Shortening Indwelling Catheterization After Vaginal Surgery for Pelvic Organ Prolapse: Results from a Prospective Randomized Trial
Abstract
1. Introduction
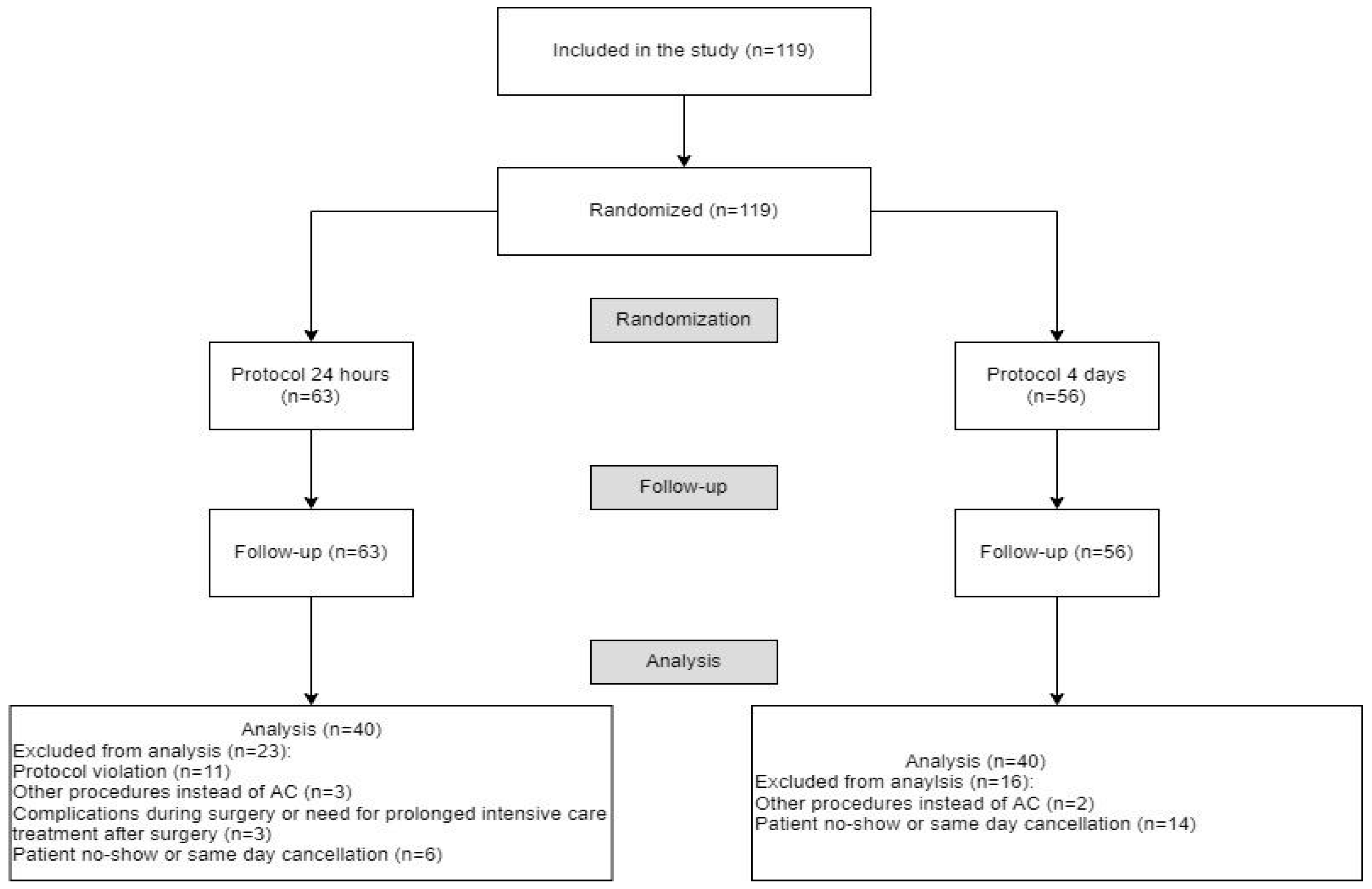
2. Materials and Methods
2.1. Study Design and Randomization
2.2. Perioperative Management
2.3. Microbiological Evaluation
2.4. Data Collection and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Postoperative Urinary Retention
3.3. Urinary Tract Infections
3.4. Duration of Catheterization and Hospital Stay
3.5. Hospitalization and Concomitant Procedures
3.6. Predictions of Hospital Stay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| POP | Pelvic organ prolapse |
| AC | Anterior colporrhaphy |
| IUC | Indwelling urinary catheter |
| POUR | Postoperative urinary retention |
| UTI | Urinary tract infection |
| TVT | Tension-free vaginal tape |
| EAU | European Association of Urology |
| ERAS | Enhanced recovery after surgery |
References
- Iglesia, C.B.; Smithling, K.R. Pelvic Organ Prolapse. Am. Fam. Physician 2017, 96, 179–185. [Google Scholar] [PubMed]
- Dietz, H.P. Pelvic organ prolapse—A review. Aust. Fam. Physician 2015, 44, 446–452. [Google Scholar] [PubMed]
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic organ prolapse. Lancet 2007, 369, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Urbankova, I.; Grohregin, K.; Hanacek, J.; Krcmar, M.; Feyereisl, J.; Deprest, J.; Krofta, L. The effect of the first vaginal birth on pelvic floor anatomy and dysfunction. Int. Urogynecol. J. 2019, 30, 1689–1696. [Google Scholar] [CrossRef]
- Blomquist, J.L.; Muñoz, A.; Carroll, M.; Handa, V.L. Association of Delivery Mode With Pelvic Floor Disorders After Child-birth. JAMA 2018, 320, 2438–2447. [Google Scholar] [CrossRef]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative review of the epidemiology, diagnosis and pathophysiology of pelvic organ prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef]
- Serdinšek, T.; But, I. Začetna obravnava bolnice z uroginekološkimi težavami. Slov. Med. J. 2019, 87, 575–586. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; The American Urogynecologic Society. Pelvic Organ Prolapse. Female Pelvic Med. Reconstr. Surg. 2019, 25, 397–408. [Google Scholar] [CrossRef]
- Barber, M.D.; Maher, C. Epidemiology and outcome assessment of pelvic organ prolapse. Int. Urogynecol. J. 2013, 24, 1783–1790. [Google Scholar] [CrossRef]
- Haya, N.; Feiner, B.; Baessler, K.; Christmann-Schmid, C.; Maher, C. Perioperative interventions in pelvic organ prolapse sur-gery. Cochrane Database Syst. Rev. 2018, 8, CD013105. [Google Scholar] [CrossRef]
- Altman, D.; Väyrynen, T.; Engh, M.E.; Axelsen, S.; Falconer, C. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N. Engl. J. Med. 2011, 364, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.C.; Umanskiy, K. Ventral Rectopexy. Clin. Colon Rectal Surg. 2021, 34, 062–068. [Google Scholar] [CrossRef]
- Weemhoff, M.; Wassen, M.M.L.H.; Korsten, L.; Serroyen, J.; Kampschöer, P.H.N.M.; Roumen, F.J.M.E. Postoperative catheterization after anterior colporrhaphy: 2 versus 5 days. A multicentre randomized controlled trial. Int. Urogynecol. J. 2011, 22, 477–483. [Google Scholar] [CrossRef]
- Geller, E.J. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int. J. Womens Health 2014, 6, 829–838. [Google Scholar] [CrossRef]
- Hakvoort, R.A.; Dijkgraaf, M.G.; Burger, M.P.; Emanuel, M.H.; Roovers, J.P.W.R. Predicting Short-Term Urinary Retention After Vaginal Prolapse Surgery. Neurourol. Urodyn. 2009, 28, 225–228. [Google Scholar] [CrossRef]
- Trowbridge, E.R.; Buchanan, L.E.; Evans, S.L.; Allen, M.N.; Chacon, H.L.; Hullfish, K.L. Pass or Fail? Postoperative Active Void-ing Trials in an Enhanced Recovery Program. Female Pelvic Med. Reconstr. Surg. 2022, 28, 436–443. [Google Scholar] [CrossRef]
- Hakvoort, R.A.; Elberink, R.; Vollebregt, A.; Ploeg, T.; Emanuel, M.H. How long should urinary bladder catheterisation be continued after vaginal prolapse surgery? A randomised controlled trial comparing short term versus long term cathe-terisation after vaginal prolapse surgery. BJOG 2004, 111, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.T.; Borawski, K.M.; South, M.M.; Weidner, A.C.; Webster, G.D.; Amundsen, C.L. A randomized, controlled trial evalu-ating 2 techniques of postoperative bladder testing after transvaginal surgery. Am. J. Obstet. Gynecol. 2007, 197, 627.e1–627.e4. [Google Scholar] [CrossRef] [PubMed]
- Moen, M.; Noone, M.; Vassallo, B. Anterior colporrhaphy: Why surgeon performance is paramount. Int. Urogynecol. J. 2014, 25, 857–862. [Google Scholar] [CrossRef]
- Baldini, G.; Bagry, H.; Aprikian, A.; Carli, F. Postoperative urinary retention: Anesthetic and perioperative considerations. Anesthesiology 2009, 110, 1139–1157. [Google Scholar] [CrossRef]
- Ceratti, R.D.N.; Beghetto, M.G. Incidence of urinary retention and relations between patient’s complaint, physical exami-nation, and bladder ultrasound. Rev. Gaucha. Enferm. 2021, 42, e20200014. [Google Scholar] [CrossRef]
- European Association of Urology. EAU Guidelines on Urological Infections; Edn presented at the EAU Annual Congress Amsterdam, the Netherlands; European Association of Urology: Arnhem, The Netherlands, 2022; ISBN 978-94-92671-16-5. [Google Scholar]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Ou, C.S.; Yeh, G.P.; der Tsai, H.; Sun, M.J. Optimal duration of urinary catheterization after anterior colporrhaphy. Int. Urogynecol. J. 2011, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kringel, U.; Reimer, T.; Tomczak, S.; Green, S.; Kundt, G.; Gerber, B. Postoperative infections due to bladder catheters after an-terior colporrhaphy: A prospective, randomized three-arm study. Int. Urogynecol. J. 2010, 21, 1499–1504. [Google Scholar] [CrossRef]
- Halpern-Elenskaia, K.; Umek, W.; Bodner-Adler, B.; Hanzal, E. Anterior colporrhaphy: A standard operation? Systematic review of the technical aspects of a common procedure in randomized controlled trials. Int. Urogynecol. J. 2018, 29, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Lensen, E.J.M.; Stoutjesdijk, J.A.; Withagen, M.I.J.; Kluivers, K.B.; Vierhout, M.E. Technique of anterior colporrhaphy: A Dutch evaluation. Int. Urogynecol. J. 2011, 22, 557–561. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, S.; Martinez Franco, E.; Martínez-Cumplido, R.; Molinet Coll, C.; Ojeda González, F.; Gómez Roig, M.D.; Tardiu, L.A. Reducing postoperative catheterisation after anterior colporrhaphy from 48 to 24 h: A randomised controlled trial. Int. Urogynecol. J. 2019, 30, 1897–1902. [Google Scholar] [CrossRef]
- Blaganje, M.; Lutfallah, F.; Deval, B. Mini-laparoscopic sacrocolpopexy for apical and posterior female pelvic organ pro-lapse. Int. Urogynecol. J. 2016, 27, 1117–1119. [Google Scholar] [CrossRef]
- Hickman, L.C.; Paraiso, M.F.R.; Goldman, H.B.; Propst, K.; Ferrando, C.A. Same-Day Discharge After Minimally Invasive Sac-rocolpopexy Is Feasible, Safe, and Associated With High Patient Satisfaction. Female Pelvic Med. Reconstr. Surg. 2021, 27, e614–e619. [Google Scholar] [CrossRef]
- Haylen, B.T.; Maher, C.F.; Barber, M.D.; Camargo, S.; Dandolu, V.; Digesu, A.; Goldman, H.B.; Huser, M.; Milani, A.L.; Moran, P.A.; et al. An International Urogynecological Associa-tion (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Organ Prolapse (POP). Neurourol. Urodyn. 2016, 35, 137–168. [Google Scholar] [CrossRef]
- Geerlings, S.E. Clinical Presentations and Epidemiology of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, UTI-0002-2012. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dong, L.; Gu, L. The timing of urinary catheter removal after gynecologic surgery: A meta-analysis of random-ized controlled trials. Medicine 2020, 99, e18710. [Google Scholar] [CrossRef]
- Sekhavat, L.; Farajkhoda, T.; Davar, R. The effect of early removal of indwelling urinary catheter on postoperative urinary complications in anterior colporrhaphy surgery. Aust. New Zealand J. Obstet. Gynaecol. 2008, 48, 348–352. [Google Scholar] [CrossRef]
- Castillo-Pino, E.; Benavides, N.; Acevedo, V.; Alonso, V. Removal time of postoperative vesical catheter in utero-vaginal prolapse surgery: A comparative study. Pelviperineology 2021, 40, 103–108. [Google Scholar] [CrossRef]
- Habboush, Y.; Guzman, N. Antibiotic Resistance; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Scheib, S.A.; Thomassee, M.; Kenner, J.L. Enhanced Recovery after Surgery in Gynecology: A Review of the Literature. J. Minim. Invasive Gynecol. 2019, 26, 327–343. [Google Scholar] [CrossRef]
- Ottesen, M.; Sørensen, M.; Rasmussen, Y.; Smidt-Jensen, S.; Kehlet, H.; Ottesen, B. Fast track vaginal surgery. Acta. Obstet. Gynecol. Scand. 2002, 81, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Lambat Emery, S.; Brossard, P.; Petignat, P.; Boulvain, M.; Pluchino, N.; Dällenbach, P.; Wenger, J.-M.; Savoldelli, G.L.; Rehberg-Klug, B.; Dubuisson, J. Fast-Track in Minimally Invasive Gynecology: A Randomized Trial Comparing Costs and Clinical Outcomes. Front. Surg. 2021, 8, 773653. [Google Scholar] [CrossRef]
- Nezhat, C.; Main, J.; Paka, C.; Soliemannjad, R.; Parsa, M.A. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS 2014, 18, e2014.00291. [Google Scholar] [CrossRef]
- Wodlin, N.B.; Nilsson, L. The development of fast-track principles in gynecological surgery. Acta Obstet. Gynecol. Scand. 2013, 92, 17–27. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative. Global guidance for surgical care during the COVID-19 pandemic. Br. J. Surg. 2020, 107, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.J.; Dobbs, T.D.; Wan, Y.I.; Laloo, R.; Hui, S.; Nepogodiev, D.; Bhangu, A.; Whitaker, I.S.; Pearse, R.M.; Abbott, T.E.F. Resource requirements for reintroducing elective surgery during the COVID-19 pandemic: Modelling study. Br. J. Surg. 2021, 108, 97–103. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative; Nepogodiev, D.; Bhangu, A. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar] [CrossRef]
- Chan, J.J.; Chen, K.K.; Choi, P.; Rojas, E.O.; Schipper, O.N.; Aiyer, A.; Netto, C.d.C.; Haleem, A.M.; Kadakia, A.R.; Vulcano, E. Impact of COVID-19 Pandemic on Patients’ Perceptions of Safety and Need for Elective Foot and Ankle Surgery in the United States. Foot Ankle Orthop. 2021, 6, 24730114211013788. [Google Scholar] [CrossRef]
- Norris, Z.A.; Sissman, E.; O’Connell, B.K.; Mottole, N.A.; Patel, H.; Balouch, E.; Ashayeri, K.; Maglaras, C.; Protopsaltis, T.S.; Buckland, A.J.; et al. COVID-19 pandemic and elective spinal surgery cancelations—What happens to the patients? Spine J. 2021, 21, 2003–2009. [Google Scholar] [CrossRef]
- Chang, J.; Wignadasan, W.; Kontoghiorghe, C.; Kayani, B.; Singh, S.; Plastow, R.; Magan, A.; Haddad, F. Restarting elective orthopaedic services during the COVID-19 pandemic. Bone Jt. Open 2020, 1, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Madanipour, S.; Al-Obaedi, O.; Ayub, A.; Iranpour, F.; Subramanian, P. Resuming elective hip and knee arthroplasty in the COVID-19 era: A unique insight into patient risk aversion and sentiment. Ann. R. Coll. Surg. Engl. 2021, 103, 104–109. [Google Scholar] [CrossRef]
- Garde-García, H.; González-López, R.; González-Enguita, C. Functional urology surgery and SARS-CoV-2: How and why surgical activity should be resumed now, adapting to the new reality. Actas Urol. Esp. 2020, 44, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, A.L.D.; Santiago, A.E.; Derchain, S.F.M.; Carvalho, J.P. Enhanced Recovery After Surgery (ERAS): New Concepts in the Perioperative Management of Gynecologic Surgery. Rev. Bras. Ginecol. Obstet. 2018, 40, 433–436. [Google Scholar] [CrossRef] [PubMed]
| Type of Surgery | 24 h Group (n = 40) | 4 Days Group (n = 40) |
|---|---|---|
| Only anterior colporrhaphy | 13 | 13 |
| Anterior and posterior colporrhaphy | 3 | 4 |
| Anterior colporrhaphy and Manchester–Fothergill surgery | 5 | 0 |
| Anterior colporrhaphy and vaginal hysterectomy | 9 | 18 |
| Anterior colporrhaphy and LAVHA | 3 | 1 |
| Anterior colporrhaphy and colpopexy on sacrospinous ligament | 3 | 0 |
| Anterior colporrhaphy and TVT | 4 | 4 |
| 24 h (n = 40) | 4 Days (n = 40) | p-Value | |
|---|---|---|---|
| Duration of catheterization (days) | 1 [1–1] | 4 [4–4] | <0.001 |
| Duration of hospitalization (days) | 3 [2–4] | 4 [4–4] | <0.001 |
| Concomitant Procedures (n = 54) | AC (n = 26) | p-Value | |
|---|---|---|---|
| All patients (days) | 3.8 (0.9) | 3.1 (1.1) | 0.010 |
| 24 h group (days) | 3.4 (1.0) | 2.4 (0.8) | 0.001 |
| 4 days group (days) | 4.2 (0.6) | 3.7 (0.9) | 0.339 |
| 24 h, AC (n = 13) | 4 Days, AC (n = 13) | p-Value | |
|---|---|---|---|
| Duration of hospitalization (days) | 2.4 (0.8) | 3.9 (0.9) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordis, T.; Kofol, A.; Blaganje, M. Shortening Indwelling Catheterization After Vaginal Surgery for Pelvic Organ Prolapse: Results from a Prospective Randomized Trial. J. Clin. Med. 2025, 14, 8295. https://doi.org/10.3390/jcm14238295
Kordis T, Kofol A, Blaganje M. Shortening Indwelling Catheterization After Vaginal Surgery for Pelvic Organ Prolapse: Results from a Prospective Randomized Trial. Journal of Clinical Medicine. 2025; 14(23):8295. https://doi.org/10.3390/jcm14238295
Chicago/Turabian StyleKordis, Tala, Ana Kofol, and Mija Blaganje. 2025. "Shortening Indwelling Catheterization After Vaginal Surgery for Pelvic Organ Prolapse: Results from a Prospective Randomized Trial" Journal of Clinical Medicine 14, no. 23: 8295. https://doi.org/10.3390/jcm14238295
APA StyleKordis, T., Kofol, A., & Blaganje, M. (2025). Shortening Indwelling Catheterization After Vaginal Surgery for Pelvic Organ Prolapse: Results from a Prospective Randomized Trial. Journal of Clinical Medicine, 14(23), 8295. https://doi.org/10.3390/jcm14238295





