Efficacy and Safety of Finerenone in Kidney Transplant Patients
Abstract
1. Introduction
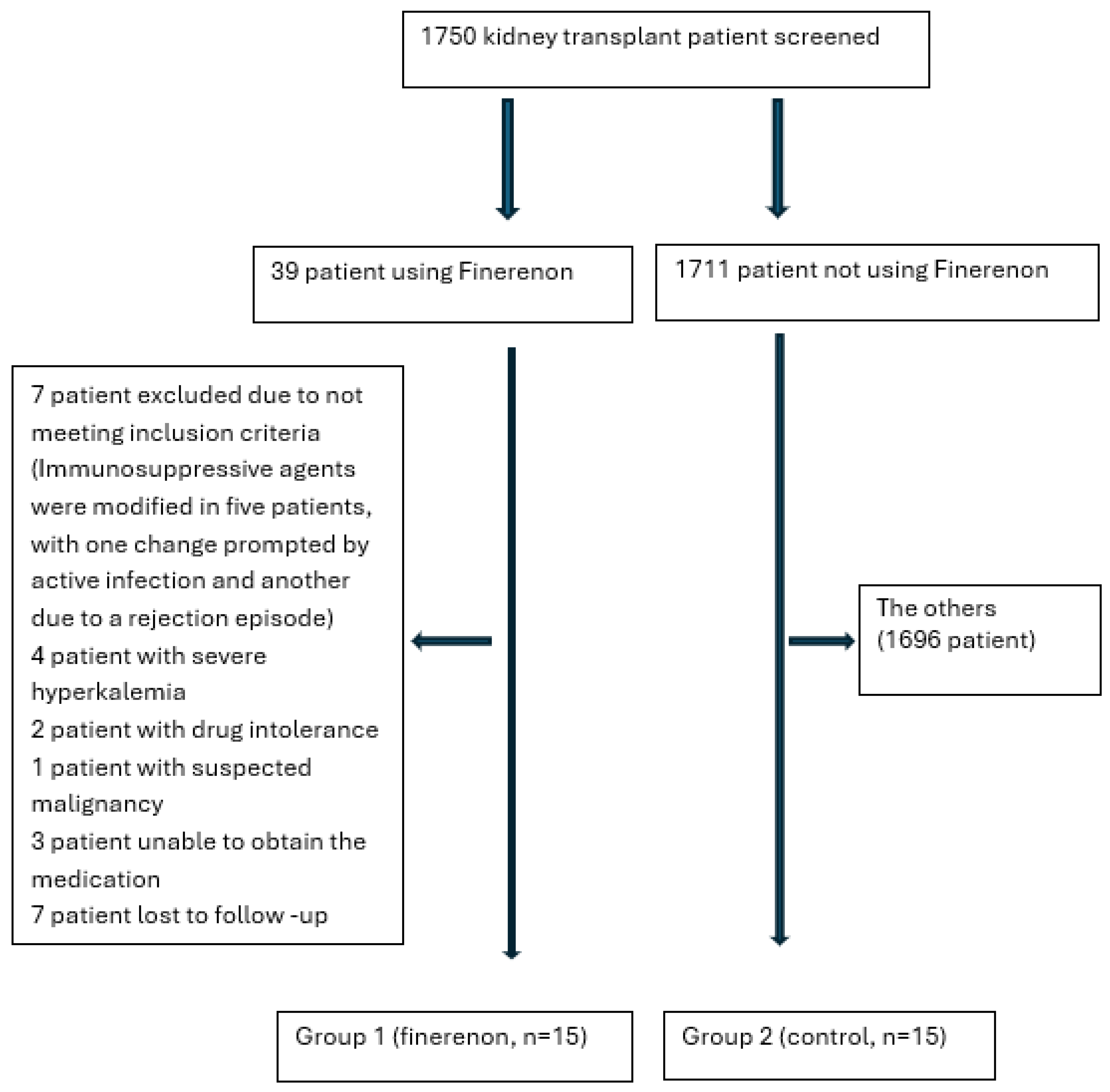
2. Materials and Methods
2.1. Participants
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Study Design
2.3. Data Collection and Follow-Up
2.4. Ethics Statement
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEIs | Angiotensin-converting Enzyme Inhibitors |
| ARBs | Angiotensin II Receptor Blockers |
| CKD | Chronic Kidney Disease |
| CKD-EPI | The Chronic Kidney Disease Epidemiology Collaboration |
| eGFR | Estimated Glomerular Filtration Rate |
| GFR | Glomerüler Filtration Rate |
| GLP-1 | Glucagon-like Peptide-1 |
| MRAs | Nonsteroidal Mineralocorticoid Receptor Antagonists |
| MR | Mineralocorticoid Receptor |
| SGLT2-Is | Sodium-glucose Co-transporter 2 Inhibitors |
References
- Chaudhry, D.; Chaudhry, A.; Peracha, J.; Sharif, A. Survival for waitlisted kidney failure patients receiving transplantation versus remaining on waiting list: Systematic review and meta-analysis. BMJ 2002, 376, e068769. [Google Scholar] [CrossRef]
- Minkovich, M.; Gupta, N.; Liu, M.; Famure, O.; Li, Y.; Selzner, M.; Lee, J.Y.; Kim, S.J.; Ghanekar, A. Impact of early surgical complications on kidney transplant outcomes. BMC Surg. 2024, 24, 165. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: Standards of care in diabetes–2024. Diabetes Care 2024, 47, S219–S230. [Google Scholar] [CrossRef]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Abdelhakim, A.M.; Abd-ElGawad, M. Impact of mineralocorticoid receptor antagonist in renal transplant patients: A systematic review and meta-analysis of randomized controlled trials. J. Nephrol. 2020, 33, 529–538. [Google Scholar] [CrossRef]
- de Sousa, M.; Guida, J.; Valle, C.D.; Camargo, L.; Rivelli, G.; Mazzali, M. Spironolactone in post-transplant proteinuria: A safe alternative therapy. Transplant. Proc. 2017, 49, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Baskin, E.; Alam Siddiqui, M.; Gülleroğlu, K.; Özdemir, B.H.; Yılmaz, A.Ç.; Çolak, M.Y.; Akdur, A.; Soy, E.A.; Moray, G.; Haberal, M. Long-term effect of eplerenone treatment in children with chronic allograft nephropathy. Pediatr. Transplant. 2023, 27, e14557. [Google Scholar] [CrossRef]
- Waanders, F.; Rienstra, H.; Boer, M.W.; Zandvoort, A.; Rozing, J.; Navis, G.; van Goor, H.; Hillebrands, J.-L. Spironolactone ameliorates transplant vasculopathy in renal chronic transplant dysfunction in rats. Am. J. Physiol. Renal Physiol. 2009, 296, F1072–F1079. [Google Scholar] [CrossRef]
- Afsar, B.; Afsar, R.E.; Caliskan, Y.; Lentine, K.L. Mineralocorticoid receptor blockage in kidney transplantation: Too much of a good thing or not? Int. Urol. Nephrol. 2025, 57, 839–854. [Google Scholar] [CrossRef]
- Buonafine, M.; Bonnard, B.; Jaisser, F. Roles of mineralocorticoid receptors in cardiovascular and cardiorenal diseases. Am. J. Hypertens. 2018, 31, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Lawatscheck, R.; Filippatos, G.; Bakris, G.L. Nonsteroidal mineralocorticoid receptor antagonism by finerenone-translational aspects and clinical perspectives across multiple organ systems. Int. J. Mol. Sci. 2022, 23, 9243. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Joseph, A.; Kintscher, U. Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders—New perspectives for combination therapy. Pharmacol. Res. 2021, 172, 105859. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Bakris, G.; E Pergola, P.; Delgado, B.; Genov, D.; Doliashvili, T.; Vo, N.; Yang, Y.F.; McCabe, J.; Benn, V.; Pitt, B. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: Results of the BLOCK-CKD study. Hypertension 2021, 78, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Jaisser, F.; Barrera-Chimal, J. Mineralocorticoid receptor antagonism for non-diabetic kidney disease. Nephrol. Dial. Transplant. 2025, 40, i29–i36. [Google Scholar] [CrossRef]
- Hemmelgarn, B.R.; Manns, B.J.; Lloyd, A.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Tonelli, M.; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010, 303, 423–429. [Google Scholar] [CrossRef]
- Fernández-Fresnedo, G.; Escallada, R.; Rodrigo, E.; De Francisco, A.L.; Cotorruelo, J.G.; De Castro, S.S.; Zubimendi, J.A.; Ruiz, J.C.; Arias, M. The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation 2002, 73, 1345–1348. [Google Scholar] [CrossRef]
- Shamseddin, M.K.; Knoll, G.A. Posttransplantation proteinuria: An approach to diagnosis and management. Clin. J. Am. Soc. Nephrol. 2011, 6, 1786–1793. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105 (Suppl. 4), S117–S314. [Google Scholar] [CrossRef]
- Agarwal, R.; Green, J.B.; Heerspink, H.J.; Mann, J.F.; McGill, J.B.; Mottl, A.K.; Rosenstock, J.; Rossing, P.; Vaduganathan, M.; Brinker, M.; et al. Finerenone with Empagliflozin in Chronic Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2025, 393, 533–543. [Google Scholar] [CrossRef]
- Currie, G.; Taylor, A.H.M.; Fujita, T.; Ohtsu, H.; Lindhardt, M.; Rossing, P.; Boesby, L.; Edwards, N.C.; Ferro, C.J.; Townend, J.N.; et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2016, 17, 127. [Google Scholar] [CrossRef]
- Nicholas, S.B.; Correa-Rotter, R.; Desai, N.R.; Guo, L.; Navaneethan, S.D.; Pantalone, K.M.; Wanner, C.; Hamacher, S.; Fatoba, S.T.; Horvat-Broecker, A.; et al. First interim results from FINE-REAL: A prospective, non-interventional, phase 4 study providing insights into the use and safety of finerenone in a routine clinical setting. J. Nephrol. 2024, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Pitt, B.; McMurray, J.J.; Pocock, S.J.; Solomon, S.D.; Pfeffer, M.A.; Zannad, F.; Rossignol, P. Steroidal MRA Across the Spectrum of Renal Function: A Pooled Analysis of RCTs. JACC Heart Fail. 2022, 10, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kang, S.-M.; Song, M.K.; Hong, N.; Youn, J.-C.; Han, S.; Jeon, E.-S.; Cho, M.-C.; Kim, J.-J.; Yoo, B.-S.; et al. Clinical Benefit of Spironolactone in Patients with Acute Decompensated Heart Failure and Severe Renal Dysfunction: Data from the Korean Heart Failure Registry. Am. Heart J. 2015, 169, 713–720.e3. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-C.; Liu, J.-S.; Hung, S.-C.; Kuo, K.-L.; Chen, Y.-H.; Tarng, D.-C.; Hsu, C.-C. Effect of Spironolactone on the Risks of Mortality and Hospitalization for Heart Failure in Pre-Dialysis Advanced Chronic kidney Disease: A Nationwide Population-Based Study. Int. J. Cardiol. 2017, 238, 72–78. [Google Scholar] [CrossRef]
- Inampudi, C.; Parvataneni, S.; Morgan, C.J.; Deedwania, P.; Fonarow, G.C.; Sanders, P.W.; Prabhu, S.D.; Butler, J.; Forman, D.E.; Aronow, W.S.; et al. Spironolactone Use and Higher Hospital Readmission for Medicare Beneficiaries with Heart Failure, LeftVentricular Ejection Fraction <45%, and Estimated Glomerular Filtration Rate <45 ml/min/1.73 m2. Am. J. Cardiol. 2014, 114, 79–82. [Google Scholar]
- Mortensen, L.A.; Jespersen, B.; Helligsoe, A.S.L.; Tougaard, B.; Cibulskyte-Ninkovic, D.; Egfjord, M.; Boesby, L.; Marcussen, N.; Madsen, K.; Jensen, B.L.; et al. Effect of Spironolactone on Kidney Function in Kidney Transplant Recipients (the SPIREN trial): A Randomized Placebo-Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 755–766. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD prognosis consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef]
- Clase, C.M.; Carrero, J.-J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Available online: https://kdigo.org/wp-content/uploads/2018/04/KDIGO-Potassium-Management-Final-publication.pdf. (accessed on 9 December 2021).
- Epstein, M.; Williams, G.H.; Weinberger, M.; Lewin, A.; Krause, S.; Mukherjee, R.; Patni, R.; Beckerman, B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2006, 1, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.-Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Desai, N.R.; Navaneethan, S.D.; Nicholas, S.B.; Pantalone, K.M.; Wanner, C.; Hamacher, S.; Gay, A.; Wheeler, D.C. Design and rationale of FINE-REAL: A prospective study of finerenone in clinical practice. J. Diabetes Complicat. 2023, 37, 108411. [Google Scholar] [CrossRef]
- Nankivell, B.J.; P’Ng, C.H.; O’Connell, P.J.; Chapman, J.R. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: Comparison of cyclosporine and tacrolimus eras. Transplantation 2016, 100, 1723–1731. [Google Scholar] [CrossRef]
- Feria, I.; Pichardo, I.; Juárez, P.; Ramírez, V.; González, M.A.; Uribe, N.; García-Torres, R.; López-Casillas, F.; Gamba, G.; Bobadilla, N.A. Therapeutic benefit of spironolactone in experimental chronic cyclosporine a nephrotoxicity. Kidney Int. 2003, 63, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Copur, S.; Mizrak, B.; Mallamaci, F.; Zoccali, C. Mineralocorticoid receptor antagonists in kidney transplantation. Eur. J. Clin. Investig. 2024, 54, e14206. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, S.M.; Bertocchio, J.-P.; Nakamura, T.; El-Moghrabi, S.; Jaisser, F.; Amador, C.A. The Mineralocorticoid Receptor on Smooth Muscle Cells Promotes Tacrolimus-Induced Renal Injury in Mice. Pharmaceutics 2023, 15, 1373. [Google Scholar] [CrossRef] [PubMed]



| Variables | Finerenon (n = 15) Median (Min–Max) | Control (n = 15) Median (Min–Max) | p |
|---|---|---|---|
| Age (years) | 42 (24–61) | 45 (19–59) | >0.05 |
| Weight (kg) | 75 (50–110) | 70 (44.60–111) | >0.05 |
| Height (cm) | 170 (153–190) | 171 (158–190) | >0.05 |
| Transplant duration (months) | 58 (13–150) | 80 (12–142) | >0.05 |
| BUN (mg/dL) | 24 (14–49) | 23(13–42) | >0.05 |
| Creatinine (mg/dL) | 1.85 (1.00–3.34) | 1.52 (1.12–2.38) | >0.05 |
| eGFR (mL/min/1.73 m2) | 41.7 (25.80–81.17) | 48.31(26.80–84.66) | >0.05 |
| Proteinuria (mg/day) | 2150 (600–5335) | 1691 (403–7112) | >0.05 |
| Uric acid (mg/dL) | 6.9 (4.75–12.11) | 6.7 (4.82–11.40) | >0.05 |
| Potassium (mmol/L) | 4.35 (3.29–5.00) | 4.22 (3.30–5.00) | >0.05 |
| ALT (U/L) | 14 (5–36) | 17 (8–33) | >0.05 |
| LDL (mg/dL) | 103 (38–257) | 103 (40–215) | >0.05 |
| TG (mg/dL) | 147 (80–787) | 101 (68–451) | >0.05 |
| Tacrolimus (ng/mL) | 7.70 (4.30–10.25) | 6.52 (3.14–9.9) | >0.05 |
| Donor Type (Living/Deceased) | 11/4 | 7/8 | >0.05 |
| Sex (F/M) | 5/10 | 9/6 | >0.05 |
| Smoking (+/−) | 5/10 | 3/12 | >0.05 |
| Etiology | |||
| HTN | 9 | 12 | |
| DM | 1 | 0 | |
| DM + HTN | 1 | 0 | |
| Others | 4 | 1 | |
| Unknown | 0 | 2 |
| Variables | GROUP 1 Median (Min–Max) | GROUP 2 Median (Min–Max) | p # | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6th Month | p * | Baseline | 6th Month | p & | ||
| BUN (mg/dL) | 24 (14–49) | 26 (17–53) | >0.05 | 23 (13–42) | 26 (18–72) | >0.05 | >0.05 |
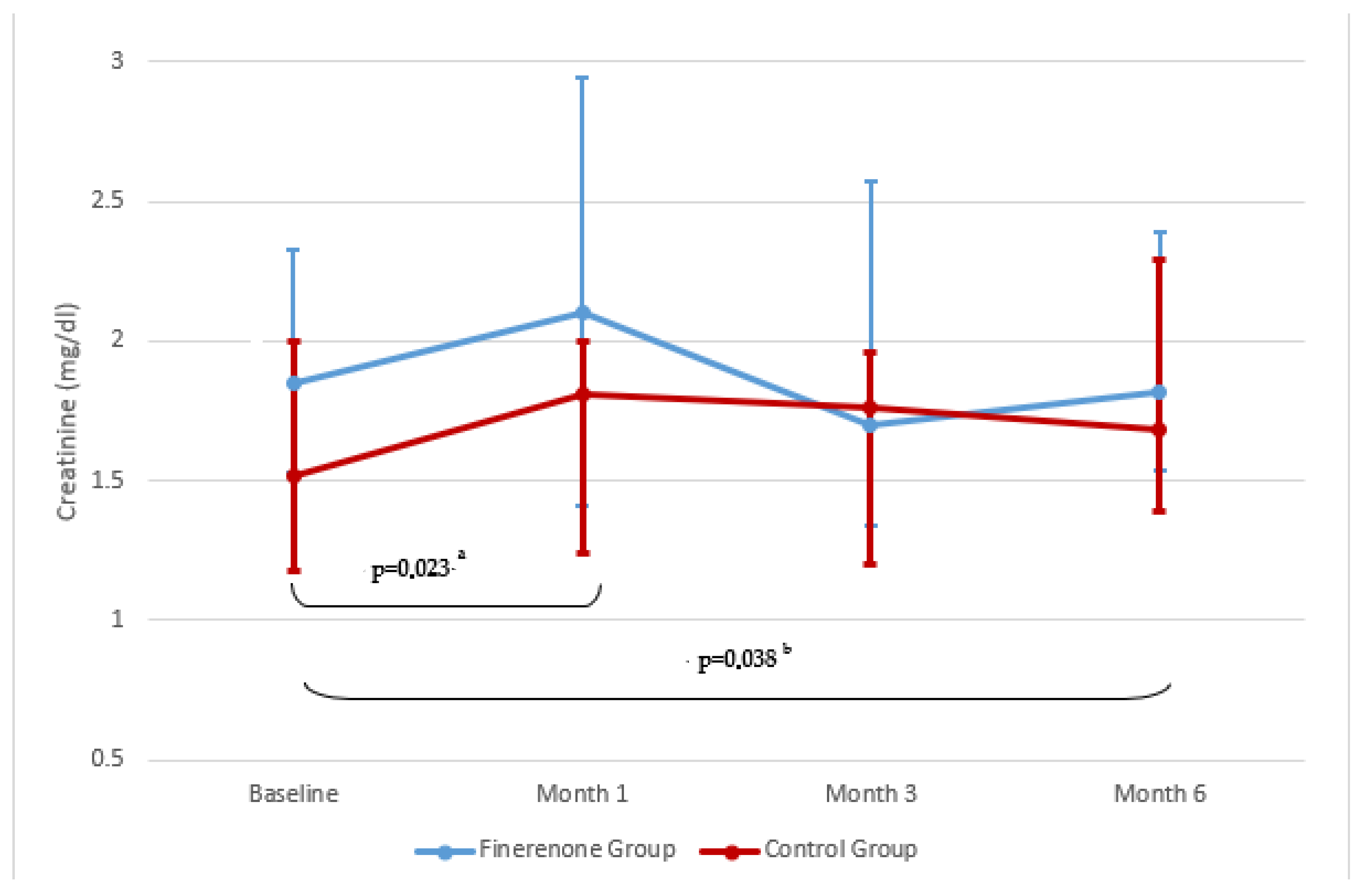
| Creatinine (mg/dL) | 1.85 (1–3.34) | 1.82 (1–3.95) | >0.05 | 1.52 (1.12–2.38) | 1.68 (0.91–4.26) | <0.05 | >0.05 |
| eGFR (mL/dk/1.73 m2) | 41.7 (25.8–81.1) | 40 (19.2–83.5) | >0.05 | 48.3 (26.80–84.66) | 48.7 (17.00–69.35) | <0.05 | >0.05 |
| Proteinuria (mg/day) | 2150 (600–5335) | 1127 (80–3848) | <0.05 | 1691(403–7112) | 1615 (420–5389) | >0.05 | >0.05 |
| Potassium (mmol/L) | 4.35 (3.29–5) | 4.32 (3.4–4.7) | >0.05 | 4.20 (3.3–5.0) | 4.4 (3.4–5.5) | >0.05 | >0.05 |
| Tacrolimus (ng/mL) | 7.7 (4.3–10.25) | 6.28 (3.66–10.5) | >0.05 | 6.52 (3.14–9.9) | 6.2 (4.7–10.2) | >0.05 | >0.05 |
| Uric acid (mg/dL) | 6.9 (4.15–12.1) | 6.27 (4.4–10.54) | >0.05 | 6.27 (4.82–11.4) | 6 (4.96–10.8) | >0.05 | >0.05 |
| ALT (IU/L) | 14 (5–36) | 17 (5–58) | >0.05 | 13 (8–33) | 16 (5–69) | >0.05 | >0.05 |
| LDL (mg/dL) | 103 (38–257) | 112 (36–191) | >0.05 | 103 (40–215) | 115 (36–207) | >0.05 | >0.05 |
| TG (mg/dL) | 147 (80–787) | 173 (50–466) | >0.05 | 101 (70–502) | 159 (37–581) | >0.05 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahvecioglu, S.; Celik, H.; Yalcinkaya, A.S.; Ayar, Y.; Aktas, N.; Akarsu, O. Efficacy and Safety of Finerenone in Kidney Transplant Patients. J. Clin. Med. 2025, 14, 8296. https://doi.org/10.3390/jcm14238296
Kahvecioglu S, Celik H, Yalcinkaya AS, Ayar Y, Aktas N, Akarsu O. Efficacy and Safety of Finerenone in Kidney Transplant Patients. Journal of Clinical Medicine. 2025; 14(23):8296. https://doi.org/10.3390/jcm14238296
Chicago/Turabian StyleKahvecioglu, Serdar, Huseyin Celik, Asena Serap Yalcinkaya, Yavuz Ayar, Nimet Aktas, and Ozger Akarsu. 2025. "Efficacy and Safety of Finerenone in Kidney Transplant Patients" Journal of Clinical Medicine 14, no. 23: 8296. https://doi.org/10.3390/jcm14238296
APA StyleKahvecioglu, S., Celik, H., Yalcinkaya, A. S., Ayar, Y., Aktas, N., & Akarsu, O. (2025). Efficacy and Safety of Finerenone in Kidney Transplant Patients. Journal of Clinical Medicine, 14(23), 8296. https://doi.org/10.3390/jcm14238296






