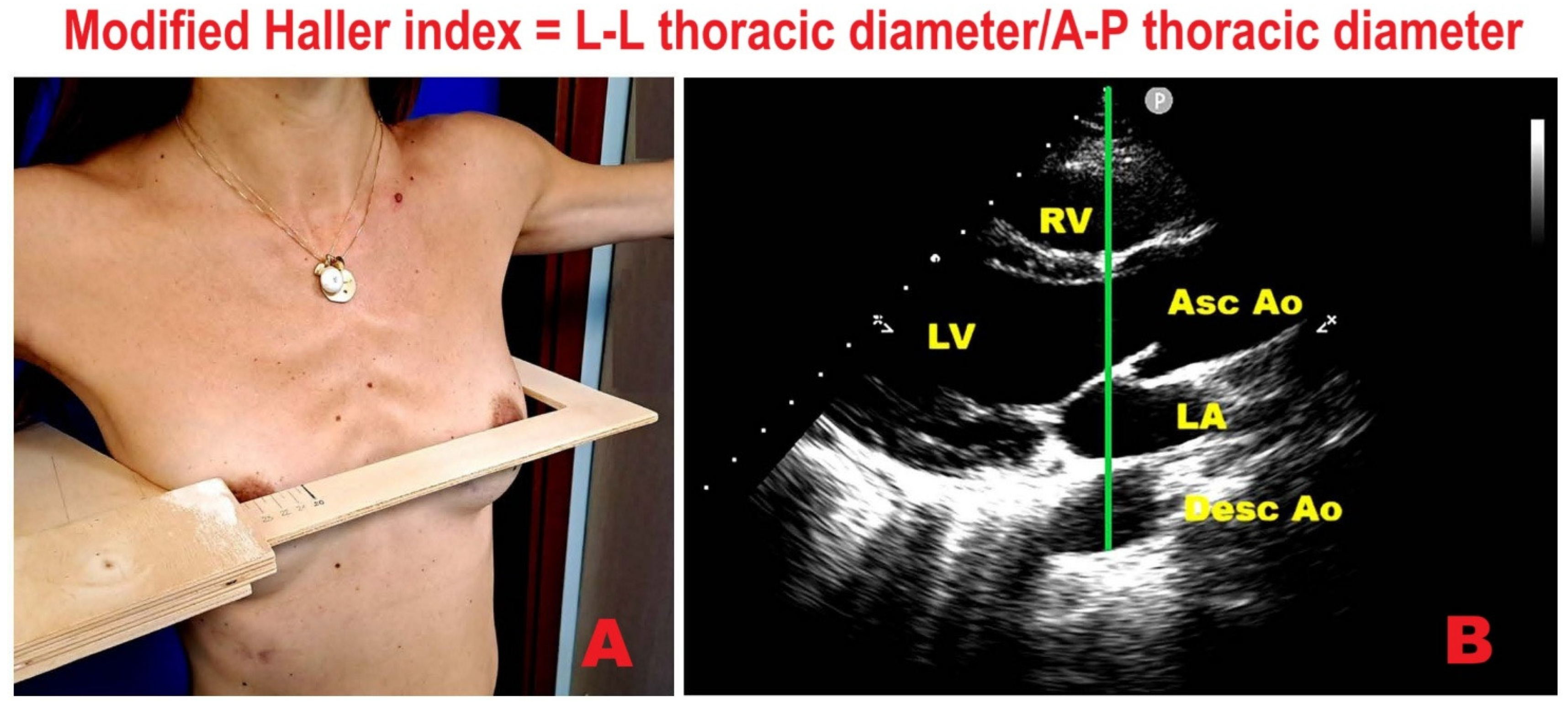
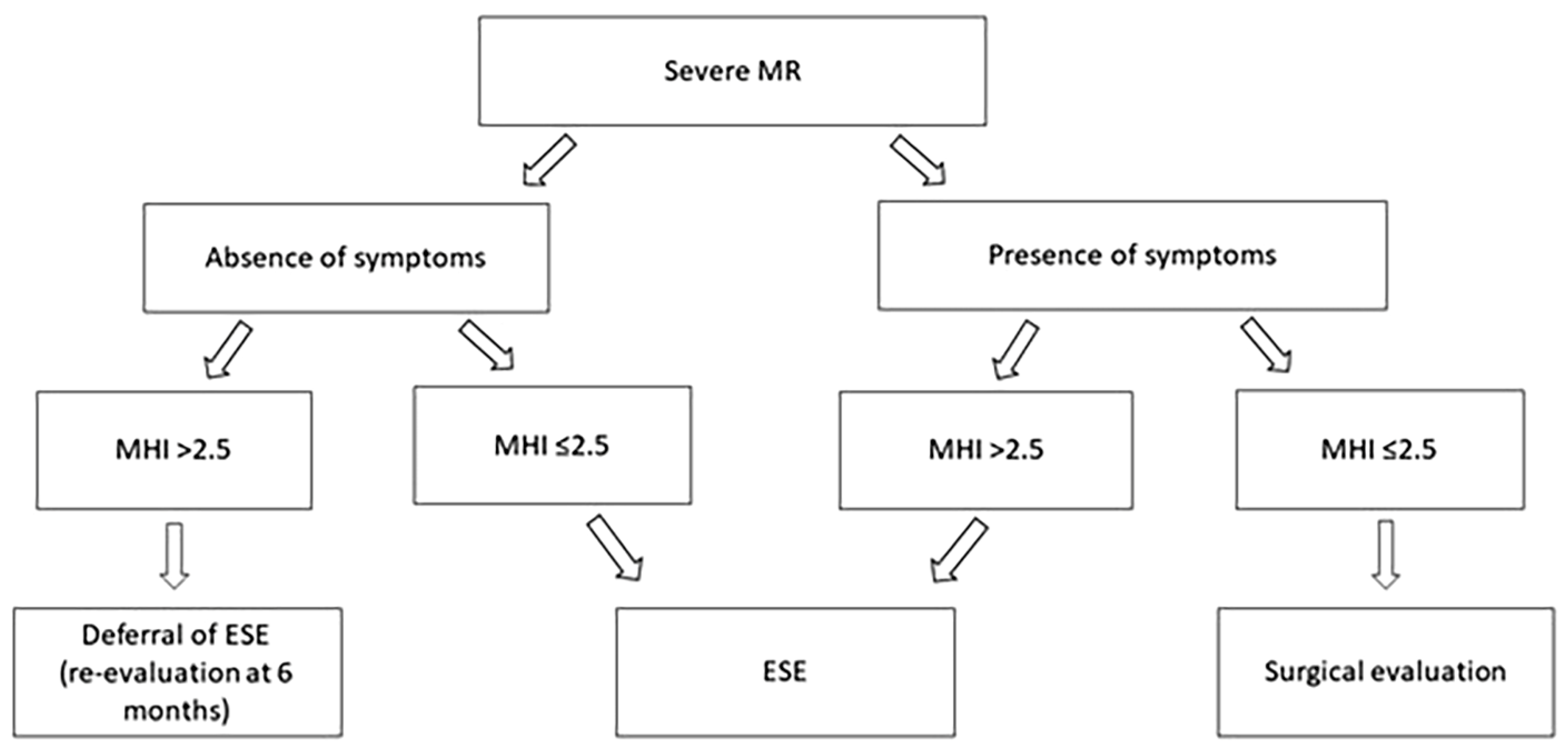
9.1. Modified Haller Index
9.2. Relationship Between Chest Wall Conformation and MAD Distance in PMR with MVP
9.3. Potential Link Between Concave Chest Morphology and a “Benign MAD Phenotype”
9.4. Influence of Chest Wall Conformation on ESE Results
9.5. Implications for Clinical Practice
9.6. Limitations of Innovative Anthropometrics in Assessing PMR Severity
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; De Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prothero, A.; Wilson, J.; Kennedy, A.; Brubert, J.; Masters, M.; Newton, J.D.; Dawkins, S.; Enriquez-Sarano, M.; Prendergast, B.D.; et al. Community Prevalence, Mechanisms and Outcome of Mitral or Tricuspid Regurgitation. Heart 2021, 107, 1003–1009. [Google Scholar] [CrossRef]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef]
- Dziadzko, V.; Dziadzko, M.; Medina-Inojosa, J.R.; Benfari, G.; Michelena, H.I.; Crestanello, J.A.; Maalouf, J.; Thapa, P.; Enriquez-Sarano, M. Causes and Mechanisms of Isolated Mitral Regurgitation in the Community: Clinical Context and Outcome. Eur. Heart J. 2019, 40, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.H.; Enriquez-Sarano, M.; Seward, J.B.; Tajik, A.J.; Schaff, H.V.; Bailey, K.R.; Frye, R.L. Clinical Outcome of Mitral Regurgitation Due to Flail Leaflet. N. Engl. J. Med. 1996, 335, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A Prospective Survey of Patients with Valvular Heart Disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Figlioli, G.; Sticchi, A.; Christodoulou, M.N.; Hadjidemetriou, A.; Amorim Moreira Alves, G.; De Carlo, M.; Praz, F.; Caterina, R.; Nikolopoulos, G.K.; Bonovas, S.; et al. Global Prevalence of Mitral Regurgitation: A Systematic Review and Meta-Analysis of Population-Based Studies. J. Clin. Med. 2025, 14, 2749. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2025, 46, 4635–4736. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Wang, C.L.; Chan, Y.H.; Wu, V.C.; Lee, H.F.; Hsiao, F.C.; Chu, P.H. Incremental Prognostic Value of Global Myocardial Work over Ejection Fraction and Global Longitudinal Strain in Patients with Heart Failure and Reduced Ejection Fraction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 348–356. [Google Scholar] [CrossRef]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and Clinical Outcome of Mitral-Valve Prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Avierinos, J.F.; Gersh, B.J.; Melton, L.J., III; Bailey, K.R.; Shub, C.; Nishimura, R.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural History of Asymptomatic Mitral Valve Prolapse in the Community. Circulation 2002, 106, 1355–1361. [Google Scholar] [CrossRef]
- Dziadzko, V.; Clavel, M.A.; Dziadzko, M.; Medina-Inojosa, J.R.; Michelena, H.; Maalouf, J.; Nkomo, V.; Thapa, P.; Enriquez-Sarano, M. Outcome and Undertreatment of Mitral Regurgitation: A Community Cohort Study. Lancet 2018, 391, 960–969. [Google Scholar] [CrossRef]
- Tsang, W. Recent Advances in Understanding and Managing Mitral Valve Disease. F1000Research 2019, 8, 1686. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anzà, C. A New Modified Anthropometric Haller Index Obtained without Radiological Exposure. Int. J. Cardiovasc. Imaging 2018, 34, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Braga, M.; Villa, M.C.; Migliori, C.; Lombardo, M. Does Chest Wall Conformation Influence Myocardial Strain Parameters in Infants with Pectus Excavatum? J. Clin. Ultrasound 2021, 49, 918–928. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Lombardo, M.; Anzà, C.; Ambrosio, G. Reduced Myocardial Strain Parameters in Subjects with Pectus Excavatum: Impaired Myocardial Function or Methodological Limitations Due to Chest Deformity? Semin. Thorac. Cardiovasc. Surg. 2021, 33, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Influence of Chest Conformation on Myocardial Strain Parameters in Healthy Subjects with Mitral Valve Prolapse. Int. J. Cardiovasc. Imaging 2021, 37, 1009–1022. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Granato, A.; Zompatori, M.; Lombardo, M. Modified Haller Index Validation and Correlation with Left Ventricular Strain in a Cohort of Subjects with Obesity and without Overt Heart Disease. Intern. Emerg. Med. 2022, 17, 1907–1919. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Esposito, V.; Caruso, C.; Nicolosi, G.L.; Bianchi, S.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Chest Conformation Spuriously Influences Strain Parameters of Myocardial Contractile Function in Healthy Pregnant Women. J. Cardiovasc. Med. 2021, 22, 767–779. [Google Scholar] [CrossRef]
- Archer, J.E.; Gardner, A.; Berryman, F.; Pynsent, P. The Measurement of the Normal Thorax Using the Haller Index Methodology at Multiple Vertebral Levels. J. Anat. 2016, 229, 577–581. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation Due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the Echocardiographic Assessment of Native Valvular Regurgitation: An Executive Summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Samad, Z.; Shaw, L.K.; Phelan, M.; Glower, D.D.; Ersboll, M.; Toptine, J.H.; Alexander, J.H.; Kisslo, J.A.; Wang, A.; Mark, D.B.; et al. Long-Term Outcomes of Mitral Regurgitation by Type and Severity. Am. Heart J. 2018, 203, 39–48. [Google Scholar] [CrossRef]
- Carpentier, A. Cardiac Valve Surgery—The “French Correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [CrossRef]
- Gavazzoni, M.; Taramasso, M.; Zuber, M.; Russo, G.; Pozzoli, A.; Miura, M.; Maisano, F. Conceiving MitraClip as a Tool: Percutaneous Edge-to-Edge Repair in Complex Mitral Valve Anatomies. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1059–1067. [Google Scholar] [CrossRef]
- Grigioni, F.; Clavel, M.A.; Vanoverschelde, J.L.; Tribouilloy, C.; Pizarro, R.; Huebner, M.; Avierinos, J.F.; Barbieri, A.; Suri, R.; Pasquet, A.; et al. The MIDA mortality risk score: Development and external validation of a prognostic model for early and late death in degenerative mitral regurgitation. Eur. Heart J. 2018, 39, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Flemming, M.A.; Oral, H.; Rothman, E.D.; Briesmiester, K.; Petrusha, J.A.; Starling, M.R. Echocardiographic Markers for Mitral Valve Surgery to Preserve Left Ventricular Performance in Mitral Regurgitation. Am. Heart J. 2000, 140, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Zhou, T.; Wang, Y.; Zhu, K.; Zhai, J.; Sun, Y.; Lai, H.; Wang, C. Mitral Valve Repair for Degenerative Mitral Regurgitation in Patients with Left Ventricular Systolic Dysfunction: Early and Mid-Term Outcomes. J. Cardiothorac. Surg. 2020, 15, 284. [Google Scholar] [CrossRef]
- Song, J.M.; Kang, S.H.; Lee, E.J.; Shin, M.J.; Lee, J.W.; Chung, C.H.; Kim, D.H.; Kang, D.H.; Song, J.K. Echocardiographic Predictors of Left Ventricular Function and Clinical Outcomes After Successful Mitral Valve Repair: Conventional Two-Dimensional Versus Speckle-Tracking Parameters. Ann. Thorac. Surg. 2011, 91, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Barbieri, A.; Rusinaru, D.; Szymanski, C.; Ferlito, M.; Tafanelli, L.; Bursi, F.; Trojette, F.; et al. Survival Implication of Left Ventricular End-Systolic Diameter in Mitral Regurgitation Due to Flail Leaflets: A Long-Term Follow-Up Multicenter Study. J. Am. Coll. Cardiol. 2009, 54, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, J.; Lai, H.; Zhu, K.; Sun, Y.; Ding, W.; Hong, T.; Wang, C. Benefits of Early Surgery on Clinical Outcomes after Degenerative Mitral Valve Repair. Ann. Thorac. Surg. 2018, 106, 1063–1070. [Google Scholar] [CrossRef]
- Witkowski, T.G.; Thomas, J.D.; Debonnaire, P.J.; Delgado, V.; Hoke, U.; Ewe, S.H.; Versteegh, M.I.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; et al. Global Longitudinal Strain Predicts Left Ventricular Dysfunction after Mitral Valve Repair. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 69–76. [Google Scholar] [CrossRef]
- Mascle, S.; Schnell, F.; Thebault, C.; Corbineau, H.; Laurent, M.; Hamonic, S.; Veillard, D.; Mabo, P.; Leguerrier, A.; Donal, E. Predictive Value of Global Longitudinal Strain in a Surgical Population of Organic Mitral Regurgitation. J. Am. Soc. Echocardiogr. 2012, 25, 766–772. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Tajik, A.J.; Schaff, H.V.; Orszulak, T.A.; Bailey, K.R.; Frye, R.L. Echocardiographic Prediction of Survival After Surgical Correction of Organic Mitral Regurgitation. Circulation 1994, 90, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Shapiro, L.M.; Wells, F.C. Mortality and Morbidity After Mitral Valve Repair: The Importance of Left Ventricular Dysfunction. J. Heart Valve Dis. 1995, 4, 460–468. [Google Scholar]
- Tribouilloy, C.; Rusinaru, D.; Szymanski, C.; Mezghani, S.; Fournier, A.; Lévy, F.; Peltier, M.; Ben Ammar, A.; Carmi, D.; Remadi, J.P.; et al. Predicting Left Ventricular Dysfunction after Valve Repair for Mitral Regurgitation Due to Leaflet Prolapse: Additive Value of Left Ventricular End-Systolic Dimension to Ejection Fraction. Eur. J. Echocardiogr. 2011, 12, 702–710. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, W.; Li, H.; Yan, G.; Wang, Y. Timing of Valve Repair for Asymptomatic Mitral Regurgitation and Preserved Left Ventricular Function. Ann. Thorac. Surg. 2021, 111, 862–870. [Google Scholar] [CrossRef]
- Kitai, T.; Okada, Y.; Shomura, Y.; Tani, T.; Kaji, S.; Kita, T.; Furukawa, Y. Timing of Valve Repair for Severe Degenerative Mitral Regurgitation and Long-Term Left Ventricular Function. J. Thorac. Cardiovasc. Surg. 2014, 148, 1978–1982. [Google Scholar] [CrossRef]
- Nair, V.V.; Das, S.; Nair, R.B.; George, T.P.; Kathayanat, J.T.; Chooriyil, N.; Radhakrishnan, R.; Thanathu Krishnan Nair, J. Mitral Valve Repair in Chronic Severe Mitral Regurgitation: Short-Term Results and Analysis of Mortality Predictors. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 506–513. [Google Scholar] [CrossRef]
- Alashi, A.; Mentias, A.; Patel, K.; Gillinov, A.M.; Sabik, J.F.; Popović, Z.B.; Mihaljevic, T.; Suri, R.M.; Rodriguez, L.L.; Svensson, L.G.; et al. Synergistic utility of brain natriuretic peptide and left ventricular global longitudinal strain in asymptomatic patients with significant primary mitral regurgitation and preserved systolic function undergoing mitral valve surgery. Circ. Cardiovasc. Imaging 2016, 9, e004451. [Google Scholar] [CrossRef]
- Cho, E.J.; Park, S.J.; Yun, H.R.; Jeong, D.S.; Lee, S.C.; Park, S.W.; Park, P.W. Predicting left ventricular dysfunction after surgery in patients with chronic mitral regurgitation: Assessment of myocardial deformation by 2-dimensional multilayer speckle tracking echocardiography. Korean Circ. J. 2016, 46, 213–221. [Google Scholar] [CrossRef]
- Mentias, A.; Naji, P.; Gillinov, A.M.; Rodriguez, L.L.; Reed, G.; Mihaljevic, T.; Suri, R.M.; Sabik, J.F.; Svensson, L.G.; Grimm, R.A.; et al. Strain echocardiography and functional capacity in asymptomatic primary mitral regurgitation with preserved ejection fraction. J. Am. Coll. Cardiol. 2016, 68, 1974–1986. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Tomsic, A.; van Wijngaarden, S.E.; Palmen, M.; Klautz, R.J.M.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Prognostic value of global longitudinal strain and etiology after surgery for primary mitral regurgitation. JACC Cardiovasc. Imaging 2020, 13, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Mascle, S.; Brunet, A.; Thebault, C.; Corbineau, H.; Laurent, M.; Leguerrier, A.; Mabo, P. Prediction of left ventricular ejection fraction 6 months after surgical correction of organic mitral regurgitation: The value of exercise echocardiography and deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Kislitsina, O.N.; Thomas, J.D.; Crawford, E.; Michel, E.; Kruse, J.; Liu, M.; Andrei, A.C.; Cox, J.L.; McCarthy, P.M. Predictors of left ventricular dysfunction after surgery for degenerative mitral regurgitation. Ann. Thorac. Surg. 2020, 109, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Pandis, D.; Sengupta, P.P.; Castillo, J.G.; Caracciolo, G.; Fischer, G.W.; Narula, J.; Anyanwu, A.; Adams, D.H. Assessment of longitudinal myocardial mechanics in patients with degenerative mitral valve regurgitation predicts postoperative worsening of left ventricular systolic function. J. Am. Soc. Echocardiogr. 2014, 27, 627–638. [Google Scholar] [CrossRef]
- Canessa, M.; Thamman, R.; Americo, C.; Soca, G.; Dayan, V. Global longitudinal strain predicts survival and left ventricular function after mitral valve surgery: A meta-analysis. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 337–342. [Google Scholar] [CrossRef]
- Balachandran, P.; Schaff, H.V.; Lahr, B.D.; Nguyen, A.; Daly, R.C.; Maltais, S.; Pislaru, S.V.; Dearani, J.A. Preoperative Left Atrial Volume Index Is Associated with Postoperative Outcomes in Mitral Valve Repair for Chronic Mitral Regurgitation. J. Thorac. Cardiovasc. Surg. 2020, 160, 661–672.e5. [Google Scholar] [CrossRef]
- Di Gioia, G.; Mega, S.; Nenna, A.; Campanale, C.M.; Colaiori, I.; Scordino, D.; Ragni, L.; Miglionico, M.; Di Sciascio, G. Should Pre-Operative Left Atrial Volume Receive More Consideration in Patients with Degenerative Mitral Valve Disease Undergoing Mitral Valve Surgery? Int. J. Cardiol. 2017, 227, 106–113. [Google Scholar] [CrossRef]
- Szymanski, C.; Bohbot, Y.; Rusinaru, D.; Touati, G.; Tribouilloy, C. Impact of Preoperative Left Atrial Dimension on Outcome in Patients in Sinus Rhythm Undergoing Surgical Valve Repair for Severe Mitral Regurgitation Due to Mitral Valve Prolapse. Cardiology 2019, 142, 189–193. [Google Scholar] [CrossRef]
- Essayagh, B.; Antoine, C.; Benfari, G.; Messika-Zeitoun, D.; Michelena, H.; Le Tourneau, T.; Mankad, S.; Tribouilloy, C.M.; Thapa, P.; Enriquez-Sarano, M. Prognostic Implications of Left Atrial Enlargement in Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Cho, G.Y.; Hwang, I.C.; Choi, H.M.; Park, J.B.; Yoon, Y.E.; Kim, H.K. Myocardial Strain in Prediction of Outcomes after Surgery for Severe Mitral Regurgitation. JACC Cardiovasc. Imaging 2018, 11, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.K.; Poulsen, S.H. Left atrial systolic function by strain analysis—A new useful prognostic tool in primary severe mitral regurgitation? Int. J. Cardiol. 2021, 322, 204–205. [Google Scholar] [CrossRef]
- Debonnaire, P.; Leong, D.P.; Witkowski, T.G.; Al Amri, I.; Joyce, E.; Katsanos, S.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left atrial function by two-dimensional speckle-tracking echocardiography in patients with severe organic mitral regurgitation: Association with guidelines-based surgical indication and postoperative (long-term) survival. J. Am. Soc. Echocardiogr. 2013, 26, 1053–1062. [Google Scholar] [CrossRef]
- Candan, O.; Ozdemir, N.; Aung, S.M.; Hatipoglu, S.; Karabay, C.Y.; Guler, A.; Gecmen, C.; Dogan, C.; Omaygenc, O.; Bakal, R.B. Atrial longitudinal strain parameters predict left atrial reverse remodeling after mitral valve surgery: A speckle tracking echocardiography study. Int. J. Cardiovasc. Imaging 2014, 30, 1049–1056. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Benfari, G.; Bisleri, G.; Maccherini, M.; Lisi, G.; Cameli, P.; Lisi, M.; Dokollari, A.; Carrucola, C.; et al. Left atrial strain as a pre-operative prognostic marker for patients with severe mitral regurgitation. Int. J. Cardiol. 2021, 324, 139–145. [Google Scholar] [CrossRef]
- Stassen, J.; van Wijngaarden, A.L.; Butcher, S.C.; Palmen, M.; Herbots, L.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Prognostic value of left atrial reservoir function in patients with severe primary mitral regurgitation undergoing mitral valve repair. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 142–151. [Google Scholar] [CrossRef]
- Cameli, M.; Pastore, M.C.; Righini, F.M.; Mandoli, G.E.; D’Ascenzi, F.; Lisi, M.; Nistor, D.; Sparla, S.; Curci, V.; Di Tommaso, C.; et al. Prognostic value of left atrial strain in patients with moderate asymptomatic mitral regurgitation. Int. J. Cardiovasc. Imaging 2019, 35, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Nistor, D.; Lisi, E.; Massoni, A.; Crudele, F.; Stricagnoli, M.; Lunghetti, S.; Mondillo, S. Left heart longitudinal deformation analysis in mitral regurgitation. Int. J. Cardiovasc. Imaging 2018, 34, 1741–1751. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Fei, H.; Yu, Y.; Ren, S.; Lin, Q.; Li, H.; Tang, Y.; Hou, Y.; Li, M. Left atrial strain reproducibility using vendor-dependent and vendor-independent software. Cardiovasc. Ultrasound 2019, 17, 9. [Google Scholar] [CrossRef]
- Le Tourneau, T.; Richardson, M.; Juthier, F.; Modine, T.; Fayad, G.; Polge, A.S.; Ennezat, P.V.; Bauters, C.; Vincentelli, A.; Deklunder, G. Echocardiography Predictors and Prognostic Value of Pulmonary Artery Systolic Pressure in Chronic Organic Mitral Regurgitation. Heart 2010, 96, 1311–1317. [Google Scholar] [CrossRef]
- Ratwatte, S.; Strange, G.; Playford, D.; Stewart, S.; Celermajer, D.S. Prevalence of pulmonary hypertension in mitral regurgitation and its influence on outcomes. Open Heart 2023, 10, e002268. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Gibbs, J.S.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.L. Left Ventricular Heart Failure and Pulmonary Hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Evans, C.F.; DeFilippi, C.R.; Hobbs, G.; Young, C.A.; Griffith, B.P.; Gammie, J.S. Pulmonary Hypertension Adversely Affects Short-and Long-Term Survival after Mitral Valve Operation for Mitral Regurgitation: Implications for Timing of Surgery. J. Thorac. Cardiovasc. Surg. 2011, 142, 1439–1452. [Google Scholar] [CrossRef]
- Nozohoor, S.; Hyllén, S.; Meurling, C.; Wierup, P.; Sjögren, J. Prognostic Value of Pulmonary Hypertension in Patients Undergoing Surgery for Degenerative Mitral Valve Disease with Leaflet Prolapse. J. Card. Surg. 2012, 27, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Murashita, T.; Okada, Y.; Kanemitsu, H.; Fukunaga, N.; Konishi, Y.; Nakamura, K.; Koyama, T. The Impact of Preoperative and Postoperative Pulmonary Hypertension on Long-Term Surgical Outcome after Mitral Valve Repair for Degenerative Mitral Regurgitation. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Genuardi, M.V.; Shpilsky, D.; Handen, A.; VanSpeybroeck, G.; Canterbury, A.; Lu, M.; Shapero, K.; Nieves, R.A.; Thoma, F.; Mulukutla, S.R.; et al. Increased Mortality in Patients with Preoperative and Persistent Postoperative Pulmonary Hypertension Undergoing Mitral Valve Surgery for Mitral Regurgitation: A Cohort Study. J. Am. Heart Assoc. 2021, 10, e018394. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, G.F.; Garcia, A.L.; Correia, P.M.; Branco, C.; Antunes, M.J. Negative Impact of Atrial Fibrillation and Pulmonary Hypertension After Mitral Valve Surgery in Asymptomatic Patients with Severe Mitral Regurgitation: A 20-Year Follow-Up. Eur. J. Cardiothorac. Surg. 2015, 48, 548–556. [Google Scholar] [CrossRef]
- Mentias, A.; Patel, K.; Patel, H.; Gillinov, A.M.; Sabik, J.F.; Mihaljevic, T.; Suri, R.M.; Rodriguez, L.L.; Svensson, L.G.; Griffin, B.P.; et al. Effect of Pulmonary Vascular Pressures on Long-Term Outcome in Patients with Primary Mitral Regurgitation. J. Am. Coll. Cardiol. 2016, 67, 2952–2961. [Google Scholar] [CrossRef]
- Barbieri, A.; Bursi, F.; Grigioni, F.; Tribouilloy, C.; Avierinos, J.F.; Michelena, H.I.; Rusinaru, D.; Szymansky, C.; Russo, A.; Suri, R.; et al. Prognostic and Therapeutic Implications of Pulmonary Hypertension Complicating Degenerative Mitral Regurgitation Due to Flail Leaflet: A Multicenter Long-Term International Study. Eur. Heart J. 2011, 32, 751–759. [Google Scholar] [CrossRef]
- Gackowski, A.; Chrustowicz, A.; Kapelak, B.; Miszalski-Jamka, T.; El-Massri, N.; Sadowski, J. Forward Stroke Volume Is Predictor of Perioperative Course in Patients with Mitral Regurgitation Undergoing Mitral Valve Replacement. Cardiol. J. 2010, 17, 386–389. [Google Scholar]
- Haddad, F.; Denault, A.Y.; Couture, P.; Cartier, R.; Pellerin, M.; Levesque, S.; Lambert, J.; Tardif, J.C. Right Ventricular Myocardial Performance Index Predicts Perioperative Mortality or Circulatory Failure in High-Risk Valvular Surgery. J. Am. Soc. Echocardiogr. 2007, 20, 1065–1072. [Google Scholar] [CrossRef]
- Le Tourneau, T.; Deswarte, G.; Lamblin, N.; Foucher-Hossein, C.; Fayad, G.; Richardson, M.; Polge, A.S.; Vannesson, C.; Topilsky, Y.; Juthier, F.; et al. Right Ventricular Systolic Function in Organic Mitral Regurgitation: Impact of Biventricular Impairment. Circulation 2013, 127, 1597–1608. [Google Scholar] [CrossRef]
- Chrustowicz, A.; Gackowski, A.; El-Massri, N.; Sadowski, J.; Piwowarska, W. Preoperative Right Ventricular Function in Patients with Organic Mitral Regurgitation. Echocardiography 2010, 27, 282–285. [Google Scholar] [CrossRef]
- Simon, R.; Oelert, H.; Borst, H.G.; Lichtlen, P.R. Influence of Mitral Valve Surgery on Tricuspid Incompetence Concomitant with Mitral Valve Disease. Circulation 1980, 62, I152–I157. [Google Scholar]
- Murashita, T.; Okada, Y.; Kanemitsu, H.; Fukunaga, N.; Konishi, Y.; Nakamura, K.; Sakon, Y.; Koyama, T. Fate of functional tricuspid regurgitation after mitral valve repair for degenerative mitral regurgitation. Circ. J. 2013, 77, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Burwash, I.G.; Lam, B.K.; Auyeung, T.; Tran, A.; Mesana, T.G.; Ruel, M. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann. Thorac. Surg. 2009, 88, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, M.; Bezante, G.P.; Di Baldassarre, A.; Clemente, D.; Cardinali, A.; Acitelli, A.; Salerni, S.; Penco, M.; Calafiore, A.M.; Gallina, S.; et al. Functional tricuspid regurgitation: An underestimated issue. Int. J. Cardiol. 2013, 168, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Yeates, A.; Marwick, T.; Deva, R.; Mundy, J.; Wood, A.; Griffin, R.; Peters, P.; Shah, P. Does moderate tricuspid regurgitation require attention during mitral valve surgery? ANZ J. Surg. 2014, 84, 63–67. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; David, C.M.; Fan, C.S.; Manlhiot, C. Tricuspid regurgitation is uncommon after mitral valve repair for degenerative diseases. J. Thorac. Cardiovasc. Surg. 2017, 154, 110–122.e1. [Google Scholar] [CrossRef]
- Essayagh, B.; Antoine, C.; Benfari, G.; Maalouf, J.; Michelena, H.I.; Crestanello, J.A.; Thapa, P.; Avierinos, J.-F.; Enriquez-Sarano, M. Functional tricuspid regurgitation of degenerative mitral valve disease: A crucial determinant of survival. Eur. Heart J. 2020, 41, 1918–1929. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Zaidi, F.; Asghar, M.S.; Aamir, M. Efficacy and safety of concomitant tricuspid repair in patients undergoing mitral valve surgery: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2022, 47, 101360. [Google Scholar] [CrossRef]
- Bakkestrøm, R.; Banke, A.; Christensen, N.L.; Pecini, R.; Irmukhamedov, A.; Andersen, M.; Borlaug, B.A.; Møller, J.E. Hemodynamic characteristics in significant symptomatic and asymptomatic primary mitral valve regurgitation at rest and during exercise. Circ. Cardiovasc. Imaging 2018, 11, e007171. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Hidaka, T.; Susawa, H.; Izumi, K.; Harada, Y.; Kinoshita, M.; Itakura, K.; Masada, K.; Kihara, Y. Exercise-stress echocardiography and effort intolerance in asymptomatic/minimally symptomatic patients with degenerative mitral regurgitation: Combined invasive–noninvasive hemodynamic monitoring. Circ. Cardiovasc. Imaging 2018, 11, e007282. [Google Scholar] [CrossRef]
- Picano, E.; Pibarot, P.; Lancellotti, P.; Monin, J.L.; Bonow, R.O. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J. Am. Coll. Cardiol. 2009, 54, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Naji, P.; Griffin, B.P.; Barr, T.; Asfahan, F.; Gillinov, A.M.; Grimm, R.A.; Rodriguez, L.L.; Mihaljevic, T.; Stewart, W.J.; Desai, M.Y. Importance of exercise capacity in predicting outcomes and determining optimal timing of surgery in significant primary mitral regurgitation. J. Am. Heart Assoc. 2014, 3, e001010. [Google Scholar] [CrossRef]
- Leung, D.Y.; Griffin, B.P.; Stewart, W.J.; Cosgrove, D.M., 3rd; Thomas, J.D.; Marwick, T.H. Left ventricular function after valve repair for chronic mitral regurgitation: Predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J. Am. Coll. Cardiol. 1996, 28, 1198–1205. [Google Scholar] [CrossRef]
- Lancellotti, P.; Cosyns, B.; Zacharakis, D.; Attena, E.; Van Camp, G.; Gach, O.; Radermecker, M.; Piérard, L.A. Importance of left ventricular longitudinal function and functional reserve in patients with degenerative mitral regurgitation: Assessment by two-dimensional speckle tracking. J. Am. Soc. Echocardiogr. 2008, 21, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Vitel, E.; Galli, E.; Leclercq, C.; Fournet, M.; Bosseau, C.; Corbineau, H.; Bouzille, G.; Donal, E. Right ventricular exercise contractile reserve and outcomes after early surgery for primary mitral regurgitation. Heart 2018, 104, 855–860. [Google Scholar] [CrossRef]
- Magne, J.; Donal, E.; Mahjoub, H.; Miltner, B.; Dulgheru, R.; Thebault, C.; Pierard, L.A.; Pibarot, P.; Lancellotti, P. Impact of exercise pulmonary hypertension on postoperative outcome in primary mitral regurgitation. Heart 2015, 101, 391–396. [Google Scholar] [CrossRef]
- Lee, R.; Haluska, B.; Leung, D.Y.; Case, C.; Mundy, J.; Marwick, T.H. Functional and prognostic implications of left ventricular contractile reserve in patients with asymptomatic severe mitral regurgitation. Heart 2005, 91, 1407–1412. [Google Scholar] [CrossRef]
- Magne, J.; Mahjoub, H.; Dulgheru, R.; Pibarot, P.; Pierard, L.A.; Lancellotti, P. Left ventricular contractile reserve in asymptomatic primary mitral regurgitation. Eur. Heart J. 2014, 35, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Lancellotti, P.; Piérard, L.A. Exercise-induced changes in degenerative mitral regurgitation. J. Am. Coll. Cardiol. 2010, 56, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Lancellotti, P.; Piérard, L.A. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010, 122, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Lancellotti, P.; O’Connor, K.; Van de Heyning, C.M.; Szymanski, C.; Piérard, L.A. Prediction of exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. J. Am. Soc. Echocardiogr. 2011, 24, 1004–1012. [Google Scholar] [CrossRef]
- Oh, J.K.; Kane, G.C. Diastolic stress echocardiography: The time has come for its integration into clinical practice. J. Am. Soc. Echocardiogr. 2014, 27, 1060–1063. [Google Scholar] [CrossRef]
- Burgess, M.I.; Jenkins, C.; Sharman, J.E.; Marwick, T.H. Diastolic stress echocardiography: Hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J. Am. Coll. Cardiol. 2006, 47, 1891–1900. [Google Scholar] [CrossRef]
- Ha, J.W.; Andersen, O.S.; Smiseth, O.A. Diastolic stress test: Invasive and noninvasive testing. JACC Cardiovasc. Imaging 2020, 13, 272–282. [Google Scholar] [CrossRef]
- Haluska, B.A.; Short, L.; Marwick, T.H. Relationship of ventricular longitudinal function to contractile reserve in patients with mitral regurgitation. Am. Heart J. 2003, 146, 183–188. [Google Scholar] [CrossRef]
- Kusunose, K.; Popović, Z.B.; Motoki, H.; Marwick, T.H. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ. Cardiovasc. Imaging 2013, 6, 167–176. [Google Scholar] [CrossRef]
- Kagiyama, N.; Toki, M.; Yuri, T.; Aritaka, S.; Hayashida, A.; Sengupta, P.P.; Yoshida, K. Physiological and prognostic differences between types of exercise stress echocardiography for functional mitral regurgitation. Open Heart 2021, 8, e001583. [Google Scholar] [CrossRef] [PubMed]
- Althunayyan, A.; Alborikan, S.; Badiani, S.; Wong, K.; Uppal, R.; Patel, N.; Petersen, S.E.; Lloyd, G.; Bhattacharyya, S. Clinical and prognostic implications of cardiopulmonary exercise stress echocardiography in asymptomatic degenerative mitral regurgitation. Am. J. Cardiol. 2023, 201, 8–15. [Google Scholar] [CrossRef]
- Citro, R.; Bursi, F.; Bellino, M.; Picano, E. The role of stress echocardiography in valvular heart disease. Curr. Cardiol. Rep. 2022, 24, 1477–1485. [Google Scholar] [CrossRef]
- Romano, S.; Kitkungvan, D.; Nguyen, D.T.; El-Tallawi, C.; Graviss, E.A.; Farzaneh-Far, A.; Shah, D.J. Implications of myocardial strain in primary mitral regurgitation—A cardiovascular magnetic resonance study. Eur. Heart J. Cardiovasc. Imaging 2024, 26, 126–134. [Google Scholar] [CrossRef]
- Liu, B.; Neil, D.A.H.; Premchand, M.; Bhabra, M.; Patel, R.; Barker, T.; Nikolaidis, N.; Billing, J.S.; Treibel, T.A.; Moon, J.C.; et al. Myocardial fibrosis in asymptomatic and symptomatic chronic severe primary mitral regurgitation and relationship to tissue characterisation and left ventricular function on cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 86. [Google Scholar] [CrossRef]
- Badau Riebel, C.I.; Agoston-Coldea, L. Left ventricular fibrosis by cardiac magnetic resonance tissue characterization in chronic mitral regurgitation patients. J. Clin. Med. 2024, 13, 3877. [Google Scholar] [CrossRef] [PubMed]
- Podlesnikar, T.; Delgado, V.; Bax, J.J. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int. J. Cardiovasc. Imaging 2018, 34, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Adams, D.H.; Pandis, D.; Robson, P.M.; Pawale, A.; Pyzik, R.; Liao, S.L.; El-Eshmawi, A.; Boateng, P.; Garg, J.; et al. Hybrid positron emission tomography/magnetic resonance imaging in arrhythmic mitral valve prolapse. JAMA Cardiol. 2020, 5, 1000–1005. [Google Scholar] [CrossRef]
- Sutton, T.M.; Stewart, R.A.; Gerber, I.L.; West, T.M.; Richards, A.M.; Yandle, T.G.; Kerr, A.J. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J. Am. Coll. Cardiol. 2003, 41, 2280–2287. [Google Scholar] [CrossRef]
- Detaint, D.; Messika-Zeitoun, D.; Avierinos, J.F.; Scott, C.; Chen, H.; Burnett, J.C., Jr.; Enriquez-Sarano, M. B-type natriuretic peptide in organic mitral regurgitation: Determinants and impact on outcome. Circulation 2005, 111, 2391–2397. [Google Scholar] [CrossRef]
- Rusinaru, D.; Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Suri, R.M.; Barbieri, A.; Szymanski, C.; Ferlito, M.; Michelena, H.; Tafanelli, L.; et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: Results from a large international multicenter study. Circ. Cardiovasc. Imaging 2011, 4, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, F.; Benfari, G.; Vanoverschelde, J.L.; Tribouilloy, C.; Avierinos, J.F.; Bursi, F.; Suri, R.M.; Guerra, F.; Pasquet, A.; Rusinaru, D.; et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J. Am. Coll. Cardiol. 2019, 73, 264–274. [Google Scholar] [CrossRef]
- Szymanski, C.; Magne, J.; Fournier, A.; Rusinaru, D.; Touati, G.; Tribouilloy, C. Usefulness of preoperative atrial fibrillation to predict outcome and left ventricular dysfunction after valve repair for mitral valve prolapse. Am. J. Cardiol. 2015, 115, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Vanoverschelde, J.L.; Grigioni, F.; Schaff, H.V.; Tribouilloy, C.; Avierinos, J.F.; Barbieri, A.; Pasquet, A.; Huebner, M.; Rusinaru, D.; et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013, 310, 609–616. [Google Scholar] [CrossRef]
- Jung, J.C.; Jang, M.J.; Hwang, H.Y. Meta-analysis comparing mitral valve repair versus replacement for degenerative mitral regurgitation across all ages. Am. J. Cardiol. 2019, 123, 446–453. [Google Scholar] [CrossRef]
- Lazam, S.; Vanoverschelde, J.L.; Tribouilloy, C.; Grigioni, F.; Suri, R.M.; Avierinos, J.F.; de Meester, C.; Barbieri, A.; Rusinaru, D.; Russo, A.; et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: Analysis of a large, prospective, multicenter, international registry. Circulation 2017, 135, 410–422. [Google Scholar] [CrossRef]
- David, T.E.; David, C.M.; Tsang, W.; Lafreniere-Roula, M.; Manlhiot, C. Long-term results of mitral valve repair for regurgitation due to leaflet prolapse. J. Am. Coll. Cardiol. 2019, 74, 1044–1053. [Google Scholar] [CrossRef]
- Donnellan, E.; Alashi, A.; Johnston, D.R.; Gillinov, A.M.; Pettersson, G.B.; Svensson, L.G.; Griffin, B.P.; Desai, M.Y. Outcomes of patients with mediastinal radiation-associated mitral valve disease undergoing cardiac surgery. Circulation 2019, 140, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Vakamudi, S.; Jellis, C.; Mick, S.; Wu, Y.; Gillinov, A.M.; Mihaljevic, T.; Cosgrove, D.M.; Svensson, L.; Cho, L. Sex differences in the etiology of surgical mitral valve disease. Circulation 2018, 138, 1749–1751. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Sorajja, P.; Vemulapalli, S.; Feldman, T.; Mack, M.; Holmes, D.R., Jr.; Stebbins, A.; Kar, S.; Thourani, V.; Ailawadi, G. Outcomes with transcatheter mitral valve repair in the United States: An STS/ACC TVT Registry report. J. Am. Coll. Cardiol. 2017, 70, 2315–2327. [Google Scholar] [CrossRef]
- Gammie, J.S.; Bartus, K.; Gackowski, A.; D’Ambra, M.N.; Szymanski, P.; Bilewska, A.; Kusmierczyk, M.; Kapelak, B.; Rzucidlo-Resil, J.; Moat, N.; et al. Beating-heart mitral valve repair using a novel ePTFE cordal implantation device: A prospective trial. J. Am. Coll. Cardiol. 2018, 71, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Spargias, K.; Chrissoheris, M.; Büllesfeld, L.; Nickenig, G.; Deuschl, F.; Schueler, R.; Fam, N.P.; Moss, R.; Makar, M.; et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: A multicentre, prospective, observational, first-in-man study. Lancet 2017, 390, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Chan, V.; Adams, D.H. Current indications for transcatheter edge-to-edge repair in a patient with primary mitral regurgitation. Circulation 2022, 146, 1263–1265. [Google Scholar] [CrossRef]
- Zilberszac, R.; Heinze, G.; Binder, T.; Laufer, G.; Gabriel, H.; Rosenhek, R. Long-term outcome of active surveillance in severe but asymptomatic primary mitral regurgitation. JACC Cardiovasc. Imaging 2018, 11, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Song, J.K.; Mahara, K.; Kuwaki, H.; Jang, J.Y.; Takeuchi, M.; Sun, B.J.; Kim, Y.J.; Miyamoto, T.; Oginosawa, Y.; et al. Basal Left Ventricular Dilatation and Reduced Contraction in Patients with Mitral Valve Prolapse Can Be Secondary to Annular Dilatation: Preoperative and Postoperative Speckle-Tracking Echocardiographic Study on Left Ventricle and Mitral Valve Annulus Interaction. Circ. Cardiovasc. Imaging 2016, 9, e005113. [Google Scholar] [CrossRef]
- Bulkley, B.H.; Roberts, W.C. Dilatation of the mitral anulus. A rare cause of mitral regurgitation. Am. J. Med. 1975, 59, 457–463. [Google Scholar] [CrossRef]
- Chandra, S.; Salgo, I.S.; Sugeng, L.; Weinert, L.; Tsang, W.; Takeuchi, M.; Spencer, K.T.; O’Connor, A.; Cardinale, M.; Settlemier, S.; et al. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images: Objective insight into complexity and planning of mitral valve repair. Circ. Cardiovasc. Imaging 2011, 4, 24–32. [Google Scholar] [CrossRef]
- Dahl, J.S.; Videbæk, L.; Poulsen, M.K.; Rudbæk, T.R.; Pellikka, P.A.; Møller, J.E. Global strain in severe aortic valve stenosis: Relation to clinical outcome after aortic valve replacement. Circ. Cardiovasc. Imaging 2012, 5, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Mignot, A.; Donal, E.; Zaroui, A.; Reant, P.; Salem, A.; Hamon, C.; Monzy, S.; Roudaut, R.; Habib, G.; Lafitte, S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: A multicenter study. J. Am. Soc. Echocardiogr. 2010, 23, 1019–1024. [Google Scholar] [CrossRef]
- Bertini, M.; Ng, A.C.; Antoni, M.L.; Nucifora, G.; Ewe, S.H.; Auger, D.; Marsan, N.A.; Schalij, M.J.; Bax, J.J.; Delgado, V. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 383–391. [Google Scholar] [CrossRef]
- Levy, P.T.; El-Khuffash, A.; Patel, M.D.; Breatnach, C.R.; James, A.T.; Sanchez, A.A.; Abuchabe, C.; Rogal, S.R.; Holland, M.R.; McNamara, P.J.; et al. Maturational patterns of systolic ventricular deformation mechanics by two-dimensional speckle-tracking echocardiography in preterm infants over the first year of age. J. Am. Soc. Echocardiogr. 2017, 30, 685–698.e1. [Google Scholar] [CrossRef]
- Bijnens, B.H.; Cikes, M.; Claus, P.; Sutherland, G.R. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur. J. Echocardiogr. 2009, 10, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.J.; Jaroszewski, D.; Gotway, M.; Ewais, M.; Wilansky, S.; Lester, S.; Unzek, S.; Appleton, C.P.; Chaliki, H.P.; Gaitan, B.D.; et al. Effects of Pectus Excavatum Repair on Right and Left Ventricular Strain. Ann. Thorac. Surg. 2018, 105, 294–301. [Google Scholar] [CrossRef]
- Truong, V.T.; Li, C.Y.; Brown, R.L.; Moore, R.A.; Garcia, V.F.; Crotty, E.J.; Taylor, M.D.; Ngo, T.M.N.; Mazur, W. Occult RV systolic dysfunction detected by CMR derived RV circumferential strain in patients with pectus excavatum. PLoS ONE 2017, 12, e0189128. [Google Scholar] [CrossRef]
- Lollert, A.; Emrich, T.; Eichstädt, J.; Kampmann, C.; Abu-Tair, T.; Turial, S.; Düber, C.; Kreitner, K.F.; Staatz, G. Differences in myocardial strain between pectus excavatum patients and healthy subjects assessed by cardiac MRI: A pilot study. Eur. Radiol. 2018, 28, 1276–1284. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The mitral annulus disjunction arrhythmic syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Mahajan, R.; Elliott, A.D.; Haqqani, H.; Lau, D.H.; Vohra, J.K.; Morton, J.B.; Semsarian, C.; Marwick, T.; Kalman, J.M.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review and meta-analysis. Heart 2019, 105, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Han, H.C.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and outcome of arrhythmic mitral valve prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.J.; Bitkover, C.Y.; Omran, A.S.; David, T.E.; Ivanov, J.; Ali, M.J.; Woo, A.; Siu, S.C.; Rakowski, H. Mitral annular disjunction in advanced myxomatous mitral valve disease: Echocardiographic detection and surgical correction. J. Am. Soc. Echocardiogr. 2005, 18, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef]
- Vaksmann, G.; Bouzguenda, I.; Guillaume, M.P.; Gras, P.; Silvestri, V.; Richard, A. Mitral annular disjunction and Pickelhaube sign in children with mitral valve prolapse: A prospective cohort study. Arch. Cardiovasc. Dis. 2023, 116, 514–522. [Google Scholar] [CrossRef]
- Figliozzi, S.; Georgiopoulos, G.; Lopes, P.M.; Bauer, K.B.; Moura-Ferreira, S.; Tondi, L.; Mushtaq, S.; Censi, S.; Pavon, A.G.; Bassi, I.; et al. Myocardial fibrosis at cardiac MRI helps predict adverse clinical outcome in patients with mitral valve prolapse. Radiology 2023, 306, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Bhagia, G.; Doyle, M.; Rayarao, G.; Williams, R.B.; Biederman, R.W.W. Mitral annular disjunction; how accurate are we? A cardiovascular MRI study defining risk. Int. J. Cardiol. Heart Vasc. 2023, 49, 101298. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Cecere, A.; Cipriani, A.; Migliore, F.; Zorzi, A.; De Lazzari, M.; Lorenzoni, G.; Cecchetto, A.; Brunetti, G.; Graziano, F.; et al. Determinants of ventricular arrhythmias in mitral valve prolapse. JACC Clin. Electrophysiol. 2024, 10, 670–681. [Google Scholar] [CrossRef]
- Blondeel, M.; L’Hoyes, W.; Robyns, T.; Verbrugghe, P.; De Meester, P.; Dresselaers, T.; Masci, P.G.; Willems, R.; Bogaert, J.; Vandenberk, B. Serial cardiac magnetic resonance imaging in patients with mitral valve prolapse—A single-center retrospective registry. J. Clin. Med. 2024, 13, 2669. [Google Scholar] [CrossRef]
- Figliozzi, S.; Stankowski, K.; Tondi, L.; Catapano, F.; Gitto, M.; Lisi, C.; Bombace, S.; Olivieri, M.; Cannata, F.; Fazzari, F.; et al. Mitral annulus disjunction in consecutive patients undergoing cardiovascular magnetic resonance: Where is the boundary between normality and disease? J. Cardiovasc. Magn. Reson. 2024, 26, 101056. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. The influence of chest wall conformation on myocardial strain parameters in a cohort of mitral valve prolapse patients with and without mitral annular disjunction. Int. J. Cardiovasc. Imaging 2023, 39, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional implication of mitral annular disjunction in mitral valve prolapse: A quantitative dynamic 3D echocardiographic study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Kwon, D.H.; Abou-Hassan, O.; Chetrit, M.; Harb, S.C.; Patel, D.; Kalahasti, V.; Popovic, Z.B.; Griffin, B.P.; Ayoub, C. Strain evaluation for mitral annular disjunction by echocardiography and magnetic resonance imaging: A case–control study. Int. J. Cardiol. 2021, 334, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.J.; Kebed, K.; Mor-Avi, V.; Rashedi, N.; Sun, D.; Patel, B.; Balkhy, H.; Lang, R.M.; Patel, A.R. Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int. J. Cardiovasc. Imaging 2020, 36, 1363–1370. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Moore, G.W.; Skoog, D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N. Engl. J. Med. 1986, 314, 535–540. [Google Scholar] [CrossRef]
- Mantegazza, V.; Tamborini, G.; Muratori, M.; Gripari, P.; Fusini, L.; Italiano, G.; Volpato, V.; Sassi, V.; Pepi, M. Mitral annular disjunction in a large cohort of patients with mitral valve prolapse and significant regurgitation. JACC Cardiovasc. Imaging 2019, 12, 2278–2280. [Google Scholar] [CrossRef]
- Wunderlich, N.C.; Ho, S.Y.; Flint, N.; Siegel, R.J. Myxomatous mitral valve disease with mitral valve prolapse and mitral annular disjunction: Clinical and functional significance of the coincidence. J. Cardiovasc. Dev. Dis. 2021, 8, 9. [Google Scholar] [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death. J. Cardiovasc. Electrophysiol. 2016, 27, 463–468. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Perazzolo Marra, M. Mitral annulus disjunction: Emerging role of myocardial mechanical stretch in arrhythmogenesis. J. Am. Coll. Cardiol. 2018, 72, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Brochhausen, C.; Turial, S.; Müller, F.K.; Schmitt, V.H.; Coerdt, W.; Wihlm, J.M.; Schier, F.; Kirkpatrick, C.J. Pectus excavatum: History, hypotheses and treatment options. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 801–806. [Google Scholar] [CrossRef]
- Nomura, K.; Ajiro, Y.; Nakano, S.; Matsushima, M.; Yamaguchi, Y.; Hatakeyama, N.; Ohata, M.; Sakuma, M.; Nonaka, T.; Harii, M.; et al. Characteristics of mitral valve leaflet length in patients with pectus excavatum: A single center cross-sectional study. PLoS ONE 2019, 14, e0212165. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Muti-Schünemann, G.E.U.; Rispoli, G.A.; Lombardo, M.; Muti, P. Does preliminary chest shape assessment improve the prognostic risk stratification of individuals with mitral annular disjunction? A case report and narrative review. J. Clin. Med. 2025, 14, 2277. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Appropriate use criteria implementation with modified Haller index for predicting stress echocardiographic results and outcome in a population of patients with suspected coronary artery disease. Int. J. Cardiovasc. Imaging 2021, 37, 2917–2930. [Google Scholar] [CrossRef]
- Magne, J.; Pibarot, P.; Sengupta, P.P.; Donal, E.; Rosenhek, R.; Lancellotti, P. Pulmonary hypertension in valvular disease: A comprehensive review on pathophysiology to therapy from the HAVEC Group. JACC Cardiovasc. Imaging 2015, 8, 83–99. [Google Scholar] [CrossRef]
- Naji, P.; Asfahan, F.; Barr, T.; Rodriguez, L.L.; Grimm, R.A.; Agarwal, S.; Thomas, J.D.; Gillinov, A.M.; Mihaljevic, T.; Griffin, B.P.; et al. Impact of duration of mitral regurgitation on outcomes in asymptomatic patients with myxomatous mitral valve undergoing exercise stress echocardiography. J. Am. Heart Assoc. 2015, 4, e001348. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Maslow, A.D.; Poppas, A. Primary mitral valve regurgitation: Update and review. Glob. Cardiol. Sci. Pract. 2017, 2017, e201703. [Google Scholar] [CrossRef]
- Katsi, V.; Georgiopoulos, G.; Magkas, N.; Oikonomou, D.; Virdis, A.; Nihoyannopoulos, P.; Toutouzas, K.; Tousoulis, D. The role of arterial hypertension in mitral valve regurgitation. Curr. Hypertens. Rep. 2019, 21, 20. [Google Scholar] [CrossRef]
- Topilsky, Y.; Michelena, H.; Bichara, V.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Mitral valve prolapse with mid-late systolic mitral regurgitation: Pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation 2012, 125, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Weissman, N.J.; Zamorano, J.L. Quantitation of mitral regurgitation. Circulation 2012, 126, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Taçoy, G.; Balcioğlu, A.S.; Arslan, U.; Durakoğlugil, E.; Erdem, G.; Ozdemir, M.; Cengel, A. Effect of metoprolol on heart rate variability in symptomatic patients with mitral valve prolapse. Am. J. Cardiol. 2007, 99, 1568–1570. [Google Scholar] [CrossRef]
- Slipczuk, L.; Rafique, A.M.; Davila, C.D.; Beigel, R.; Pressman, G.S.; Siegel, R.J. The role of medical therapy in moderate to severe degenerative mitral regurgitation. Rev. Cardiovasc. Med. 2016, 17, 28–39. [Google Scholar] [CrossRef]
- Nicolosi, G.L. The strain and strain rate imaging paradox in echocardiography: Overabundant literature in the last two decades but still uncertain clinical utility in an individual case. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e297–e305. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Prognostic value of modified Haller index in patients with suspected coronary artery disease referred for exercise stress echocardiography. J. Cardiovasc. Echogr. 2021, 31, 85–95. [Google Scholar] [CrossRef] [PubMed]





| Category | Criteria |
|---|---|
| Qualitative | MV morphology (flail leaflet, papillary rupture, severe retraction, perforation); Large central jet (>50% LA) or eccentric jet; Large systolic flow convergence; Dense holosystolic CW Doppler jet |
| Semiquantitative | Vena contracta ≥ 7 mm; Systolic pulmonary vein flow reversal; Dominant E-wave (>1.2 m/s); TVI mitral/TVI aortic > 1.4 |
| Quantitative | PISA radius ≥ 1 cm; EROA ≥ 40 mm2; Regurgitant volume ≥ 60 mL/beat; Regurgitant fraction ≥ 50% |
| Structural | LVESD ≥ 40 mm; LA diameter ≥ 55 mm or volume ≥ 60 mL/m2 |
| Modality/Marker | Strengths | Limitations | Current Gaps/Future Needs |
|---|---|---|---|
| Traditional TTE indices (LVESD, LVEF, LA size, PH, RV indices, FTR) | Widely available; guideline-endorsed thresholds; strong prognostic validation in large cohorts; reproducible LV diameters. | Reflect late disease; load dependent; interobserver variability; confounded by comorbidities (e.g., hypertension, diastolic dysfunction); limited sensitivity for early dysfunction. | Refined cut-offs for earlier intervention; multiparametric risk models; standardized RV/FTR quantification. |
| Speckle tracking echocardiography (GLS, LASr, RV strain) | Sensitive to subclinical dysfunction; incremental prognostic value over LVEF; detects earlier atrial/ventricular remodeling. | Image quality dependent; vendor/software variability; lack of universal reference values; load dependent; atrial strain limited by thin wall. | Harmonization across vendors; multicenter validation; integration into surgical decision algorithms. |
| Exercise stress echocardiography | Provides dynamic assessment of MR severity, contractile reserve, and exercise-induced PH; unmasks latent symptoms; predicts outcomes beyond resting echo. | Underutilized; dependent on patient effort; no consensus on stress cut-offs (e.g., stress LVEF, ΔGLS, sPAP); variability in protocols; transient hemodynamics difficult to capture. | Standardized protocols and thresholds; broader implementation in asymptomatic PMR; multicenter trials linking ESE metrics to outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Baravelli, M. Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Symptomatic Individuals with Primary Mitral Regurgitation? A Narrative Review of Traditional and Innovative Prognostic Indicators. J. Clin. Med. 2025, 14, 8297. https://doi.org/10.3390/jcm14238297
Sonaglioni A, Nicolosi GL, Lombardo M, Baravelli M. Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Symptomatic Individuals with Primary Mitral Regurgitation? A Narrative Review of Traditional and Innovative Prognostic Indicators. Journal of Clinical Medicine. 2025; 14(23):8297. https://doi.org/10.3390/jcm14238297
Chicago/Turabian StyleSonaglioni, Andrea, Gian Luigi Nicolosi, Michele Lombardo, and Massimo Baravelli. 2025. "Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Symptomatic Individuals with Primary Mitral Regurgitation? A Narrative Review of Traditional and Innovative Prognostic Indicators" Journal of Clinical Medicine 14, no. 23: 8297. https://doi.org/10.3390/jcm14238297
APA StyleSonaglioni, A., Nicolosi, G. L., Lombardo, M., & Baravelli, M. (2025). Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Symptomatic Individuals with Primary Mitral Regurgitation? A Narrative Review of Traditional and Innovative Prognostic Indicators. Journal of Clinical Medicine, 14(23), 8297. https://doi.org/10.3390/jcm14238297