Abstract
Over the past decade, chronic lymphocytic leukaemia (CLL) treatment has shifted from chemoimmunotherapy to targeted oral agents, predominantly Bruton’s tyrosine kinase inhibitors (BTKis) and the BCL-2 inhibitor venetoclax. These therapies have significantly improved outcomes and are now established as first-line treatment options. However, CLL remains incurable, and resistance or intolerance to both drug classes (double-refractory disease) is an emerging challenge. This has driven the development of novel therapeutic strategies, including non-covalent BTKis such as pirtobrutinib and nemtabrutinib, which retain activity in BTK C481-mutated disease. Next-generation BCL-2 inhibitors (sonrotoclax, lisaftoclax) and BTK degraders are promising in early clinical trials. Immunotherapeutic approaches, such as bispecific T-cell engagers, CD20/CD3 antibodies, and CAR-T cell therapies, provide additional options for high-risk patients. Although PI3K inhibitors remain under investigation, their role is yet to be defined due to safety concerns. Minimal residual disease (MRD)-guided, fixed-duration regimens represent a significant paradigm shift toward personalised treatment and potentially deeper remissions. Ongoing clinical studies are expected to introduce new effective therapies that may further transform the management of CLL in the coming years.
1. Introduction
Chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) is the most common form of leukaemia in adults in Europe and North America, with an incidence of 5.1 cases per 100,000 individuals per year [1,2,3]. The disease mainly affects older individuals, with a median age at diagnosis of about 70 years [2,4]. CLL is an indolent, mature lymphoproliferative malignancy characterised by a progressive accumulation of monoclonal B lymphocytes that coexpress CD5, CD19, CD23 and CD20 antigens in peripheral blood (PB), lymph nodes, spleen, and bone marrow (BM). However, some patients present with an atypical CLL immunophenotype, characterised by the absence of expression of one or more surface antigens, most commonly CD5 and CD23 [5]. The cytogenetic characteristics of CLL are heterogeneous, with most patients presenting with deletion 13q, trisomy 12, deletion 11q and notably 17p deletion, which is strongly associated with poor prognosis and resistance to standard therapies [6]. In addition to cytogenetics, TP53 mutations and the immunoglobulin heavy-chain variable region (IGHV) mutational status are key prognostic markers [7]. Patients with TP53 aberrations and unmutated IGHV (umIGHV) generally have inferior outcomes, especially when treated with chemoimmunotherapy (CIT) [2].
For many years, the standard treatment for progressive and symptomatic disease in treatment-naïve (TN) and relapsed/refractory (RR) patients has been CIT with cytotoxic drugs (fludarabine, bendamustine, cyclophosphamide, and chlorambucil) and CD20 monoclonal antibodies (rituximab and obinutuzumab). One of the most commonly used regimens is the combination of fludarabine with cyclophosphamide and rituximab (FCR), especially in younger, fit patients [8]. A long-term (19.0-year) follow-up of 300 patients treated with FCR revealed that the median progression-free survival (PFS) was 14.6 years for patients with mutated IGHV (mIGHV) and 4.2 years for those with umIGHV [9]. It was found that FCR remains an acceptable treatment option for selected patients, as only 9% of the mIGHV group showed progression beyond 10 years. However, treatment with FCR carries a 1.5–3% risk of developing treatment-related myelodysplastic syndrome (MDS) or acute myeloid leukaemia (AML) [9,10]. This is why, in major guidelines, CIT is no longer recommended for treating CLL patients [11].
Over the past decade, targeted oral therapies—primarily BTKis and the BCL-2 inhibitor venetoclax—have replaced CIT as the standard of care for TN-CLL. Although these agents significantly improve outcomes, CLL remains incurable, and relapse or resistance eventually occurs in most patients [12,13]. As treatment is increasingly guided by targeted drugs rather than CIT, optimal sequencing of therapies has become a major clinical challenge. Patients may relapse after covalent BTKis (cBTKis), venetoclax-based regimens, or combinations thereof, and emerging resistance also occurs following cellular and bispecific antibody therapies. Subsequent treatment decisions depend on prior therapy, response duration, genetic risk factors, and resistance mechanisms. For example, patients relapsing after venetoclax-based fixed-duration therapy may respond to BTKis. At the same time, those progressing on cBTKis may benefit from venetoclax–rituximab or a non-covalent BTKi (ncBTKi) such as pirtobrutinib [4,12,14].
This article discusses the mechanism of action, clinical applications, and safety of novel drugs for CLL (Figure 1). Using Pubmed, Web of Science, and Google Scholar, a literature search was performed for articles published in English. Additional relevant publications were obtained by reviewing the references from the chosen articles.
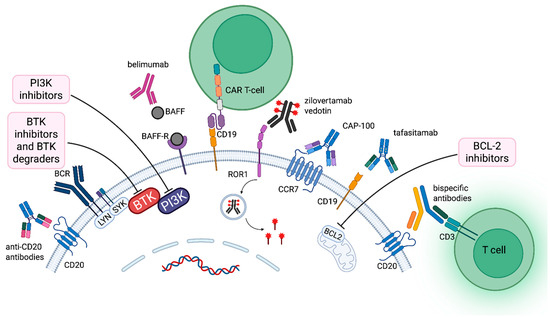
Figure 1.
Therapeutic targets and emerging treatment strategies in chronic lymphocytic leukemia. Key membrane-bound proteins and signaling components targeted by novel therapies in CLL, including CD20, CD19, ROR1, BAFF-R, CCR7, BCR, BTK, PI3K, and BCL2. Therapeutic modalities include mAbs, BsAbs, ADCs, CAR-T cells, and small-molecule inhibitors. Abbreviations: ROR1 (Receptor tyrosine kinase-like orphan receptor 1), BAFF-R (B-cell activating factor receptor), CCR7 (C-C motif chemokine receptor 7), BCR (B-cell receptor), BTK (Bruton’s tyrosine kinase), PI3K (Phosphoinositide 3-kinase), BCL2 (B-cell lymphoma 2), mAbs (monoclonal antibodies), BsAbs (bispecific antibodies), ADCs (antibody–drug conjugates). Created in BioRender. Pula, A. (2025) https://BioRender.com/4cl4q8c (accessed on 24 October 2025).
2. BTK Inhibitors
BTKis have become essential drugs in treating CLL [15]. These agents are classified into two types based on their binding mechanism: covalent (irreversible) and non-covalent (reversible). Covalent BTKis approved in CLL include ibrutinib, acalabrutinib, and zanubrutinib. They bind permanently to the C481 residue of the BTK active site. Non-covalent BTKis, such as pirtobrutinib and nemtabrutinib, bind reversibly to BTK and remain effective even if C481S mutations are present (Table 1).

Table 1.
BTK inhibitors approved or under investigation for the treatment of CLL.
2.1. Covalent BTK Inhibitors
Covalent BTKis, including first-generation ibrutinib and second-generation acalabrutinib and zanubrutinib, are established targeted therapies in CLL [15]. In monotherapy they are administered continuously until progression or unacceptable toxicity and have demonstrated superior PFS and OS over CIT, including FCR regimens [18,19,39]. However, their use is limited by adverse events, particularly cardiovascular toxicity such as atrial fibrillation, hypertension, and bleeding [40]. Second-generation inhibitors were developed to improve tolerability while maintaining efficacy [41,42,43]. Clinical trials have confirmed their favourable safety and efficacy profiles in both TN and R/R CLL [24,25,27,28,44,45,46,47].
2.1.1. Ibrutinib
Ibrutinib (Imbruvica, Johnson & Johnson, New Brunswick, NJ, USA) significantly improved PFS and OS compared with chlorambucil or CIT in both TN and RR CLL patients (Table 1) [21,22,23]. The RESONATE trial (phase 3) evaluated RR CLL and demonstrated superior efficacy of ibrutinib over ofatumumab, with a median PFS of 44.1 months versus 8.1 months [48]. The RESONATE-2 trial (phase 3) enrolled TN patients aged ≥65 years and showed that ibrutinib achieved an ORR of 86% and a median PFS of 8.9 years, significantly outperforming chlorambucil [17].
Moreover, two randomised trials (E1912 and FLAIR) demonstrated the superiority of ibrutinib-based therapy over FCR in TN CLL patients [18,19]. In the E1912 study, ibrutinib plus rituximab (IR) significantly improved PFS (HR 0.37; p < 0.001) and OS (HR 0.47; p = 0.018) compared with FCR, with benefit observed in both mIGHV and umIGHV subgroups [18]. The IR arm also showed longer OS than the FCR arm (HR, 0.47; p = 0.018). The FLAIR trial included TN patients with a median age of 62 years and a WHO performance status of 2 or less [19]. Median PFS had not been reached after a median follow-up of 53 months in the IR arm and 67 months for FCR (HR, 0.44; p < 0.0001). However, the IR and FCR arms achieved similar PFS for patients with mIGHV (HR 0.64, p = 0.15).
The A041202 study in older patients showed that ibrutinib monotherapy and IR yielded superior PFS compared with BR, with no added benefit from rituximab [20]. In TP53-aberrant CLL, ibrutinib monotherapy demonstrated a median PFS of 53 months [49]. These findings established continuous BTK inhibition as standard first-line therapy. The FDA approved Ibrutinib monotherapy in 2014 for treating patients with CLL who have received at least one prior therapy and in 2016 for treating TN CLL. The FDA also approved ibrutinib combined with obinutuzumab for TN CLL in 2019 and rituximab in 2020. However, despite its efficacy, long-term therapy with ibrutinib is limited by cardiovascular toxicity [40,50].
2.1.2. The Second-Generation BTK Inhibitors
Second-generation cBTKis, including acalabrutinib and zanubrutinib, were developed to improve tolerability while maintaining or enhancing efficacy compared with first-generation BTKis [41,42,43]. These agents are more selective for BTK, resulting in fewer off-target effects and reduced cardiovascular toxicity.
Acalabrutinib
Acalabrutinib (Calquence, AstraZeneca, Cambridge, UK) demonstrated superior efficacy compared with CIT in TN CLL. In the ELEVATE-TN phase 3 trial, acalabrutinib plus obinutuzumab achieved an ORR of 94% and significantly prolonged PFS, with median PFS not reached versus 22.6 months for chlorambucil–obinutuzumab (ChlO) (HR 0.10; p < 0.0001) [24]. In RR CLL, the ASCEND trial reported significantly longer PFS for acalabrutinib than idelalisib–rituximab or BR (HR 0.31) [25]. This BTKi also demonstrated non-inferior PFS and lower rates of atrial fibrillation compared with ibrutinib in the ELEVATE-RR trial [26,46]. Acalabrutinib was approved by FDA in monotherapy or combined with obinutuzumab for CLL in 2019.
Zanubrutinib
Zanubrutinib (Brukinsa, BGB-3111, BeOne Medicines, Cambridge, MA, USA) has shown improved depth and durability of response compared with standard therapy. In the SEQUOIA trial, zanubrutinib significantly prolonged PFS compared with BR (median PFS not reached vs. 44.1 months; HR 0.29; p = 0.0001) [28]. Moreover, in the ALPINE trial, zanubrutinib demonstrated superior PFS (24-month PFS 78.4% vs. 65.9%; HR 0.65) and higher ORR (83.5% vs. 74.2%) compared with ibrutinib in RR CLL, with fewer cardiac adverse events [29]. In January 2023, the FDA approved zanubrutinib for the first-line treatment of CLL/SLL.
Both acalabrutinib and zanubrutinib are now preferred over first-generation BTKis due to improved safety profiles and sustained efficacy in high-risk CLL. Several other next-generation cBTKis, including orelabrutinib and tirabrutinib, are currently being investigated for CLL and other B-cell malignancies [15].
Orelabrutinib
Orelabrutinib (ICP-022, Hibruka, InnoCare, Beijing, China) is a next-generation cBTKi that demonstrated in vitro greater selectivity against BTK than ibrutinib, with a more than 90% BTK inhibition rate [31]. A phase II study reported its activity on B-cell malignancies, including 80 patients with RR CLL. Treatment achieved a 93.8% ORR. At a median follow-up of 32.3 months, the median PFS had not been achieved, while the 30-month PFS and OS rates were 70.9% (95% CI, 59.5–79.6) and 81.3% (95% CI, 70.8–88.2), respectively [30]. Essentially, no cases of AF or severe hypertension were observed. Orelabrutinib was approved in China for previously treated CLL/SLL and is currently authorised for that indication.
Tirabrutinib
Tirabrutinib (Velexbru, ONO/GS-4059, Ono Pharmaceutical Co., Ltd., Osaka, Japan) is another next-generation cBTKi, more selective than ibrutinib, and is being investigated in B-cell malignancies. In a phase 1 trial, tirabrutinib was well tolerated in RR mature B-cell lymphoid malignancies [51]. This drug was studied in CLL with entospletinib, with or without obinutuzumab, and demonstrated therapeutic activity with good tolerability [32]. This BTKi demonstrates superior blood–brain barrier penetration compared with ibrutinib and may represent a promising therapeutic option for CLL patients with central nervous system involvement [33]. It has received approval in Japan for treating primary central nervous system lymphoma. Reversible non-covalent BTKis, pirtobrutinib and nemtabrutinib, do not require C481 for their antineoplastic activity and can be effective in patients refractory to covalent BTK inhibitors.
2.2. Non-Covalent BTK Inhibitors
Non-covalent BTKis are next-generation, reversible agents that effectively inhibit BCR signalling in both TN and ibrutinib-refractory CLL. Unlike cBTKis, they do not depend on binding to the C481 residue, which allows them to maintain activity in patients who have developed resistance due to C481 mutations. Acquired mutations in BTK or its downstream mediator PLCγ2 have been identified in most cases resistant to ibrutinib, acalabrutinib, and zanubrutinib [52,53,54]. Non-covalent BTKis maintain efficacy irrespective of the C481S mutation [34,35]. Pirtobrutinib (LOXO-305) and nemtabrutinib are the most advanced ncBTKis tested in clinical trials.
2.2.1. Pirtobrutinib
Pirtobrutinib (LOXO-305, Jaypirca, Eli Lilly, Indianapolis, IN, USA) received FDA approval in December 2023 to treat patients with CLL who had previously received both a cBTKis and a BCL-2 inhibitor [34]. This approval was based on data from the phase 1/2 BRUIN trial, which enrolled patients with heavily pretreated CLL or SLL who had progressed after cBTK exposure. [34]. However, the FDA approval specifically relates to the subgroup that had received both a BTKi and a BCL-2 inhibitor. In this study, pirtobrutinib demonstrated meaningful clinical activity, achieving an ORR of 73.3% and a median PFS of 19.6 months among 247 patients with RR CLL/SLL [44]. The treatment was generally well tolerated; the most common adverse events were infections (71.0%), bleeding (42.6%), and neutropenia (32.5%) [44]. Other observed adverse events included hypertension (14.2%), atrial fibrillation or flutter (3.8%), and major haemorrhage (2.2%) [34].
Several ongoing clinical trials are further evaluating pirtobrutinib in CLL and SLL. A phase 2 trial assesses the safety and efficacy of three dosing regimens in patients with RR CLL or SLL who have received one to three prior lines of therapy, including cBTKi, with study completion anticipated in 2028 (#NCT06466122). Another phase 1/2 trial (#NCT03740529) is investigating pirtobrutinib in patients with CLL, SLL, and non-Hodgkin lymphoma (NHL) who are refractory or intolerant to standard-of-care therapies, with an estimated completion date in 2028.
In phase 3 studies, pirtobrutinib is being compared with ibrutinib in patients with CLL/SLL carrying del (17p) (BRUIN CLL-314; #NCT05254743), with BR in TN patients (BRUIN CLL-313; #NCT05023980), and with investigator’s choice of idelalisib plus rituximab or BR in previously treated patients (BRUIN CLL-321; #NCT04666038). Additionally, pirtobrutinib is being evaluated in combination regimens, including phase 2 trials with venetoclax in patients resistant to cBTKis (#NCT06466122) and in TN patients with MRD assessment (#NCT05677919). Further studies include pirtobrutinib combined with obinutuzumab in TN CLL (#NCT06333262) and a phase 3 trial assessing the triplet regimen of pirtobrutinib, venetoclax, and rituximab (PVR) versus venetoclax and rituximab (VR) in RR CLL/SLL (BRUIN CLL-322; #NCT04965493). A time-limited triplet combination of pirtobrutinib, venetoclax, and obinutuzumab is also being investigated in TN CLL and in patients with Richter transformation (RT) (#NCT05536349).
Overall, the expanding body of clinical evidence supports pirtobrutinib as a highly promising therapeutic option for patients with CLL across multiple lines of therapy, particularly those with resistance to cBTKis.
2.2.2. Nemtabrutinib
Nemtabrutinib (MK-1026, ARQ-531, Merck, Rahway, NJ, USA) is another ncBTKi with a mechanism similar to pirtobrutinib, which reversibly inhibits both wild-type and C481-mutated BTK. Nemtabrutinib was tested in 47 patients with RR disease, including 29 with CLL, 17 with B-cell NHL, and one with Waldenström macroglobulinemia (WM), at doses ranging from 5 to 75 mg once daily in 28-day cycles [35]. An ORR was noted in 75% of CLL patients receiving the compound at a daily dose of 65 mg. Grade ≥ 3 treatment-emergent adverse events (TAES) were observed in 37 patients (89%), most frequently neutropenia (23.4%), febrile neutropenia (14.9%), and pneumonia (14.9%) [36]. Several late-phase clinical trials are ongoing to evaluate nemtabrutinib in CLL/SLL. The phase 2 BELLWAVE-003 trial (#NCT04728893) is assessing the efficacy and safety of nemtabrutinib in various B-cell malignancies, including CLL/SLL, at the recommended phase 2 dose, with study completion expected in 2027. In parallel, the phase 3 open-label randomized BELLWAVE-011 trial (#NCT06136559) is comparing nemtabrutinib (65 mg once daily) with the investigator’s choice of ibrutinib or acalabrutinib in TN patients with CLL/SLL. Results from this study are not yet available.
Further, nemtabrutinib is being investigated in multiple phase 3 studies across different lines of therapy. The BELLWAVE-008 trial (NCT05624554) compares nemtabrutinib with CIT in TN CLL/SLL patients without TP53 aberrations. The BELLWAVE-010 trial (NCT05947851) evaluates nemtabrutinib in combination with venetoclax versus venetoclax plus rituximab (VR) in patients with RR CLL/SLL as second-line therapy, with an estimated study completion in 2033. In addition, NCT05458297 (MK-2140-006) is investigating zilovertamab vedotin alone and in combination with nemtabrutinib in patients with aggressive and indolent B-cell malignancies, including CLL, with completion expected in 2027. Preclinical data support combination strategies. In a CLL mouse model, nemtabrutinib combined with venetoclax significantly prolonged survival compared with ibrutinib plus venetoclax, highlighting its potential synergy and supporting clinical evaluation of this combination [15,55].
2.2.3. Rocbrutinib
Covalent BTKi and nc BTKi are effective in CLL treatment; however, second-site T474 gatekeeper mutations (GM) are commonly observed in patients resistant to ncBTKi such as pirtobrutinib [38]. Rocbrutinib,(LP-168, Hansoh Pharma (Lianyungang, Jiangsu, China) and Lupeng Pharma (Guangzhou, Guangdong, China) is a selective next-generation BTKi that irreversibly targets wild-type BTK, reversibly targets C481 mutant BTK (C481S, C481F, and C481R), and irreversibly targets other non-C481 mutations, such as the GM T474I, with preclinical activity in C481 and T474 mutants and clinical activity in RR CLL. In a phase 1 trial, 10 patients who had previously received cBTKi and ncBTKi treatment were subjected to ibrutinib treatment. With a median follow-up of 14 months (range 2.6–20.1), responses were observed in 9 patients. The ORR across all dose levels was 77.8% (7/9), with all being PR or PR-L [38].
3. BTK Degraders
Proteolysis-targeting chimaeras (PROTACS) can potentially overcome acquired resistance to BTKis in B-cell malignancies. They offer improved target selectivity, rapid and sustained BTK depletion, and greater potency in BTK inhibition degradation [56,57]. BTK degraders (BTKdeg) are targeted protein degraders that induce the degradation of BTK [58]. BTKdeg can potentially prevent signal transduction from BCR receptor and may be clinically helpful in BTKi intolerance or resistance in CLL. BGB-16673, Nx-2127, Nx-5948, NRX-0492, HZ-Q1060, ABBV-101, and AC676 have shown significant BTK degradation in preclinical studies and early clinical trials (Table 2) [57]. These BTKis have demonstrated favourable safety profiles and are considered novel therapeutic agents for patients with BTKi-resistant diseases [59].

Table 2.
BTK degraders investigated in CLL.
3.1. BGB-16673
BGB-16673 (BeOne Medicines, Cambridge, MA, USA) is a bivalent BTKdeg that induces BTK degradation by specifically binding to BTK and the E3 ligase. It exhibits a unique on-target resistant mutation profile and could overcome many BTK resistance mutations. BGB-16673 showed promising preclinical BTK degradation activity. In the phase 1 study (CaDAnCe-101), BGB-16673 was safe and well-tolerated in the heavily pretreated population of patients with RR CLL/SLL [60]. In 49 response-evaluable patients, BGB-16673 showed significant antitumour activity, including in patients with BTKi–resistant mutations and those previously exposed to cBTK, ncBTK, and BCL2 inhibitors. The median time to first response was 2.8 months (2.6–8.3 months). ORR was 77.6% (38/49) and CR/CRi 4.1% (2/49). The best response was observed in patients treated with 200 mg administered orally once daily in 28-day cycles (ORR 93.8%). Promising activity was also seen in RT, with an ORR of 58.3% (7/12) and a CR of 8.3% (1/12). A Phase 1b/2 study of BGB-16673 in combination with sonrotoclax, zanubrutinib, mosunetuzumab, or glofitamab in patients with RR B-cell malignancies is ongoing (#NCT06634589). A phase 3 study evaluating the safety and efficacy of BGB-16673 compared to pirtobrutinib in adults with RR CLL/SLL is underway (#NCT06973187). In parallel, the phase 3 study (CaDAnCe-302) of BGB-16673 compared to the investigator’s choice in participants with CLL/SLL previously exposed to both BTK and BCL2 inhibitors (#NCT06846671) was recently initiated. Two phase 1/2 open-label, dose-escalation, and dose-expansion clinical trials test BGB-16673 in patients with CLL and other B-cell malignancies (#NCT05294731 and #NCT05006716). The studies are expected to be completed in 2027 and 2028, respectively.
3.2. Bexobrutideg
Bexobrutideg (NX-5948, Nurix Therapeutics Inc., San Francisco, CA, USA) is a chimeric targeting molecule (CTM) that induces BTK protein degradation by the cereblon E3 ligase (CRBN) complex without degradation of other cereblon neo-substrates. In a phase 1a/b study, 87 patients with different B-cell malignancies, including 34 patients with CLL, were included [61]. Prior therapies included BTKi (97.1%), pirtobrutinib (23.5%), BCL-2 inhibitors (91.2%), BTKi + BCL2 inhibitors (88.2%), chemotherapy/CIT (79.4%), PI3K inhibitors (32.4%), and CAR-T (5.9%). Bexobrutideg was well tolerated across all tested doses, consistent with previously reported safety profiles. The most common TEAEs were purpura/contusions (44.1%), thrombocytopenia (23.5%), petechiae (29.4%), fatigue (20.6%), and neutropenia (17.6%). There was no new onset of atrial fibrillation/flutter or hypertension. In 30 response-evaluable patients with CLL, the ORR was 76.7%, with 22 responses ongoing at data cut-off. Additionally, 29 out of 34 patients remained on treatment.
3.3. NX-2127
NX-2127 (Nurix Therapeutics, Inc., San Francisco, CA, USA) is a BTKdeg with concomitant immunomodulatory activity mediating the degradation of transcription factors IKZF1 and IKZF3 through molecular glue interactions with the cereblon E3 ubiquitin ligase complex [62]. Treatment with NX-2127 induced >80% degradation of BTK in CLL patients. NX-2127 degrades common BTK resistance mutants, including BTKC481S [63]. In the first-in-human clinical trial, deep and sustained degradation of BTK was achieved in daily oral dosing at 100 mg [64]. In heavily pretreated patients with RR CLL and NHL, encouraging and sustained responses were observed with a manageable safety profile. The safety and anticancer activity of NX-2127 are being assessed in a first-in-human phase 1a/1b clinical trial involving patients with R/R B-cell malignancies (#NCT04830137). The study is expected to be completed in 2026.
3.4. AC676
AC676 (Accutar Biotech., Cranbury, NJ, USA) is an orally bioavailable BTKdeg designed to treat B-cell malignancies [65]. It was designed as a chimeric degrader to specifically target and degrade BTK using a proprietary Protein–Protein Interaction Targeting Chimeras (PPI-TAC) platform by Accutar Biotech. AC676 recruits BTK by linking a BTK ligand to the cereblon E3-ligase recruiting ligand, bringing it into proximity with cereblon, which then induces ubiquitination and degradation of BTK proteins, including those with resistance mutations such as C481 and kinase-dead variants L528. As a result, AC676 may be an effective treatment for CLL patients progressing on both cBTKis and ncBTKis. However, AC676 does not degrade cereblon neo-substrates, which is expected to limit toxicity, particularly neutropenia, as a consequence of the treatment. AC676-001 is being investigated in a phase 1, dose-escalation study in patients with RR B-cell malignancies (ClinicalTrials.gov ID NCT05780034). AC676 is administered as a single agent orally once daily on a 28-day cycle, with doses ranging from 50 mg to 600 mg [65]. The study is estimated to be completed in 2026.
3.5. NRX-0492
NRX-0492 (Nurix Therapeutics Inc., San Francisco, CA, USA) is a targeted protein degrader that binds a non-covalent BTK-binding domain to an adaptor protein, cereblon of the E3 ubiquitin ligase complex. NRX-0492 demonstrated activity against WT BTK and C481 mutants in CLL in vitro and in vivo [58]. In PDX CLL in vivo models, NRX-0492–induced BTK degradation and inhibited CLL cell activation, proliferation, and expansion. Clinical studies testing NRX-0492 have been initiated (#NCT04830137).
3.6. HZ-Q1060 and HZ-Q1070
HZ-Q1060 and HZ-Q1070 (Hangzhou HealZen Therapeutics Co., Hangzhou, China) were developed as novel BTK-PROTAC agents within the DaTProD® platform and have been validated as promising candidates for further clinical development [69]. HZ-Q1060 can selectively degrade BTK in lymphoma cell lines, human PBMCS, and intracellular mutant BTK-C481S protein. The drug was also investigated in the subcutaneously transplanted tumour model of OCI-LY10 mice, where tumour growth was entirely inhibited by HZ-Q1060 when administered orally every day for 14 days. HZ-Q1070 is another promising BTKdeg with excellent pharmacokinetic properties for clinical use development [67]. It exhibited tumour growth inhibition in cell experiments and a mouse tumour model. Moreover, HZ-Q1070 avoided degradation of Aiolos and Ikaros, essential for NK cell activity [66]. A phase I study of HZ-Q1070 in patients with RR B-cell malignancies, including CLL/SLL, WM, mantle cell lymphoma (MCL), follicular lymphoma (FL), marginal zone lymphoma (MZL), diffuse large B-cell lymphoma (DLBCL) and primary central nervous system lymphoma (PCNSL), is in progress (#CTR20240471).
3.7. ABBV-101
ABBV-101 (AbbVie Inc., Chicago, IL, USA) is a reversible BTKdeg with high selectivity and activity against BTK wild type and multiple therapy-resistant mutant forms in preclinical studies. ABBV-101 has shown efficacy in vitro and in vivo in BTK-WT and cBTKi-resistant (BTK-C481S) systemic mouse CLL and human DLBCL models. Moreover, ABBV-101 induces complete tumour regressions in multiple non-GCB DLBCL PDX models, showing deeper and longer responses than covalent and reversible inhibitors. Significantly, the BCL-2 inhibitor enhances the efficacy of ABBV-101 in CLL and DLBCL models. ABBV-101 is investigated in a phase 1, open-label, multicenter study in patients with B-cell lymphoid malignancies, including CLL (#NCT05753501) [68].
4. BCL-2 Inhibitors
BCL-2 inhibitors target the BCL-2 protein, which is overexpressed in CLL and other haematological malignancies. Venetoclax is the first BCL-2 inhibitor approved for the treatment of CLL. Second-generation BCL-2 inhibitors, sonrotoclax, lisaftoclax, mesutoclax, and ABBV-453 are currently under investigation in CLL (Table 3).

Table 3.
BCL-2 inhibitors approved or potentially useful for the treatment of CLL.
4.1. Venetoclax
Venetoclax (formerly ABT-199, Venclyxto, AbbVie Inc., Chicago, IL, USA) is a first-in-class BCL-2 inhibitor that blocks BCL-2 signalling and induces apoptosis [79]. Single-drug venetoclax treatment was initially approved for CLL patients with 17p deletion in 2016 [80]. It should be administered continuously when given as a single agent. The more commonly used option is time-limited treatment with venetoclax combined with CD20 antibodies or BTKis [81,82,83,84]. Such regimens can induce undetectable MRD at the end of treatment, which is an independent predictor of improved OS in patients with TN or RR CLL/SLL.
In the phase 3 MURANO trial, treatment with fixed-duration venetoclax plus rituximab (VR) for up to 2 years demonstrated durable remissions in patients with RR CLL [70]. VR resulted in superior PFS and OS compared with BR. In the final analysis, with a median follow-up of 7 years, the median PFS was 54.7 months with VR and 17.0 months with BR. Among 25 patients retreated with VR, the median PFS was 23 months, and the best ORR was 72% [70].
In 2019, the FDA approved venetoclax in combination with obinutuzumab (VenO) for TN CLL based on the CLL14 phase 3 randomised trial, comparing VenO with ChlO in 432 TN patients with coexisting conditions [84]. Significantly higher PFS was observed at 24 months for patients who received VenO (82.2%) compared with those who received ChlO (64.1%) (HR 0.33; p < 0.0001). VenO was also more beneficial in high-risk subgroups, including patients with TP53 deletion and those with umIGHV. A six-year follow-up confirmed that fixed-duration VenO offers greater long-term PFS than ChlO treatment [71]. At a median observation time of 76.4 months, median PFS was 76.2 months for VenO and 36.4 months for ChlO (HR, 0.40; p < 0.0001). Additionally, over 60% of patients treated with VenO did not require second-line treatment. In the VenO arm, del (17p) and umIGHV were independent prognostic factors for shorter PFS. These findings confirm that the fixed-duration VenO regimen is an effective option for previously untreated patients with CLL.
4.2. Combination of BTKi and Venetoclax
The efficacy and tolerability of venetoclax combined with BTK inhibitors have also been established, offering another fixed-duration alternative for patients with CLL [39,85,86,87,88,89,90]. In the CAPTIVATE phase 2 study, 159 patients aged ≤70 years with previously untreated CLL received three one-month cycles of ibrutinib, followed by 12 cycles of ibrutinib plus venetoclax (I + V) [85]. The CR rate was 55%, with the best undetectable MRD rates being 77% in PB and 60% in BM; the 24-month PFS and OS rates were 95% and 98%, respectively. The most common grade 3 or higher AEs were neutropenia (33%) and hypertension (6%).
The phase 3 GLOW study compared I + V with ChlO in 211 previously untreated patients with CLL [86,87,88]. The patients’ median age was 71 years, and the median CIRS score was 9. At a median follow-up of 27.7 months, PFS events were observed in 22 I + V patients and 67 ChlO patients. At a 54-month follow-up, the median follow-up PFS rates were 65.8% for I + V and 19.1% for ChlO. In the I + V arm, patients with umIGHV demonstrated shorter PFS (58%) than those with mIGHV (90%). At 54 months, the I + V arm also achieved better estimated OS (84.5%) than the ChlO arm (63.1%).
The GAIA/CLL13 study compared CIT with FCR or BR with VR, VenO, or VenO plus ibrutinib [39]. At month 15, the VenO (86.5%) and VenO plus ibrutinib (92.2%) groups were significantly more likely to present undetectable MRD than the FCR or BR group (52.0%). No significant difference was noted between the VR group and the CIT arm (57%). PFS at three years was higher in the VenO plus ibrutinib group (90.5%) and VenO group (87.7%) than in the CIT group (75.5%) or the VR group (80.8%) [39].
The FLAIR phase 3 randomised trial compared I + V and ibrutinib with FCR in TN CLL [91]. The duration of I + V treatment was related to MRD status: at a median of 43.7 months, disease progression was observed in 12 patients in the I + V arm and 75 patients in the FCR arm (p < 0.001). Nine deaths were noted in the I + V group and 25 in the FCR group. At three years, 58.0% of the patients in the I + V arm had stopped therapy due to undetectable MRD. These findings support using VenO or I + V combinations in the first-line treatment of patients with CLL.
Acalabrutinib plus venetoclax, with or without obinutuzumab, was also compared with CIT in previously untreated patients in the AMPLIFY phase 3 study [92]. Patients were randomly assigned to receive acalabrutinib plus venetoclax, acalabrutinib plus venetoclax and obinutuzumab, or CIT with FCR or BR. The estimated 36-month PFS rates at a median follow-up of 40.8 months were 76.5%, 83.1%, and 66.5%, respectively (p = 0.004). The estimated 36-month OS rates were 94.1%, 87.7%, and 85.9%.
The arm D SEQUOIA study evaluated zanubrutinib in combination with venetoclax in patients with TN CLL/SLL with del (17p) [93]. The early result included 35 of approximately 80 planned patients with a median follow-up of 9.7 months. The initial efficacy assessment three months after starting zanubrutinib indicated an ORR of 96.8% (30/31 patients). Zanubrutinib plus venetoclax was well tolerated, and no new safety signals were reported.
4.3. Second-Generation BCL-2 Inhibitors
New BCL-2 inhibitors active in lymphoid neoplasms are currently under investigation. The most advanced in clinical trials include sonrotoclax (BGB-11417), lisaftoclax (APG-2575), ABBV-453, and mesutoclax (ICP-248) [74,94,95,96,97].
4.3.1. Sonrotoclax
Sonrotoclax (BGB-11417, BeOne Medicines, Cambridge, MA, USA) is a more selective and more pharmacologically potent inhibitor of BCL-2 than venetoclax. Moreover, it has a shorter half-life and does not accumulate in the body observed [94]. Moreover, sonrotoclax demonstrated significantly greater efficacy in xenograft mouse models and superior activity compared to venetoclax in vitro, in inhibiting both WT Bcl-2 and the G101V mutant. Currently, sonrotoclax is undergoing clinical evaluation as a monotherapy and in combination with other drugs. Sonrotoclax was investigated in combination with zanubrutinib in TN patients with CLL [73]. Among 108 evaluated patients, the ORR was 100% (CR 41% for the 160 mg dose and 42% for the 320 mg dose). No progression was observed at the 320 mg dose. Treatment was well tolerated, with no cases of laboratory or clinical TLS, and only one patient discontinued treatment due to a TEAE. A phase 3 study (CELESTIAL-TNCLL, BGB-11417-301) with this combination is currently ongoing (#NCT06073821). A single-arm, open-label, multicentre phase 2 clinical trial is assessing the efficacy of sonrotoclax in 100 adult patients with RR CLL/SLL in China. The estimated study completion date is in 2027. In parallel, sonrotoclax is also being studied with zanubrutinib in three clinical trials (#NCT06637501, #NCT06073821, and #NCT06367374).
4.3.2. Lisaftoclax
Lisaftoclax (APG-2575, Ascentage Pharma, Suzhou, China, and Rockville, MD, USA) is a novel, specific BCL-2 inhibitor active in patients with TN and RR CLL/SLL, including those with 17p deletion and progressive disease after BTKi therapy [98]. Lisaftoclax used alone or in combination with acalabrutinib or rituximab had a manageable safety profile and was active in patients with previously untreated or RR CLL/SLL. In the first-in-human open-label trial in patients with RR CLL, lisaftoclax treatment was associated with an ORR of 63.6% and a rapid reduction in absolute lymphocyte count, even at the lowest doses, with response on the first day of dosing. In patients with CLL/SLL and 17p deletion, an ORR was 66.7% [74]. Lisaftoclax combined with acalabrutinib or rituximab also had a manageable safety profile and was active in patients with previously untreated or RR CLL/SLL [99]. Several ongoing studies evaluate the efficacy and safety profiles of lisaftoclax in CLL and other haematological malignancies. A global multicentre, open-label, randomised, registrational phase 3 clinical trial is currently recruiting approximately 400 participants with CLL who have been previously treated with BTKis to assess the safety and efficacy of lisaftoclax in combination with BTKis. The estimated study completion date is 2027, with an anticipated primary completion date in October 2025 (#NCT06104566). Another global, multicenter, randomised, open-label, phase 3 confirmatory trial is also recruiting participants with newly diagnosed CLL to investigate the safety and efficacy of lisaftoclax in combination with acalabrutinib. This study is expected to be completed in 2028 (#NCT0502404528).
4.3.3. Surzetoclax
Surzetoclax (ABBV-453, AbbVie Inc., Chicago, IL, USA) is a next-generation BCL-2 inhibitor [93]. The activity of ABBV-453 as a monotherapy was investigated in an in vivo subcutaneous xenograft model of CLL. The study demonstrated superior growth inhibition of the RS4;11 xenograft compared with sonrotoclax or lisaftoclax at equivalent doses and schedules [75]. ABBV-453 has the potential to be the best-in-class next-generation BCL-inhibitor and is actively being investigated in phase 1 clinical trials in RR CLL (#NCT06291220) and RR multiple myeloma (#NCT05308654).
4.3.4. LOXO-338
LOXO-338 (Eli Lilly, Indianapolis, IN, USA) is a novel inhibitor of BCL-2, designed to inhibit Bcl-2 over Bcl-xL selectively and to avoid dose-limiting thrombocytopenia associated with Bcl-xL inhibition [94]. A phase 1 clinical trial tests the safety and effectiveness of LOXO-338 in patients with advanced B-cell malignancies, including CLL, who have already received standard treatment therapy (#NCT05024045) [76]. In total, 27 patients were enrolled, including 10 with CLL/SLL and 17 with NHL. The ORR was 19%, and disease was controlled in 67%. TEAEs were reported in 23 patients (85%), including anaemia (22%) and fatigue (22%). Serious TEAEs and TLS were not observed [76].
4.3.5. Mesutoclax
Mesutoclax (ICP-248, InnoCare Pharma, Beijing, China) is a novel, orally bioavailable BCL-2 selective inhibitor. In a phase 1 study, 68 patients were enrolled in the dose escalation and expansion study (#NCT05728658) [78]. Among the BTK-naïve patients, the ORR for RR CLL/SLL and RR MCL patients was 100%, including CR 14.3% for RR CLL/SLL and 71.4% for RR MCL. In 43% of MCL patients, undetectable MRD was reported. Among the BTK-treated patients, the ORR for RR CLL/SLL and RR MCL patients was 100% and 78.9%, respectively, and the CRR were 30.0% and 26.3%, respectively, of which uMRD was reported in 20% of CLL/SLL and 16% of MCL patients. The median PFS of RR MCL patients who had previously received treatment with BTKis was 8.3 months. The PFS was not reached among BTK-naïve RR CLL/SLL and RR MCL patients and BTK-treated RR CLL/SLL patients. In another dose optimisation study in patients with TN CLL/SLL, mesutoclax plus orelabrutinib treatment is guided by MRD status (#NCT06378138). The preliminary efficacy and safety data of mesutoclax + orelabrutinib for CLL/SLL were recently presented (ICP-CL-01203/#NCT06378138) [77]. The patients are randomly assigned 1:1 for treatment with mesutoclax (100 mg or 125 mg once daily, cycles 3–14) after two cycles of orelabrutinib (150 mg once daily, cycles 1–17). Mesutoclax was started with a 5-week ramp-up schedule to the target doses (5/10 mg, 25 mg, 50 mg, 75 mg, 100/125 mg) to control TLS. Orelabrutinib is administered continuously for undetectable MRD at cycle 17. In the first report, 42 patients were included. The median treatment duration was 7.5 months; all patients were on treatment. The ORR was 100%, and an early high uMRD rate at 12 weeks of target therapy was observed in patients receiving the 125 mg dose. No progression was seen in either cohort. No clinical or laboratory TLS occurred. The most common TEAEs were neutropenia, thrombocytopenia, and upper respiratory tract infections. A phase 3 registration study is planned to evaluate the efficacy and safety of the combination of orelabrutinib and mesutoclax at 125 mg QD.
5. PI3K Inhibitors
Phosphoinositide 3-kinases (PI3Ks) signalling regulates growth, survival, differentiation, activation, and apoptosis of B and T cells. PI3K inhibitors have been extensively studied in many cancers, including B-cell malignancies and CLL [97,98]. For RR CLL patients with limited options, PI3K inhibitors like idelalisib combined with rituximab may be considered [100]. However, the toxicity of this treatment is high [100,101]. The FDA approved three PI3K inhibitors, idelalisib, duvelisib and umbralisib, to treat CLL and/or other lymphoid malignancies (Table 4).

Table 4.
PI3K inhibitors approved or potentially effective for treating CLL.
5.1. Idelalisib
Idelalisib (GS-1101, CAL-101, Zydelig, Gilead Sciences, Inc., Foster City, CA, USA) was the first-in-class PI3Kδ inhibitor registered for treating RR CLL. In a multicentre, phase 3 randomised trial conducted in patients with RR CLL, idelalisib and rituximab were compared to rituximab alone [102]. This study was stopped early due to the advantage of the idelalisib-treated arm over the control arm. After a median follow-up of 18 months, PFS was 20.3 months in the rituximab plus idelalisib arm and 6.5 months in the rituximab arm. However, the toxicity of this treatment was high [122]. Idelalisib causes fatigue, diarrhoea, nausea, chills, and skin changes, which are mostly low-grade in severity. Also, like other PI3K inhibitors, idelalisib can cause hepatotoxicity, diarrhoea, colitis, skin changes, and infections.
5.2. Duvelisib
Duvelisib (IPI-145, INK1197, Copiktra, Secura Bio, Inc., Las Vegas, NV, USA) is a selective dual inhibitor of Pi3kδγ. In the phase 2 DYNAMO trial (#NCT01476657), among 28 patients with CLL/SLL refractory to rituximab and chemotherapy or radioimmunotherapy, the ORR was 68%, and all were PR [103]. In a phase 3 DUO trial (#NCT02004522), duvelisib was compared with ofatumumab in patients with RR CLL/SLL [123]. The PFS was significantly longer in patients treated with duvelisib (13.3 months) than in the ofatumumab arm (9.9 months). The ORR was also higher in the duvelisib group (74% vs. 45%) [124]. The most common non-haematological adverse events in patients treated with duvelisib were diarrhoea (51%), pyrexia (29%), nausea (23%), cough (21%) and colitis (13%). Duvelisib was approved by the FDA in 2018 and the EMA in 2021 for treating RR CLL/SLL patients after at least two prior therapies.
5.3. Umbralisib
Umbralisib (Ukoniq, TG Therapeutics, Morrisville, NC, USA) is a next-generation inhibitor of PI3Kδ and casein kinase-1ε (CK1ε) with a lower incidence of autoimmune complications [107,108,125]. In a phase 2 study, umbralisib was evaluated in 51 patients with CLL who were intolerant to prior BTKis (n = 44) or PI3Kδis (n = 7), and 48 were evaluable for response [106]. Median PFS was 23.5 months, and ORR was 44%. The most common adverse events were rash (27%), arthralgia (18%), and atrial fibrillation (16%), while the most common grade ≥ 3 AEs were neutropenia (18%), leukocytosis (14%), thrombocytopenia (12%), pneumonia (12%), and diarrhoea (8%). Umbralisib received fast-track FDA approval for the treatment of CLL in combination with an anti-CD20 antibody in 2020. However, the developer withdrew this combination for the treatment of CLL based on updated OS data from the UNITY-CLL trial in 2022.
5.4. Novel PI3K Inhibitors
Novel PI3K inhibitors investigated in patients with CLL include zandelisib, parsaclisib, BGB-10188, tenalisib, ACP-319, HMPL-689, SHC014748M, TQ-B3525, and linperlisib.
5.4.1. Zandelisib
Zandelisib (PWT143, ME-401, Mei Pharma, San Diego, CA, USA) is a selective, potent PI3Kδ inhibitor with prolonged target inhibition and a high volume of distribution, indicating high tissue exposure [109]. The drug is in clinical development for treating patients with B-cell lymphoid malignancies, including RR CLL and B-cell NHL. In a phase 1 study of zandelisib, used alone or in combination with rituximab, durable objective responses were observed in RR indolent B-cell malignancies (FL, CLL/SLL, MZL and DLBCL; #NCT02914938) [111]. The ORR was 83%, including 89% in CLL/SLL (100% in monotherapy and 83% in the combination group), with a median DOR not reached. The drug was well tolerated, with no safety differences observed between the monotherapy and rituximab combination groups. A phase II study evaluated zandelisib in RR FL and MZL [110]. The ORR was 75.4% and the CR was 24.6%. At least one Grade ≥ 3 TEAE was reported in 55.7% of patients, including transaminase elevation (8.2%), cutaneous reactions (3.3%), and diarrhoea, enterocolitis, and lung infections (1.6% each). A phase 2 trial has also been initiated combining zandelisib with rituximab and venetoclax in patients with RR CLL (CORAL) (#NCT05209308).
5.4.2. Parsaclisib
Parsaclisib (INCB50465, IBI-376, Incyte, Wilmington, DE, USA) is a highly selective PI3Kδ inhibitor. In the first-in-human phase 1/2 CITADEL-101 study, parsaclisib demonstrated antitumor activity in RR B-cell NHL (#NCT02018861) [113]. The drug was studied alone or in combination with itacitinib (Janus kinase 1 inhibitor) or CIT with rituximab, ifosfamide, carboplatin, and etoposide in patients with RR B-cell malignancies (#NCT02018861) [113]. Parsaclisib demonstrated durable responses and a manageable safety profile in RR MZL [112]. Parsaclisib is currently under evaluation in combination with the anti-CD19 monoclonal antibody tafasitamab in patients with RR CLL and RR NHL as part of a phase 1/2 study (#NCT04809467).
5.4.3. BGB-10188
BGB-10188 (BeOne Medicines, Cambridge, MA, USA) is a highly selective inhibitor of PI3Kδ, with an improved safety profile compared with other PI3Kis. BGB-10188 is being investigated as a monotherapy and in combination with zanubrutinib or PD-1 antibody tislelizumab in patients with CLL and other B-cell NHLs in a phase 1/2 clinical trial (#NCT04282018) [126].
5.4.4. Tenalisib
Tenalisib (GDC-0032, RP6530, Rhizen Pharmaceuticals SA, Basel, Switzerland) is a dual PI3K δ/γ inhibitor with demonstrated antitumor activity in a T cell leukaemia xenograft model in mice and in patient-derived primary cutaneous T cell lymphoma (CTCL) cells [115]. A phase 2 study has also been initiated investigating the efficacy and safety of tenalisib in patients with RR CLL after at least one prior therapy (#NCT04204057).
5.4.5. ACP-319
ACP-319 (AMG 319, Acerta Pharma BV, Oss, The Netherlands/AstraZeneca, Cambridge, UK) is a novel selective PI3Kδ inhibitor with promising results in early preclinical and clinical studies. The first human study of ACP-319 evaluated the drug’s safety, tolerability, and pharmacokinetics in 28 heavily pretreated patients with RR CLL (n = 25) and RR NHL [116]. Among 24 evaluable patients with CLL and 3 with NHL, all showed a lymph node reduction of more than 50% as their best response. Responses were observed in all high-risk cytogenetic subgroups of CLL. A Phase 1 study of ACP-319 combined with acalabrutinib in RR CLL is ongoing (#NCT02157324).
5.4.6. Amdizalisib
Amdizalisib (HMPL-689, HUTCHMED, Hong Kong, China) is a novel PI3Kδ inhibitor [117]. In the phase study, 75 patients with CLL and other NHL had received at least one dose of HMPL-689 [127]. The ORR was 51.7%, and the median time to response (TTR) was 1.9 months. The most common TEAEs were neutropenia, increases in ALT and AST, leukopenia, hypertriglyceridaemia, pneumonia, and upper respiratory tract infection. The most common grade 3 or higher adverse events were neutropenia, pneumonia, and rash.
5.4.7. SHC014748M
SHC014748M (Nanjing Sanhome Pharmaceutical, Nanjing, Jiangsu, China) is a potent, selective PI3Kδ isoform inhibitor that treats NHL and CLL/SLL [118]. SHC014748M demonstrated promising preclinical antitumor activity in B-cell NHL and CLL [119]. The Phase 1 study SHC014748M in patients with CLL and other indolent B-cell haematologic malignancies has been initiated (#NCT03588598).
5.4.8. TQ-B3525
TQ-B3525 (Chia Tai Tianqing Pharmaceutical Group, Lianyungang, Jiangsu Province, China) is a selective PI3Kα/δ inhibitor. TQ-B3525 has shown promising early clinical activity and a favourable safety profile. In a phase 1 study involving 27 patients with RR lymphoma and 13 patients with advanced solid tumours, the most common all-grade adverse events were hyperglycaemia (65.0%), increased glycosylated haemoglobin (35.0%), diarrhoea (32.5%), grade ≥ 3 hyperglycaemia (10.0%), and grade ≥ 3 hypertension (3.8%) [128]. In a phase II study, TQ-B3525 was investigated in 82 patients with RR FL after at least two prior therapies. The ORR was 88.0% with 34.2% achieving CR. With a median follow-up of 13.3 months, median PFS was 18.5 months and the 24-month OS rate was estimated at 86.1% [120]. TQ-B3525 showed a favourable safety profile, with Grade 3 or higher treatment-related adverse events observed in 63 (76.8%) cases, including neutropenia (22.0%), hyperglycemia (19.5%), and diarrhoea (13.4%). DLT was grade 3 hyperglycemia, observed in three patients. Among 23 lymphoma patients, the ORR was 60.9%, and the median PFS was not reached. TQ-B3525 in patients with RR CLL/SLL is ongoing (#NCT04808570).
5.4.9. Linperlisib
Linperlisib (YY-20394, Shanghai Yingli Pharmaceutical C, Shanghai, China) is a PI3Kδ inhibitor structurally distinct from idelalisib, with reduced activity against PI3Kγ. This results in a kinase inhibition profile that is more selective for Pi3kδ by nearly two orders of magnitude [121]. In a phase 1 study, 25 patients with B-cell haematological malignancies received 20–200 mg of YY-20394 daily (#NCT03757000). YY-20394 was well-tolerated with encouraging preliminary efficacy. The ORR was 64.0%, including five patients with CR, 11 with PR, and 2 with stable disease. The median PFS was 255 days. The most common drug-related adverse events included neutropenia (44.0%), pneumonia (16.0%), hyperuricemia (12.0%), lymphocytopenia (8.0%), leukopenia (8.0%), and pneumonitis (8.0%). Further development of this agent is justified.
6. Monoclonal Antibodies
Monoclonal antibody (mAb)-based therapeutic strategies have transformed the treatment of CLL. Rituximab, approved in 1997, was the first CD20-specific mAb authorised for treating lymphoid malignancies. Subsequently, a new generation of CD20 mAbs was developed and used for CLL treatment, both as monotherapy and, more commonly, in combination with chemotherapy or targeted drugs. Developing novel antibodies, immunotoxins, and bispecific antibodies might further enhance the efficacy of targeted therapies for CLL (Table 5) [129].
6.1. Novel Monoclonal Antibodies
6.1.1. Belimumab
The tumour necrosis factor (TNF) family member B-cell-activating factor (BAFF) plays a crucial role in the survival of both healthy and malignant B cells. High BAFF serum levels have been shown in patients with rheumatoid arthritis and SLE related to aberrant B cell activation [130]. Moreover, elevated levels of BAFF have also been detected in patients with B-cell lymphoma. BAFF promotes the survival of malignant B-cells. It has been linked to resistance against agents such as ibrutinib and venetoclax by inhibiting apoptosis and preserving mitochondrial integrity. Belimumab (Benlysta, GSK, London, UK), an anti-BAFF antibody, was approved for treating patients with SLE and is currently being evaluated in the BeliVeR trial (#NCT05069051). This study assesses whether adding belimumab to rituximab and venetoclax proves more effective than using rituximab and venetoclax alone in patients with RR CLL.

Table 5.
Novel monoclonal antibodies investigated in CLL.
Table 5.
Novel monoclonal antibodies investigated in CLL.
| Drug | Characteristics | Key Clinical Trials in CLL | Reference |
|---|---|---|---|
| Belimumab (Benlysta, GSK London, UK) | Recombinant human IgG-1λ mAb that inhibits BAFF | BeliVeR trial evaluated whether adding belimumab rituximab and venetoclax is more effective than using rituximab and venetoclax alone in RR CLL | #NCT05069051 |
| Tafasitamab-cxix (MOR00208, MONJUVI, MorphoSys US Inc., Boston, MA, USA) | Humanised CD19 mAb with an engineered Fc region to enhance Fcγ receptor binding affinity | Phase 1 trial demonstrated efficacy and an acceptable safety in RR CLL [131] Phase II COSMOS study evaluated tafasitamab combined with idelalisib or venetoclax in patients with RR CLL/SLL previously treatedwith BTKi [132] | [131,132] |
| CAP-100 (Catapult Therapeutics, Lelystad, The Netherlands) | Humanised IgG1 mAb against CCR7, with potential immunomodulating and antineoplastic activity | Phase 1b (CAP-100-1) study evaluated safety and preliminary activity of CAP-100 in RR CLL | #NCT04704323 |
| Cirmtuzumab (UC-961, University of California, San Diego, CA, USA) | Humanised mAb that inhibits the signalling of the ROR1 | Phase 1 study of cirmtuzumab alone in RR CLL [133] Phase 1/2 study of cirmtuzumab + ibrutinib in RR MCL and RR or TN CLL [134] | [133,134] #NCT02222688, #NCT03088878 |
| Zilovertamab vedotin (MK-2140, VLS-101, Merck & Co., Rahway, NJ, USA) | ADC that inhibits the signalling of the ROR1 | Phase 1/2 study evaluated zilovertamab vedotin with ibrutinib in patients with MCL and CLL | [135] #NCT03088878 |
Abbreviations: ADC—antibody-drug conjugate; BAFF—B-cell activating factor; BTKi—BTK inhibitor; CCR7—chemokine receptor 7; CLL—chronic lymphocytic leukemia; CR—complete response; IKZF—IKAROS family zinc finger; mAb—monoclonal antibody; MCL—mantle cell lymphoma; ROR1—receptor tyrosine kinase-like orphan receptor 1; RR—relapsed and refractory; TN—treatment-naïve.
6.1.2. Tafasitamab
Tafasitamab-cxix (MOR00208, MONJUVI, MorphoSys US Inc., Boston, MA, USA) is a humanised CD19 monoclonal antibody with an engineered Fc region to enhance Fcγ receptor binding affinity. A phase 1 trial demonstrated preliminary efficacy and an acceptable safety profile of MOR00208 in patients with RR CLL [131]. In a phase IIa study, MOR00208 monotherapy demonstrated promising clinical activity in patients with RR B-cell NHL, including DLBCL and FL, and an acceptable safety profile [136]. Responses were noted in 10 (22%) of 46 patients refractory to rituximab. In a phase II COSMOS study, tafasitamab-cxix was combined with idelalisib or venetoclax in patients with RR CLL/SLL receiving a BTKi therapy or intolerant of such therapy [132].
The combination of tafasitamab-cxix with venetoclax or idelalisib was well tolerated and showed promising antitumour activity in patients with RR CLL who had previously discontinued treatment with ibrutinib. Among the 24 patients included, 11 received idelalisib and 13 received venetoclax. The most common adverse events were anaemia, neutropenia, and infusion-related reactions. The highest ORR was 90.9% in patients receiving idelalisib and 76.9% in those receiving venetoclax. In 2020, the FDA granted accelerated approval to tafasitamab-cxix, in combination with lenalidomide, for patients with RR DLBCL not otherwise specified, arising from low-grade lymphoma, who are not eligible for autologous stem cell transplant. In 2025, approval was granted for patients with RR FL, along with lenalidomide and rituximab lymphoma.
6.1.3. CAP-100
CAP-100 (Catapult Therapeutics, Lelystad, The Netherlands) is a humanised immunoglobulin G1 (IgG1) mAb against CC-chemokine receptor 7 (CCR7), with potential immunomodulating and antineoplastic properties. It blocks the ligand-binding site and signalling of CCR7 [137]. In a preclinical study, CAP-100 inhibits CCR7-induced migration, extravasation, homing, and survival in CLL samples [138,139]. CAP-100 triggers potent tumour cell killing, mediated by host immune mechanisms. CCR7 expression is down-modulated in CLL patients treated with ibrutinib and venetoclax [140]. Phase 1b of the trial (expansion phase) evaluated the safety and preliminary clinical benefit of CAP-100 monotherapy to support the design of future trials investigating CAP-100 either as monotherapy or in a combination setting with approved treatments for CLL. This phase 1b (#NCT04704323) was initiated and showed a favourable toxicity profile. CAP-100 and ibrutinib have complementary non-overlapping mechanisms of action, potentially allowing for combination therapy [141].
6.1.4. ROR1 Inhibitors
ROR1 is an oncofetal protein expressed on the surface of CLL cells but is mostly absent on normal B cells [142,143,144]. For patients with CLL, self-renewing, neoplastic B cells express ROR1 in 95% of patients. High-level leukemia cell expression of ROR1 is associated with a poor prognosis. ROR1 acts as a receptor for Wnt5a, which can stimulate ROR1-dependent activation of Rho-GTPases in leukemia cells [143]. Inhibition of ROR1 signaling may have therapeutic activity in patients with CLL and other neoplasms [133]. Two mAbs that inhibit the ROR1 onco-embryonic kinase-like receptor were investigated in CLL: cirmtuzumab and zilovertamab vedotin (ZV).
Cirmutuzumab
Cirmtuzumab (UC-961, Pharmacyclics LLC, Sunnyvale, CA, USA/University of California, San Diego, CA, USA/California Institute for Regenerative Medicine, Oakland, CA, USA) is a humanised mAb that inhibits ROR1 signalling stemness signatures [133]. In a phase 1 study, 26 patients with RR CLL received four biweekly infusions of cirmtuzumab at doses ranging from 15 μg/kg to 20 mg/kg (#NCT02222688) [133]. Cirmtuzumab was well tolerated without dose-limiting toxicity (DLT) or serious AEs). Overall, 22 patients were evaluated for response but did not meet criteria for CR or PR. However, the median time to next treatment (TTNT) after CLL progression was 8.6 months. These results suggest that cirmtuzumab is a safe inhibitor of ROR1/Wnt5a signalling, suitable for further studies both alone and in combination with other agents. Cirmtuzumab in combination with ibrutinib was evaluated in a phase 1/2 study in patients with RR MCL and TN or RR CLL (#NCT03088878) [134]. In 17 evaluable patients with MCL, ORR was 82% and CR 41%, with response durations ranging from 8 to 28 months. In 34 evaluable patients with CLL, an ORR of 91% and a CR of 3% were achieved. The estimated 24-month PFS was 95% for RR patients and 87% for TN patients. The most common all-grade TEAEs in MCL and CLL patients included diarrhoea (41%), contusion (39%), fatigue (39%), urinary infection (31%), hypertension (25%), and arthralgia (23%) [134].
Zilovertamab Vedotin
Zilovertamab vedotin (MK-2140, also known as VLS-101, Merck & Co., NJ, USA, ZV) is an antibody-drug conjugate (ADC) that inhibits the signalling of the ROR1 receptor [142]. ZV consists of a fully humanised mAb (UC-961), a proteolytically cleavable amidocaproyl-valine-citrulline-para-aminobenzoate linker (mc-vc-PAB), and a monomethyl auristatin E (MMAE) cytotoxin. In preclinical studies, ZV inhibited Wnt5a-enhanced invasiveness of CLL cells. Moreover, the combination of zanubrutinib and ZV showed an additive effect in inhibiting matrigel invasiveness of CLL cells [145]. These results support the use of combined ZV and zanubrutinib in treating patients with CLL. In a phase 1 study, no significant clinical AEs were observed, and objective tumour responses were seen in 47% of heavily pretreated patients with MCL, including 4 PR and 3 CR, and in 3 of 5 patients with DLBCL (60%; one PR and two CR). However, objective responses were not observed in patients with CLL and other lymphoid malignancies. In heavily pretreated patients, ZV showed no unexpected toxicities and demonstrated evidence of antitumour activity, providing clinical proof of concept for the selective targeting of ROR1 as a potential new approach to cancer therapy (#NCT03833180) [146]. In a phase 1/2 study (#NCT03088878), ZV was evaluated in combination with ibrutinib in patients with MCL and CLL [135]. This combination was well tolerated and showed promising clinical responses in patients with MCL and CLL. For patients with R/R MCL treated with zilovertamab plus ibrutinib, the ORR was 89.3%, CRR 43%, median duration of response (DOR) was 34.1 months, and the OS rate at 25 months was 70%. In patients with RR CLL and a median of 2 previous lines of treatment, ibrutinib alone induced an ORR of 91.2% (CR rate, 8.8%). In patients treated with zilovertamab plus ibrutinib, ORR was 93.8% and CR 0%, with PFS at 24 months being 95%. For patients with TP53 mutations/del (17p), the response rate was 100% at 42 months [135].
7. T-Cell Engagers
T-cell engagers are a type of antibody that simultaneously bind to T-cells and a tumour-specific antigen on B-cells, leading to their destruction. This approach is a promising therapeutic strategy for hematological malignancies, especially in RR B-cell malignancies, including CLL [147]. Bispecific antibodies (BsAb) are more advanced in development and some have been approved for treating B-cell NHL. They have been approved for RR FL (mosunetuzumab) and RR DLBCL (glofitamab). Epcoritamab, a CD3×CD20 BsAb, is approved as a single agent for RR DLBCL and FL [148,149]. Various BsAbs targeting molecules CD3 and CD20 are currently under investigation for CLL (Table 6). However, the use of BsAbs in CLL/SLL remains experimental. More than 10 trials investigating BsAbs targeting CLL are ongoing [150].

Table 6.
T-cell engagers examined in CLL.
7.1. Epcoritamab
Epcoritamab (Tepkinly, AbbVie Inc., Chicago, IL, USA) was investigated as monotherapy in 40 patients with heavily pretreated RR CLL in the EPCORE CLL-1 study (#NCT04623541) [151]. All patients had prior BTKi therapy, and most exhibited high-risk disease characteristics. The ORR was 67%, including 33% CR. The median PFS was 12.8 months, and the median OS was not reached. Additionally, 9 out of 12 (75%) evaluable responders had undetectable MRD. The most common adverse events were cytokine release syndrome (CRS) (96%), diarrhoea (48%), peripheral oedema (48%), fatigue (43%), and injection-site reactions (43%). Kater et al. treated seven patients with RR CLL using epcoritamab administered subcutaneously. The most frequently reported TEAEs were CRS (100%), fatigue (71%), injection-site reactions (43%), and nausea (43%). Responses were observed in 3 of 5 pts [152]. The cytotoxicity of epcoritamab in CLL is enhanced by concurrent BTK or BCL-2 targeting [153]. Epcoritamab is being tested in a phase 1/2 trial (AETHER) evaluating the safety and efficacy of 2 regimens that combine epcoritamab and venetoclax in patients with RR CLL/SLL (#NCT05791409). The study is estimated to be completed in 2032 (#NCT04623541).
7.2. Mosunetuzumab
Mosunetuzumab (Lunsumio, BTCT4465A, GO29781, Roche, Basel, Switzerland) is another CD3×CD20 humanized BsAb approved for FL and investigated in patients with RR RT [154]. Fixed-duration mosunetuzumab monotherapy showed activity-induced ORR in 40% of patients and CR in 20%, with an acceptable safety profile in 20 patients with RR RT. An open-label phase 1/2 dose-escalation study is evaluating the safety, efficacy, and pharmacokinetics of escalating doses of mosumetuzumab alone and in combination with atezolizumab (anti-PD-L1) in patients with RR CLL and B-cell NHL and is ongoing (#NCT02500407). A phase 1b open-label, multicenter clinical trial is evaluating the safety, efficacy, and pharmacokinetics of mosunetuzumab alone and in combination with venetoclax in patients with RR CLL has also been initiated (#NCT05091424). The estimated completion date of the study is 2030.
7.3. Glofitamab
Glofitamab (Columvi, Roche, Basel, Switzerland) is a CD20×CD3 bispecific antibody that engages and redirects T cells to eliminate B cells [155]. Fixed-duration glofitamab monotherapy can lead to durable CR and has a manageable safety profile in RT patients. Carlo-Stella et al. treated 11 heavily pretreated RT patients (median of three prior therapies) with glofitamab monotherapy [156]. The ORR was 63.6% and the CR rate was 45.5%. CRS was mostly low grade, occurring in 72.7% of patients, and glofitamab-related neurological adverse events were observed in five patients. No fatal adverse events or those leading to discontinuation were reported. In another study, glofitamab is being evaluated alone or in combination with polatuzumab vedotin, pirtobrutinib, or atezolizumab as a potential treatment for RT (#NCT06043674).
7.4. Plamotamab
Plamotamab (XmAb-13676, Xencor, Inc., San Diego, CA, USA) is a human Fc domain-containing BsAb that binds CD3 and CD20. This agent was investigated in a phase 1 clinical trial evaluating the safety and tolerability of plamotamab in patients with CD20-expressing RR NHL (#NCT02924402) [157]. Efficacy was evaluated in 53 patients with RR NHL. The ORR was noted in 43% of them. The most common adverse event was CRS, which developed in 63% of patients. No severe or life-threatening neurotoxicity was observed.
7.5. GB261
GB261 (CND261, Genor Biopharma, Shanghai, China) is the first highly differentiated CD20×CD3 bispecific T cell engager designed to maintain Fc effector function, i.e., antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) to kill the target cell [163]. Furthermore, the “imbalanced” design of GB261 incorporates de-tuned CD3 binding to reduce CRS incidence and enhance the safety features of the Fc effector function. Extensive preclinical studies have demonstrated that GB261 offers a highly advantageous balance of safety and efficacy. In an ongoing first-in-human dose-escalation study conducted in heavily pretreated B-NHL patients, GB261 exhibited a favourable safety profile, with CRS being mild, transient, and less frequent than in other CD20×CD3 BsAbs. In the first-in-human dose-escalation phase 1 study, 22 patients were available for efficacy assessment. The median follow-up duration was 4.5 months, and the ORR was 73% (16/22), including a CR rate of 45.5% (10/22) CR [158]. A continuation of this study in B-cell NHL and CLL, covering phase 2a and phase 2b, is ongoing (#NCT04923048).
7.6. Other T-Cell Engagers Investigated in CLL
BsAbs targeting molecules other than CD3 and CD20 are also being investigated in clinical trials for CLL.
7.6.1. NVG-111
NVG111 (NovalGen, London, UK) is a humanised, first-in-class tandem scFv, ROR1xCD3 bispecific antibody that binds a unique epitope on the Frizzled domain of ROR1 and redirects T cell activity via the CD3 binder. It promotes optimal T cell interaction and efficient destruction of tumour target cells while minimising cytokine production [159]. In the first-in-human Phase 1 clinical trial of NVG111 (#NCT04763083), 12 patients with RR CLL and MCL were included, and 11 were evaluated for efficacy. Eight patients received NVG-111 combined with ibrutinib, while others received NVG-111 alone. Clinical responses were observed in six (55%) patients; the median PFS was 18.7 months. Moreover, three CLL patients achieved undetectable MRD in PB [159].
7.6.2. JNJ-75348780
NJ-75348780 (Johnson & Johnson, New Brunswick, NJ, USA) is a human bispecific antibody containing two binding sites, one for the tumour-associated antigen (TAA) CD22 and one for the T-cell surface antigen CD3 [160]. CD22 is often overexpressed in B-lymphoid malignancies. A Phase 1 study of JNJ-75348780 in patients with NHL and CLL was initiated in 2020 (#NCT04540796), and the results should be available this year.
7.6.3. AZD5492
AZD5492 (AstraZeneca, Cambridge, UK) is a novel, first-in-class, humanised, asymmetric trispecific monoclonal IgG1 antibody. It features two Fab binding domains to CD20, one VHH binding domain to the T-cell receptor, and one VHH binding domain to a CD8 co-receptor. Preclinical evaluation of AZD5492, a new CD8-guided T cell engager for B-cell NHL indications, shows that AZD5492 preferentially engages CD8+ T cells via CD8/TCR binding. This leads to the formation of an immunological synapse with CD20+ target cells, resulting in T-cell activation and the killing of target B cells [164]. AZD5492 kills B cells through preferential engagement of CD8+ T cells and reduced CD4+ T cell activation and, as a consequence, cytokine production. In NSG humanised mice with engrafted B-cell tumours, AZD5492 showed dose-dependent anti-tumour activity, similar to conventional bivalently linked CD20×CD3 T-cell engagers with significantly lower systemic cytokine production. In cynomolgus monkeys, AZD5492 was well tolerated and caused a significant and sustained reduction in CD19+ and CD20+ B cells in the blood and lymphoid tissues. In a phase 1/2 multicenter dose escalation and expansion study, the TITANium study, AZD5492 is being tested in patients with CD20+ mature B-cell lymphoid malignancies, including CLL (#NCT06542250) [161]. The study aims to assess the safety, efficacy, pharmacokinetics, and immunogenicity of AZD5492 (#NCT06542250).
7.6.4. C312
CC312 (Cytocares, Shanghai, China) is a trispecific T-cell engager that targets the B-cell surface antigen CD19, the T-cell antigen CD3, and the T-cell co-stimulatory molecule CD28 on T-cells, which leads to redirected T-cell cytotoxicity towards CD19-positive malignant B cells [165]. Phase 1 safety study of CC312 in patients with RR CD19-positive B-cell hematologic malignancies, including CLL and MCL, was initiated in 2023 (#NCT06037018).
7.6.5. Nebratamig
Nebratamig (GNC-035, Biokin Pharma, Chengdu, Sichuan, China) is an octavalent, tetra-specific T-cell engager that targets ROR1, PDL1, 4-1BB, and CD3 [162]. Nebratamig binds to CD3 T-cells to redirect them to malignant B cells expressing ROR-1. The GNC-035 can also redirect T-cell cytotoxicity towards PDL1 high-expressing cells, demonstrating its potential to convert cancer cell adaptive resistance into drug sensitivity. Additionally, the GNC-035 can engage 41-BB in a non-cytolytic manner. GNC-035 is currently being evaluated as a single agent in clinical trials enrolling patients with various ROR1-positive hematopoietic malignancies and solid tumours. An open-label, multicentre, phase Ib/II clinical trial was conducted to assess the safety, tolerability, pharmacokinetics/pharmacodynamics, and antitumour activity of nebratamig in patients with RR CLL and other haematological malignancies (#NCT05944978).
8. Chimeric Antigen Receptor T-Cell Therapy
Chimeric Antigen Receptor T (CAR) T-cell therapy is a therapeutic approach that involves the adoptive transfer of T cells with anti-tumour activity to the patient, inducing tumour regression [166,167,168]. In this treatment, T-cells collected from a patient are ex vivo modified to express an engineered chimeric antigen receptor against a specific antigen on tumour cells. The genetically modified CAR-T cells are expanded ex vivo and then infused into the patient, where they eliminate target neoplastic cells. The most commonly used target antigen in CLL is CD19, which is highly expressed and relatively specific for this leukaemia [147]. CD19-targeted CAR T-cells are approved for DLBCL patients [169]. In a retrospective analysis of CLL patients treated with CD19-targeted CAR-T cells, ORR was 70% and a CR rate was 30%, with a median PFS of 12 months [167]. These findings highlight the potential of CAR-T cell therapy in improving outcomes for patients with CLL and its aggressive complications [169].
Second-generation CAR T-cell therapies, such as lisocabtagene maraleucel (liso-cel; Breyanzi), are very effective treatments for B-cell neoplasms, including CLL. However, CLL’s responses to these cell-based treatments were lower than those in other indolent B-cell lymphoma diseases [170]. CAR T-cells can be particularly useful in patients with disease progression post-BTK and BCL2 inhibitors. Of the CD19 CAR T-cells currently approved for clinical use in B-cell malignancies, only brexucabtagene autoleucel (Brexu-cel), and lisocabtagene maraleucel (Liso-cel) have been investigated in CLL [159,160,161,162,163,164,165,166,167,168].
8.1. Lisocabtagene Maraleucel
Liso-cel,(Breyanzi, Bristol Myers Squibb, Princeton, NJ, USA) an autologous CD19-directed CAR T-cell therapy, was studied in patients with RR CLL/SLL in the TRANSCEND CLL 004 study [171,172]. The response rate was 44%, including 20% of CR. It has been recently shown that a lower disease burden correlates with a higher chance of achieving a response and that liso-cel is also effective in high-risk CLL with high-risk features [173]. CD19-directed CAR T-cell therapy can induce long-lasting responses and possibly even a cure, as shown in some patients with RR CLL. The long-term potential and clonal stability of the infused CAR T-cell were observed in CLL patients. In one study, CART cells were detectable more than ten years after infusion in two patients with sustained remission [174]. Adding ibrutinib may leverage the compound’s off-target effects, resulting in a positive overall impact on treatment outcomes. It increases the persistence of activated T cells in vivo, reduces the Treg/CD4+ T cell ratio, diminishes CLL cell immunosuppression, and enhances CD19-directed CAR T-cell expansion while decreasing CRS [175,176]. A phase 1 study administered liso-cel and ibrutinib to 19 RR CLL patients after ibrutinib failure. An 83% ORR was observed, while NGS assessed MRD negativity in BM, which was seen in 61% of patients. The 1-year PFS and OS rates were 59% and 86%, respectively [176]. The FDA has recently approved Liso-cel as the first second-generation CAR T-cell product for patients with RR CLL/SLL who have relapsed after two or more lines of therapy, including BTKis and BCL-2is. However, it has not yet been approved in Europe.
8.2. Brexucabtagene Autoleucel
Davids et al. evaluated brexucabtagene autoleucel (Brexu-cel, KTE-X19, Kite Pharmaceuticals, Inc., Santa Monica, CA, USA), a CD19-targeted CAR T-cell therapy, in 15 heavily pretreated patients with RR CLL in the phase 1 ZUMA-8 clinical trial [177,178]. At a median follow-up of 24.3 months, seven patients responded (47%), including one CR (7%). However, durable clinical responses were mainly limited to patients with low tumour burden. One grade 4 CRS and grade ≥ 3 neurologic events occurred in 3 patients (20%).
8.3. JCAR014
In the phase 1/2 study (#NCT01865617), Liang et al. treated 49 patients with JCAR014 (Juno Therapeutics, Seattle, WA, USA/Bristol-Myers Squibb/Princeton, NJ, USA), a novel CAR T-cell therapy, including 19 with concurrent ibrutinib. At a median follow-up of 79.6 months, the median PFS for all included patients was 8.9 months, and the median OS was 25.0 months (95% CI, 11.5–62.1). The 6-year PFS rate was 17.8% (95% CI, 9.7–32.8%), and the 6-year OS rate was 31.2%. A 6-year DOR was estimated at 26.4% [179].
8.4. New Generation of CAR T-Cell Therapies
A new generation of CAR T-cell therapy has shown promising efficacy and safety in a phase 1/2 study of heavily pretreated patients with CLL [180]. Third-generation CAR T-cell therapies mitigate the exhaustion observed with second-generation CAR T-cells by modifying the CAR vector, which enables enhanced and faster expansion responses [181]. Dergis et al. performed the first phase 1/2 trial evaluating third-generation CAR T-cell therapy (HD-CAR-1) targeting CD19 in 9 heavily pretreated patients with RR CLL and B-cell lymphoma (#NCT03676504) [169]. All patients were on BTKi and received at least one venetoclax-based treatment. HD-CAR-1 demonstrated high efficacy and exceptionally low treatment-specific toxicity. Six patients (67%) obtained a CR, including five (83%) with undetectable MRD. The 2-year PFS and OS rates were 30% and 69%, respectively. HD-CAR-1 products of responders contained significantly more CD4+ T cells than non-responders. In non-responders, a strong enrichment of effector memory-like CD8 + T cells with high CD39 and/or CD197 expression was observed (#NCT03676504).
CAR T-cell therapy can cause acute toxicities such as CRS and immune effector cell-associated neurotoxicity syndrome (ICANS). Additionally, secondary T-cell lymphoma is a potential complication of CAR T-cell therapy [182,183].
9. Conclusions
Over the past decade, the treatment landscape for CLL has undergone a profound shift, transitioning from traditional chemotherapy to targeted oral therapies. BTKis and venetoclax-based regimens have significantly enhanced survival outcomes and enabled time-limited, MRD-guided treatment strategies. However, CLL remains incurable, and disease resistance—particularly in double-refractory patients—poses an emerging clinical challenge.
Novel therapeutic agents, including ncBTKis, next-generation BCL-2 inhibitors, and BTKdeg, demonstrate promising activity in heavily pretreated and high-risk groups. Furthermore, immunotherapeutic approaches such as bispecific antibodies and CAR T-cell therapy offer additional options for selected patients with refractory disease.
Future CLL management will increasingly rely on personalised treatment approaches based on genetic and molecular biomarkers, MRD status, and individual risk profiles. Ongoing clinical trials are expected to expand therapeutic options further, potentially improving long-term disease control and quality of life. Although a cure remains out of reach, the rapid development of new agents moves the field closer to achieving durable, treatment-free remission in many patients.
Author Contributions
T.R. designed the study and wrote the first draft. All authors contributed to writing and reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Edward Lowczowski, a native English speaker from the Medical University of Lodz, Poland, for language assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. Guidelines for diagnosis, indications for treatment, response assessment and supportive management of chronic lymphocytic leukemia. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef]
- Teras, L.R.; DeSantis, C.E.; Cerhan, J.R.; Morton, L.M.; Jemal, A.; Flowers, C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 2016, 66, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic Lymphocytic Leukemia: 2025 Update on the Epidemiology, Pathogenesis, Diagnosis, and Therapy. Am. J. Hematol. 2025, 100, 450–480. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Krawczyńska, A.; Cebula-Obrzut, B.; Urbaniak, M.; Iskierka-Jażdżewska, E.; Robak, P. Atypical chronic lymphocytic leukemia-the current status. Cancers 2023, 15, 4427. [Google Scholar] [CrossRef]
- Baliakas, P.; Jeromin, S.; Iskas, M.; Puiggros, A.; Plevova, K.; Nguyen-Khac, F.; Davis, Z.; Rigolin, G.M.; Visentin, A.; Xochelli, A.; et al. Cytogenetic complexity in chronic lymphocytic leukemia: Definitions, associations, and clinical impact. Blood 2019, 133, 1205–1216. [Google Scholar] [CrossRef]
- Hus, I.; Giannopoulos, K.; Jamroziak, K.; Wolowiec, D.; Roliński, J.; Robak, T. Diagnostic and therapeutic recommendations of the Polish Society of Haematologists and Transfusiologists and Polish Adult Leukemia Group-CLL for chronic lymphocytic leukemia in 2025. Acta Haematol. Pol. 2025, 56, 143–172. [Google Scholar] [CrossRef]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grunhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Thompson, P.A.; Bazinet, A.; Wierda, W.G.; Tam, C.S.; O’Brien, S.M.; Saha, S.; Peterson, C.B.; Plunkett, W.; Keating, M.J. Sustained remissions in CLL after frontline FCR treatment with very-long-term follow-up. Blood 2023, 142, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Bahlo, J.; Fink, A.M.; Goede, V.; Herling, C.D.; Cramer, P.; Langerbeins, P.; von Tresckow, J.; Engelke, A.; Maurer, C.; et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016, 127, 208–215. [Google Scholar] [CrossRef]
- Robak, T. Is this the end for immunochemotherapy in relapsed/refractory chronic lymphocytic leukemia? Leuk. Lymphoma 2023, 64, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Seymour, J.F. Update on the management of relapsed/refractory chronic lymphocytic leukemia. Blood Cancer J. 2024, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Iskierka-Jażdżewska, E.; Obracaj, A.; Urbaniak, M.; Robak, T. New Treatment options for newly-diagnosed and relapsed chronic lymphocytic leukemia. Curr. Treat. Options Oncol. 2022, 23, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Zygmunciak, P.; Robak, T.; Puła, B. Treatment of double-refractory chronic lymphocytic leukemia-an unmet clinical need. Int. J. Mol. Sci. 2024, 25, 1589. [Google Scholar] [CrossRef]
- Robak, T.; Witkowska, M.; Smolewski, P. The Role of Bruton’s Tyrosine Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions. Cancers 2022, 14, 771. [Google Scholar] [CrossRef]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.P.; Owen, C.; Tedeschi, A.; Sarma, A.; Patten, P.E.; Grosicki, S.; McCarthy, H.D.; Offner, F.; et al. Final analysis of the RESONATE-2 study: Up to 10 years of follow-up of first-line ibrutinib treatment for CLL/SLL. Blood 2025, 146, 2168–2176. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef]
- Hillmen, P.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): Interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol. 2023, 24, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Perez Burbano, G.; Ruppert, A.S.; Miller, C.; Heerema, N.A.; Zhao, W.; Wall, A.; Ding, W.; Bartlett, N.L.; Brander, D.M.; et al. Follow-up from the A041202 study shows continued efficacy of ibrutinib regimens for older adults with CLL. Blood 2024, 143, 1616–1627. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef]
- Robak, T.; Stilgenbauer, S.; Tedeschi, A. Front-line treatment of CLL in the era of novel agents. Cancer Treat. Rev. 2017, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Seymour, J.F.; Byrd, J.C.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Brown, J.R.; Munir, T.; Mato, A.; et al. Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood 2023, 142, 687–699. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Šimkovič, M.; Kriachok, I.; Illés, Á.; de la Serna, J.; Dolan, S.; Campbell, P.; et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: Final ASCEND trial Results. Hemasphere 2022, 6, e801. [Google Scholar] [CrossRef]
- Shadman, M.; Munir, T.; Robak, T.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Šimkovič, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib versus bendamustine and rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma: Median 5-year follow-up of SEQUOIA. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2025, 43, 780–787. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, K.; Wang, T.; Yang, S.; Liu, L.; Hu, Y.; Zhang, W.; Ding, K.; Zhou, J.; Gao, S.; et al. Orelabrutinib in relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma patients: Multi-center, single-arm, open-label, phase 2 study. Am. J. Hematol. 2023, 98, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Witkowska, M.; Wolska-Washer, A.; Robak, T. The preclinical discovery and development of orelabrutinib as a novel treatment option for B-cell lymphoid malignancies. Expert Opin. Drug Discov. 2023, 18, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Kutsch, N.; Pallasch, C.; Tausch, E.; Böhme, V.; Ritgen, M.; Liersch, R.; Wacker, A.; Jacobs, G.; Trappe, R.U.; Dreger, P.; et al. Efficacy and Safety of the Combination of Tirabrutinib and Entospletinib With or Without Obinutuzumab in Relapsed Chronic Lymphocytic Leukemia. Hemasphere 2022, 6, e692. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.; Nayak, L.; Grommes, C. Bruton’s tyrosine kinase (BTK) inhibitors for the treatment of primary central nervous system lymphoma (PCNSL): Current progress and latest advances. Leuk. Lymphoma 2024, 65, 882–894. [Google Scholar] [CrossRef]
- Mato, A.R.; Woyach, J.A.; Brown, J.R.; Ghia, P.; Patel, K.; Eyre, T.A.; Munir, T.; Lech-Maranda, E.; Lamanna, N.; Tam, C.S.; et al. Pirtobrutinib after a Covalent BTK Inhibitor in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 389, 33–44. [Google Scholar] [CrossRef]
- Woyach, J.A.; Stephens, D.M.; Flinn, I.W.; Bhat, S.A.; Savage, R.E.; Chai, F.; Eathiraj, S.; Reiff, S.D.; Muhowski, E.M.; Granlund, L.; et al. First-in-Human Study of the Reversible BTK Inhibitor Nemtabrutinib in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia and B-Cell Non-Hodgkin Lymphoma. Cancer Discov. 2024, 14, 66–75. [Google Scholar] [CrossRef]
- Kipps, T.; Awan, F.T.; Eichhorst, B.; Herishanu, Y.; Jurczak, W.; Lavie, D.; Martinez-Calle, N.; Masszi, A.; Owen, C.; Poulsen, C.; et al. Efficacy and safety of nemtabrutinib in relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma: Cohort J of the phase 2 BELLWAVE-003 study. J. Clin. Oncol. 2025, 43, TPS7088. [Google Scholar] [CrossRef]
- Tadmor, T.; Eyre, T.A.; Benjamini, O.; Chaudhry, A.; Shen, J.; Leng, S.; Farooqui, M.Z.H.; Lavie, D. Nemtabrutinib Versus Ibrutinib or Acalabrutinib for Untreated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: The Phase 3, Open-Label, Randomized Bellwave-011 Study. Blood 2024, 144, 1875.2. [Google Scholar] [CrossRef]
- Woyach, J.A.; Brander, D.M.; Hu, B.; Rogers, K.A.; Omer, Z.; Stephens, D.M.; Sitlinger, A.; Tan, F.; Chen, Y.; Anthony, S.P.; et al. LP-168 (Rocbrutinib), a Novel Covalent and Non-Covalent Next-Generation Inhibitor of Bruton’s Tyrosine Kinase: Updates on the Phase 1 Trial and Initial Results of the CLL Gatekeeper Mutation Cohort. Blood 2024, 144, 4622. [Google Scholar] [CrossRef]
- Eichhorst, B.; Niemann, C.U.; Kater, A.P.; Fürstenau, M.; von Tresckow, J.; Zhang, C.; Robrecht, S.; Gregor, M.; Juliusson, G.; Thornton, P.; et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 1739–1754. [Google Scholar] [CrossRef]
- Quartermaine, C.; Ghazi, S.M.; Yasin, A.; Awan, F.T.; Fradley, M.; Wiczer, T.; Kalathoor, S.; Ferdousi, M.; Krishan, S.; Habib, A.; et al. Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2023, 5, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Wolska-Washer, A.E.; Robak, T. Acalabrutinib in treatment of patients with chronic lymphocytic leukemia including those at high genetic risk. Acta Haematol. Pol. 2025, 56, 172–186. [Google Scholar] [CrossRef]
- Puła, B.; Iskierka-Jażdżewska, E.; Jamroziak, K.; Giannopoulos, K.; Wróbel, T.; Robak, T.; Hus, I. Expert opinion on use of acalabrutinib for chronic lymphocytic leukemia treatment. Acta Haematol. Pol. 2024, 55, 130–136. [Google Scholar] [CrossRef]
- Majeranowski, A.; Lebiedziński, F.; Okrój, M.; Osowski, J.; Mital, A. Zanubrutinib: A novel therapeutic option for the treatment of B-cell neoplasms. Acta Haematol. Pol. 2023, 54, 53–64. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Patel, K.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Hughes, M.; et al. Acalabrutinib ± Obinutuzumab Vs Obinutuzumab + Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia: 6-Year Follow-up of Elevate-TN. Blood 2023, 142, 636. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022, 36, 1171–1175. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Hillmen, P.; Eichhorst, B.; Brown, J.R.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Kazmierczak, M.; Zhou, K.; Šimkovič, M.; et al. Zanubrutinib Versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: Interim analysis of a randomized phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 1035–1045. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; O’Brien, S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; Barr, P.M.; et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood 2019, 133, 2031–2042. [Google Scholar] [CrossRef]
- Ahn, I.E.; Tian, X.; Wiestner, A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N. Engl. J. Med. 2020, 383, 498–500. [Google Scholar] [CrossRef]
- Szmit, S.; Hus, I.; Giannopoulos, K.; Jamroziak, K.; Robak, T. Recommendations on cardiac safety during ibrutinib therapy. Acta Haematol. Pol. 2023, 54, 3–5. [Google Scholar] [CrossRef]
- Walter, H.S.; Rule, S.A.; Dyer, M.J.; Karlin, L.; Jones, C.; Cazin, B.; Quittet, P.; Shah, N.; Hutchinson, C.V.; Honda, H.; et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016, 127, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lama, T.G.; Kyung, D.; O’Brien, S. Mechanisms of ibrutinib resistance in chronic lymphocytic leukemia and alternative treatment strategies. Expert Rev. Hematol. 2020, 13, 871–883. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Puła, B. A review of resistance mechanisms to bruton’s kinase inhibitors in chronic lymphocytic leukemia. Int. J. Mol. Sci. 2024, 25, 5246. [Google Scholar] [CrossRef]
- Woyach, J.A.; Jones, D.; Jurczak, W.; Robak, T.; Illés, A.; Kater, A.P.; Ghia, P.; Byrd, J.C.; Seymour, J.F.; Long, S.; et al. Characterization of mechanisms of resistance in previously treated chronic lymphocytic leukemia (CLL) from a head-to-head trial of acalabrutinib versus ibrutinib. Hematol. Oncol. 2023, 41, 249–250. [Google Scholar] [CrossRef]
- Cool, A.; Nong, T.; Montoya, S.; Taylor, J. BTK inhibitors: Past, present, and future. Trends Pharmacol. Sci. 2024, 45, 691–707. [Google Scholar] [CrossRef]
- Sabakhtarishvili, G.; Alshebli, M.; Bajwa, O.; Tabbara, I.A. Bruton Tyrosine Kinase Degraders: Current Concepts. Am. J. Clin. Oncol. 2025, 48, 257–261. [Google Scholar] [CrossRef]
- Salvaris, R.T.; Brennan, J.; Lewis, K.L. BTK is the target that keeps on giving: A review of BTK-degrader drug development, clinical data, and future directions in CLL. Cancers 2025, 17, 557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Harris, H.M.; Chen, J.; Judy, J.; James, G.; Kelly, A.; McIntosh, J.; Tenn-McClellan, A.; Ambing, E.; Tan, Y.S.; et al. NRX-0492 degrades wild-type and C481 mutant BTK and demonstrates in vivo activity in CLL patient-derived xenografts. Blood 2023, 141, 1584–1596. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, J.; Jiang, Y.; Li, J.; Shi, W. Next-generation Bruton tyrosine kinase inhibitors and degraders in the treatment of B-cell malignancies: Advances and challenges. Ann. Hematol. 2025, 104, 3929–3941. [Google Scholar] [CrossRef]
- Thompson, M.C.; Parrondo, R.D.; Frustaci, A.M.; Allan, J.N.; Ghia, P.; Mocanu, I.; Tam, C.S.; Trotman, J.; Ahn, I.E.; Stilgenbauer, S.; et al. Preliminary efficacy and safety of the bruton tyrosine kinase degrader BGB-16673 in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma: Results from the phase 1 CaDAnCe-101 Study. Blood 2024, 144, 885. [Google Scholar] [CrossRef]
- Shah, N.N.; Omer, Z.; Collins, G.P.; Forconi, F.; Danilov, A.; Byrd, J.C.; El-Sharkawi, D.; Searle, E.; Alencar, A.J.; Ma, S.; et al. Efficacy and safety of the Bruton’s tyrosine kinase (BTK)degrader NX-5948 in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): Updated results from an ongoing phase 1a/b study. Blood 2024, 144, 884. [Google Scholar] [CrossRef]
- Robbins, D.W.; Noviski, M.A.; Tan, Y.S.; Konst, Z.A.; Kelly, A.; Auger, P.; Brathaban, N.; Cass, R.; Chan, M.L.; Cherala, G.; et al. Discovery and Preclinical Pharmacology of NX-2127, an Orally Bioavailable Degrader of Bruton’s Tyrosine Kinase with Immunomodulatory Activity for the Treatment of Patients with B Cell Malignancies. J. Med. Chem. 2024, 67, 2321–2336. [Google Scholar] [CrossRef]
- Montoya, S.; Bourcier, J.; Noviski, M.; Lu, H.; Thompson, M.C.; Chirino, A.; Jahn, J.; Sondhi, A.K.; Gajewski, S.; Tan, Y.S.M.; et al. Kinase-impaired BTK mutations are susceptible to clinical-stage BTK and IKZF1/3 degrader NX-2127. Science 2024, 383, eadi5798. [Google Scholar] [CrossRef]
- Mato, A.R.; Wierda, W.G.; Ai, W.Z.; Flinn, I.W.; Tees, M.; Patel, M.R.; Patel, K.; O’Brien, S.; Bond, D.A.; Roeker, L.E.; et al. NX-2127-001, a First-in-Human Trial of NX-2127, a Bruton’s Tyrosine Kinase-Targeted Protein Degrader, in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia and B-Cell Malignancies. Blood 2022, 140, 2329–2332. [Google Scholar] [CrossRef]
- Tees, M.T.; Bond, D.A.; Khan, N.; Awan, F.T.; Xu, Q.; Zhang, H.; Brown, G.; Woyach, J.A.; Patel, M. Aphase 1 study of AC676, a novel BTK chimeric degrader, in patients with B-cell malignancies. Blood 2024, 144, 4422.1. [Google Scholar] [CrossRef]
- Song, Y.; Lin, N.; Liu, X.; Zhou, Y.; Wu, Y.; Luo, X.; Xie, J.; Ma, X.; Hu, M.; Zhang, Y.; et al. Preclinical and first-in-human phase I study of Bruton tyrosine kinase degrader HZ-Q1070 in patients with recurrent or refractory B-cell malignancies. Blood 2024, 144, 6286. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Wu, Y.; Luo, X.; Xie, J.; Jin, X.; Li, J.; Zhou, X. Abstract 3450: Discovery and preclinical study of novel BTK degrader HZ-Q1070. Cancer Res. 2023, 83, 3450. [Google Scholar] [CrossRef]
- Chong, E.A.; Yuda, J.; Izutsu, K.; Fletcher, L.B.; Assaily, W.; Sahtout, M.; Badawi, M.; Burke, J.M.; Dean, J.P.; Stevenson, C.; et al. A first-in-human study of the potent and highly selective BTK degrader ABBV-101 in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2024, 42, TPS7091. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Wu, Y.; Luo, X.; Xie, J.; Jin, X.; Yang, H.; Jia, L.; Zhou, X. Discovery and Preclinical Study of Novel BTK Degrader HZ-Q1060. Blood 2022, 140, 4942. [Google Scholar] [CrossRef]
- Kater, A.P.; Harrup, R.; Kipps, T.J.; Eichhorst, B.; Owen, C.J.; Assouline, S.; Lamanna, N.; Robak, T.; de la Serna, J.; Jaeger, U.; et al. The MURANO study: Final analysis and retreatment/crossover substudy results of VenR for patients with relapsed/refractory CLL. Blood 2025, 145, 2733–2745. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Robrecht, S.; Zhang, C.; Olivieri, S.; Chang, Y.M.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.A.; et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized phase 3 CLL14 study. Blood 2024, 144, 1924–1935. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Cheah, C.Y.; Anderson, M.A.; Lasica, M.; Verner, E.; Opat, S.S.; Ma, S.; Weinkove, R.; Cordoba, R.; Ghia, P.; et al. Sonrotoclax and Zanubrutinib as frontline treatment for CLL demonstrates high MRD clearance rates with good tolerability: Data from an ongoing phase 1/1b Study BGB-11417-101. Blood 2024, 144, 1012. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Chen, Z.; Huang, B.; Paulus, A.; Collins, M.C.; Fu, L.T.; Li, M.; Ahmad, M.; Men, L.; Wang, H.; et al. Novel BCL-2 Inhibitor lisaftoclax in relapsed or refractory chronic lymphocytic leukemia and other hematologic malignancies: First-in-human open-label trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 2385–2393. [Google Scholar] [CrossRef]
- Phillips, D.C.; Robles-Cardona, L.; Alvey, C.; Ingram, J.; Lam, L.T.; Yang, Z.; Riehm, J.J.; Chen, J.; Kurnick, M.; Kovar, P.; et al. Abbv-453: A highly potent and selective next generation small molecule inhibitor of BCL-2. Blood 2024, 144, 4966. [Google Scholar] [CrossRef]
- Kwiatek, M.; Murthy, G.S.G.; Hoffmann, M.; Tessoulin, B.; Danilov, A.; Alencar, A.J.; Shah, N.N.; Ghesquieres, H.; Le Gouill, S.; Jurczak, W.; et al. A First-in-human phase I study of LOXO-338, an oral selective Bcl-2 inhibitor, in patients with advanced hematologic malignancies. Clin. Lymphoma Myeloma Leuk. 2025, 25, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, Z.; Liao, A.; Yu, W.; Peng, H.; Zhou, F.; Huang, X.; Cui, G.; Ge, J.; Li, F.; et al. MRD guided mesutoclax plus orelabrutinib for CLL/SLL demonstrates high response and early mrd clearance with good tolerability: Preliminary analysis from an ongoing phase II dose optimization study. In Proceedings of the European Hematology Association (EHA) 2025 Congress, Milan, Italy, 12–15 June 2025. [Google Scholar]
- YI, S.; Zhou, K.; Jin, J.; Li, Z.; Zou, L.; Li, Z.; Ge, J.; Wang, L.; Mi, J.-Q.; Yang, Y.; et al. Preliminary safety and efficacy data of ICP-248, a novel BCL2 inhibitor, in patients with relapsed or refractory B-cell malignancies. J. Clin. Oncol. 2025, 43, 7038. [Google Scholar] [CrossRef]
- Korycka-Wolowiec, A.; Wolowiec, D.; Kubiak-Mlonka, A.; Robak, T. Venetoclax in the treatment of chronic lymphocytic leukemia. Expert Opin. Drug Metab. Toxicol. 2019, 15, 353–366. [Google Scholar] [CrossRef]
- Borg, M.A.; Clemmons, A. Venetoclax: A novel treatment for patients with del(17p) chronic lymphocytic leukemia. J. Adv. Pract. Oncol. 2017, 8, 647–652. [Google Scholar]
- Soboń, A.; Drozd-Sokołowska, J.; Paszkiewicz-Kozik, E.; Popławska, L.; Morawska, M.; Tryc-Szponder, J.; Bołkun, Ł.; Rybka, J.; Pruszczyk, K.; Juda, A.; et al. Clinical efficacy and tolerability of venetoclax plus rituximab in patients with relapsed or refractory chronic lymphocytic leukemia-a real-world analysis of the Polish Adult Leukemia Study Group. Ann. Hematol. 2023, 102, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; D’Rozario, J.; Owen, C.; Assouline, S.; Lamanna, N.; Robak, T.; de la Serna, J.; Jaeger, U.; et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 2022, 140, 839–850. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]
- Niemann, C.U.; Munir, T.; Moreno, C.; Owen, C.; Follows, G.A.; Benjamini, O.; Janssens, A.; Levin, M.D.; Robak, T.; Simkovic, M.; et al. Fixed-duration ibrutinib-venetoclax versus chlorambucil-obinutuzumab in previously untreated chronic lymphocytic leukaemia (GLOW): 4-year follow-up from a multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol. 2023, 24, 1423–1433. [Google Scholar] [CrossRef]
- Munir, T.; Moreno, C.; Owen, C.; Follows, G.; Benjamini, O.; Janssens, A.; Levin, M.D.; Osterborg, A.; Robak, T.; Simkovic, M.; et al. Impact of Minimal Residual Disease on Progression-Free Survival Outcomes After Fixed-Duration Ibrutinib-Venetoclax Versus Chlorambucil-Obinutuzumab in the GLOW Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3689–3699. [Google Scholar] [CrossRef]
- Fürstenau, M.; Kater, A.P.; Robrecht, S.; von Tresckow, J.; Zhang, C.; Gregor, M.; Thornton, P.; Staber, P.B.; Tadmor, T.; Lindström, V.; et al. First-line venetoclax combinations versus chemoimmunotherapy in fit patients with chronic lymphocytic leukaemia (GAIA/CLL13): 4-year follow-up from a multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol. 2024, 25, 744–759. [Google Scholar] [CrossRef]
- Munir, T.; Hillmen, P. Chronic lymphocytic leukemia therapy guided by measurable residual disease. Reply. N. Engl. J. Med. 2024, 390, 1634–1635. [Google Scholar] [CrossRef]
- Munir, T.; Cairns, D.A.; Bloor, A.; Allsup, D.; Cwynarski, K.; Pettitt, A.; Paneesha, S.; Fox, C.P.; Eyre, T.A.; Forconi, F.; et al. Chronic lymphocytic leukemia therapy guided by measurable residual disease. N. Engl. J. Med. 2024, 390, 326–337. [Google Scholar] [CrossRef]
- Brown, J.R.; Miller, K.; Munugalavadla, V. fixed-duration acalabrutinib combinations in untreated chronic lymphocytic leukemia. Reply. N. Engl. J. Med. 2025, 392, 2181. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.N.; Helbig, D.; Mulvey, E.; Nazir, S.; Stewart, S.; Lapinta, M.L.; Rutherford, S.C.; Ruan, J.; Leonard, J.P.; Martin, P.; et al. Zanubrutinib and venetoclax as initial therapy for CLL/SLL with obinutuzumab triplet consolidation in patients with minimal residual disease positivity (BruVenG). Blood 2023, 142, 3285. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, H.; Hu, N.; Liu, Y.; Sun, H.; Yu, D.; Qin, L.; Shi, G.; Wang, F.; Xin, L.; et al. Discovery of the Clinical Candidate Sonrotoclax (BGB-11417), a Highly Potent and Selective Inhibitor for Both WT and G101V Mutant Bcl-2. J. Med. Chem. 2024, 67, 7836–7858. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Wang, Q.; Feng, Y.; Xing, H.; Yang, X.; Guo, Y.; Guo, Y.; Sun, H.; Liu, X.; et al. Sonrotoclax overcomes BCL2 G101V mutation–induced venetoclax resistance in preclinical models of hematologic malignancy. Blood 2024, 143, 1825–1836. [Google Scholar] [CrossRef]
- Tam, C.S.; Anderson, M.A.; Lasica, M.; Verner, E.; Opat, S.S.; Ma, S.; Weinkove, R.; Cordoba, R.; Soumerai, J.; Ghia, P.; et al. Combination treatment with sonrotoclax (BGB-11417), a second-generation BCL2 Inhibitor, and zanubrutinib, a Bruton tyrosine kinase (BTK) inhibitor, is well tolerated and achieves deep responses in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma (TN-CLL/SLL): Data from an ongoing phase 1/2 study. Blood 2023, 142, 327. [Google Scholar] [CrossRef]
- Deng, J.; Paulus, A.; Fang, D.D.; Manna, A.; Wang, G.; Wang, H.; Zhu, S.; Chen, J.; Min, P.; Yin, Y.; et al. Lisaftoclax (APG-2575) Is a novel BCL-2 inhibitor with robust antitumor activity in preclinical models of hematologic malignancy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 5455–5468. [Google Scholar] [CrossRef]
- Zhou, K.; Sun, M.; Wei, X.; Yi, S.; Qi, J.; Nian, W.; Cui, G.; Wang, J.; Zhang, X.; Cen, H.; et al. Updated efficacy and safety results of lisaftoclax (APG-2575) inpatients (Pts) with Heavily pretreated chronic lymphocytic leukemia (CLL): Pooled Analyses of Two Clinical Trials. Blood 2023, 142, 1900. [Google Scholar] [CrossRef]
- Davids, M.S.; Chanan-Khan, A.; Mudenda, B.; Nogaieva, L.; Kriachok, I.; Usenko, H.; Ivanov, V.; Kyselova, O.; Perekhrestenko, T.; Muzhychuk, I.; et al. Lisaftoclax (APG-2575) Safety and activity as monotherapy or combined with acalabrutinib or rituximab in patients (pts) with treatment-naïve, relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (R/R CLL/SLL): Initial data from a phase 2 global study. Blood 2022, 140, 2326–2328. [Google Scholar] [CrossRef]
- Skånland, S.S.; Brown, J.R. PI3K inhibitors in chronic lymphocytic leukemia: Where do we go from here? Haematologica 2023, 108, 9–21. [Google Scholar] [CrossRef]
- Hus, I.; Puła, B.; Robak, T. PI3K Inhibitors for the treatment of chronic lymphocytic leukemia: Current status and future perspectives. Cancers 2022, 14, 1571. [Google Scholar] [CrossRef]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final results of a randomized, phase III Study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef]
- Flinn, I.W.; Miller, C.B.; Ardeshna, K.M.; Tetreault, S.; Assouline, S.E.; Mayer, J.; Merli, M.; Lunin, S.D.; Pettitt, A.R.; Nagy, Z.; et al. DYNAMO: A Phase II Study of Duvelisib (IPI-145) in Patients With Refractory Indolent Non-Hodgkin Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; Cherry, M.A.; Maris, M.B.; Matous, J.V.; Berdeja, J.G.; Patel, M. Combination trial of duvelisib (IPI-145) with rituximab or bendamustine/rituximab in patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia. Am. J. Hematol. 2019, 94, 1325–1334. [Google Scholar] [CrossRef]
- Flinn, I.W.; O’Brien, S.; Kahl, B.; Patel, M.; Oki, Y.; Foss, F.F.; Porcu, P.; Jones, J.; Burger, J.A.; Jain, N.; et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood 2018, 131, 877–887. [Google Scholar] [CrossRef]
- Mato, A.R.; Ghosh, N.; Schuster, S.J.; Lamanna, N.; Pagel, J.M.; Flinn, I.W.; Barrientos, J.C.; Rai, K.R.; Reeves, J.A.; Cheson, B.D.; et al. Phase 2 study of the safety and efficacy of umbralisib in patients with CLL who are intolerant to BTK or PI3Kδ inhibitor therapy. Blood 2021, 137, 2817–2826. [Google Scholar] [CrossRef]
- Maharaj, K.; Powers, J.J.; Achille, A.; Mediavilla-Varela, M.; Gamal, W.; Burger, K.L.; Fonseca, R.; Jiang, K.; Miskin, H.P.; Maryanski, D.; et al. The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 2020, 4, 3072–3084. [Google Scholar] [CrossRef]
- Burris, H.A., III; Flinn, I.W.; Patel, M.R.; Fenske, T.S.; Deng, C.; Brander, D.M.; Gutierrez, M.; Essell, J.H.; Kuhn, J.G.; Miskin, H.P.; et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: An open-label, phase 1, dose-escalation, first-in-human study. Lancet. Oncol. 2018, 19, 486–496. [Google Scholar] [CrossRef]
- Moreno, O.; Wood, J. Absorption, distribution, and binding profile of ME-401, a potent and selective oral small-molecule inhibitor of phosphatidylinositol 3-Kinase δ (PI3Kδ) inanimal and B-cell lymphoma models. Target. Oncol. 2019, 14, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Munakata, W.; Kumode, T.; Goto, H.; Fukuhara, N.; Shimoyama, T.; Takeuchi, M.; Kawakita, T.; Kubo, K.; Sawa, M.; Uchida, T.; et al. A phase II study of zandelisib in patients with relapsed or refractory indolent non-Hodgkin lymphoma: ME-401-K02 study. Br. J. Haematol. 2025, 206, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zelenetz, A.D.; Reddy, N.; Jagadeesh, D.; Stathis, A.; Salman, H.S.; Soumerai, J.D.; Kenkre, V.P.; Asch, A.S.; Llorin-Sangalang, J.; Li, J.; et al. Tolerability and durable respones of the PI3Kδ inhibitor ME-401 administered on an intermittent schedule in relapsed/refractory (R/R) follicular lymphoma (FL) and other B-cell malignancies. J. Clin. Oncol. 2020, 38, 8016. [Google Scholar] [CrossRef]
- Phillips, T.J.; Avigdor, A.; Gurion, R.; Patti, C.; Corradini, P.; Tani, M.; Mehta, A.; Lossos, I.S.; Zinzani, P.L.; Thieblemont, C.; et al. A phase 2 study of the PI3Kδ inhibitor parsaclisib in relapsed and refractory marginal zone lymphoma (CITADEL-204). Blood Adv. 2024, 8, 867–877. [Google Scholar] [CrossRef]
- Forero-Torres, A.; Ramchandren, R.; Yacoub, A.; Wertheim, M.S.; Edenfield, W.J.; Caimi, P.; Gutierrez, M.; Akard, L.; Escobar, C.; Call, J.; et al. Parsaclisib, a potent and highly selective PI3Kδ inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood 2019, 133, 1742–1752. [Google Scholar] [CrossRef]
- Yang, X.; Yang, X.; Cui, X.; Su, D.; Wu, Y.; Sun, X.; Wang, J.; Bai, H.; Wei, W.; Li, J.; et al. Abstract 664: BGB-10188, a highly selective PI3Kδ inhibitor with improved safety profile and superior anti-tumor activities in vivo. Cancer Res. 2020, 80, 664. [Google Scholar] [CrossRef]
- Lewis, J.; Girardi, M.; Vakkalanka, S.; Viswanadha, S.; Bertoni, F. RP6530, a dual PI3Kδ/γ inhibitor, attenutates AKT phosphorylation and induces apoptosis in primary cutaneous T cell lymphoma (CTCL) cells. Blood 2013, 122, 4418. [Google Scholar] [CrossRef]
- Lanasa, M.; Glenn, M.; Mato, A.; Allgood, S.; Wong, S.; Amore, B.; Means, G.; Stevens, E.; Yan, C.; Friberg, G.; et al. First-In-Human Study of AMG 319, a Highly Selective, Small Molecule Inhibitor of PI3Kδ, In Adult Patients With Relapsed Or Refractory Lymphoid Malignancies. Blood 2013, 122, 678. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Dai, X.; He, J.; Liang, J.; Yu, Y.; Yu, J.; Yang, N.; Wang, L.; Cai, Y.; et al. Abstract 5454: Amdizalisib (HMPL-689), a highly selective PI3Kδ inhibitor, exhibits potent anti-tumor activity in pre-clinical B-cell lymphoma models. Cancer Res. 2022, 82, 5454. [Google Scholar] [CrossRef]
- Guo, F.; Liu, B.; Li, X.; Wang, H.; Zhu, X.; Su, Y.; He, C.; Zhu, M.; Ding, J.; Xu, Y.; et al. Mass balance, metabolic disposition, and pharmacokinetics of a novel selective inhibitor of PI3Kδ [(14)C] SHC014748M in healthy Chinese subjects following oral administration. Cancer Chemother. Pharmacol. 2023, 91, 143–156. [Google Scholar] [CrossRef]
- Fan, L.; Wang, C.; Zhao, L.; Wang, Z.; Zhang, X.; Liu, X.; Cao, L.; Xu, W.; Li, J. SHC014748M, a novel selective inhi-bitor of PI3Kδ, demonstrates promising preclinical antitumor activity in B cell lymphomas and chronic lymphocytic leukemia. Neoplasia 2020, 22, 714–724. [Google Scholar] [CrossRef]
- Wang, H.; Feng, J.; Liu, Y.; Qian, Z.; Gao, D.; Ran, X.; Zhou, H.; Liu, L.; Wang, B.; Fang, M.; et al. Phase II study of novel orally PI3Kα/δ inhibitor TQ-B3525 in relapsed and/or refractory follicular lymphoma. Signal Transduct. Target. Ther. 2024, 9, 99. [Google Scholar] [CrossRef]
- Jiang, B.; Qi, J.; Song, Y.; Li, Z.; Tu, M.; Ping, L.; Liu, Z.; Bao, H.; Xu, Z.; Qiu, L. Phase 1 clinical trial of the PI3Kδ inhibitor YY-20394 in patients with B-cell hematological malignancies. J. Hematol. Oncol. 2021, 14, 130. [Google Scholar] [CrossRef]
- Coutre, S.E.; Barrientos, J.C.; Brown, J.R.; de Vos, S.; Furman, R.R.; Keating, M.J.; Li, D.; O’Brien, S.M.; Pagel, J.M.; Poleski, M.H.; et al. Management of adverse events associated with idelalisib treatment: Expert panel opinion. Leuk. Lymphoma 2015, 56, 2779–2786. [Google Scholar] [CrossRef]
- Flinn, I.W.; Hillmen, P.; Montillo, M.; Nagy, Z.; Illés, Á.; Etienne, G.; Delgado, J.; Kuss, B.J.; Tam, C.S.; Gasztonyi, Z.; et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 2018, 132, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kuss, B.J.; Hillmen, P.; Montillo, M.; Moreno, C.; Essell, J.; Lamanna, N.; Nagy, Z.; Tam, C.S.; Stilgenbauer, S.; et al. Efficacy and Safety of Duvelisib Following Disease Progression on Ofatumumab in Patients with Relapsed/Refractory CLL or SLL in the DUO Crossover Extension Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2096–2103. [Google Scholar] [CrossRef]
- Gribben, J.G.; Jurczak, W.; Jacobs, R.W.; Grosicki, S.; Giannopoulos, K.; Wrobel, T.; Zafar, S.F.; Cultrera, J.L.; Kambhampati, S.; Danilov, A.; et al. Umbralisib Plus Ublituximab (U2) Is Superior to Obinutuzumab Plus Chlorambucil (O+Chl) in Patients with Treatment Naïve (TN) and Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): Results from the Phase 3 Unity-CLL Study. Blood 2020, 136, 37–39. [Google Scholar] [CrossRef]
- Yang, X.; Bai, H.; Yuan, X.; Yang, X.; Liu, Y.; Guo, M.; Hu, N.; Jiang, B.; Lian, Z.; Ma, Z.; et al. A highly selective PI3Kδ inhibitor BGB-10188 shows superior preclinical anti-tumor activities and decreased on-target side effects on colon. Neoplasia 2024, 57, 101053. [Google Scholar] [CrossRef]
- Cao, J.; Li, Z.; Zhou, J.; Zhang, Q.; Chen, Y.; Zhu, Z.; Li, L.; Feng, R.; Li, F.; Xu, B.; et al. A phase Ib study result of HMPL-689, a PI3Kδ inhibitor, in Chinese patients with relapsed/refractory lymphoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 773–785. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, W.; Li, S.; Wang, Y.; Sun, P.; Zhou, P.; Zheng, Y.; Zhan, J.; Li, Z. Safety and efficacy of TQ-B3525, a novel and selective oral PI3K α/δ inhibitor, in Chinese patients with advanced malignancies: A phase I dose-escalation and expansion trial. J. Clin. Oncol. 2020, 38, 8058. [Google Scholar] [CrossRef]
- Witkowska, M.; Majchrzak, A.; Robak, P.; Wolska-Washer, A.; Robak, T. The role of antibody therapies in treating relapsed chronic lymphocytic leukemia: A review. Expert Opin. Biol. Ther. 2024, 24, 1233–1244. [Google Scholar] [CrossRef]
- Stohl, W.; Metyas, S.; Tan, S.M.; Cheema, G.S.; Oamar, B.; Xu, D.; Roschke, V.; Wu, Y.; Baker, K.P.; Hilbert, D.M. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: Longitudinal observations. Arthritis Rheum. 2003, 48, 3475–3486. [Google Scholar] [CrossRef]
- Woyach, J.A.; Awan, F.; Flinn, I.W.; Berdeja, J.G.; Wiley, E.; Mansoor, S.; Huang, Y.; Lozanski, G.; Foster, P.A.; Byrd, J.C. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood 2014, 124, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Staber, P.B.; Jurczak, W.; Greil, R.; Vucinic, V.; Middeke, J.M.; Montillo, M.; Munir, T.; Neumeister, P.; Schetelig, J.; Stilgenbauer, S.; et al. Tafasitamab combined with idelalisib or venetoclax in patients with CLL previously treated with a BTK inhibitor. Leuk. Lymphoma 2021, 62, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Widhopf, G.F., II; Ghia, E.M.; Kidwell, R.L.; Hasan, M.K.; Yu, J.; Rassenti, L.Z.; Chen, L.; Chen, Y.; Pittman, E.; et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 2018, 22, 951–959.e953. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, M.Y.; Siddiqi, T.; Barrientos, J.C.; Wierda, W.G.; Isufi, I.; Tuscano, J.M.; Lamanna, N.; Subbiah, S.; Koff, J.L.; et al. Phase 1/2 study of cirmtuzumab and ibrutinib in mantle cell lymphoma (MCL) or chronic lymphocytic leukemia (CLL). J. Clin. Oncol. 2021, 39, 7556. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, M.Y.; Siddiqi, T.; Rhodes, J.M.; Wierda, W.G.; Isufi, I.; Tuscano, J.M.; Lamanna, N.; Subbiah, S.; Koff, J.L.; et al. Phase 1/2 Study of Zilovertamab and ibrutinib in mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), or marginal zone lymphoma (MZL). Blood 2022, 140, 566–568. [Google Scholar] [CrossRef]
- Jurczak, W.; Zinzani, P.L.; Gaidano, G.; Goy, A.; Provencio, M.; Nagy, Z.; Robak, T.; Maddocks, K.; Buske, C.; Ambarkhane, S.; et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1266–1272. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Cuesta-Mateos, C.; Brown, J.R.; Terrón, F.; Muñoz-Calleja, C. Oflymph nodes and CLL cells: Deciphering therole of CCR7 in the pathogenesis of CLL and understanding its potential as therapeutic target. Front. Immunol. 2021, 12, 662866. [Google Scholar] [CrossRef]
- Cuesta-Mateos, C.; Juárez-Sánchez, R.; Mateu-Albero, T.; Loscertales, J.; Mol, W.; Terrón, F.; Muñoz-Calleja, C. Targeting cancer homing into the lymph node with a novel anti-CCR7 therapeutic antibody: The paradigm of CLL. mAbs 2021, 13, 1917484. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Albero, T.; Marcos-Jimenez, A.; Delgado-Wicke, P.; Terrón, F.; Loscertales, J.; López-Matencio, J.M.S.; Muñoz-Calleja, C.; Cuesta-Mateos, C. Evaluation of the novel therapeutic anti-CCR7 antibody CAP-100 as an add-on therapy in chronic lymphocytic leukemia patients receiving venetoclax. Hematol. Oncol. 2023, 41, 869–876. [Google Scholar] [CrossRef]
- Mateu-Albero, T.; Juárez-Sánchez, R.; Loscertales, J.; Mol, W.; Terrón, F.; Muñoz-Calleja, C.; Cuesta-Mateos, C. Effect of ibrutinib on CCR7 expression and functionality in chronic lymphocytic leukemia and its implication for the activity of CAP-100, a novel therapeutic anti-CCR7 antibody. Cancer Immunol. Immunother. 2022, 71, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J. ROR1: An orphan becomes apparent. Blood 2022, 140, 1583–1591. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ghia, E.M.; Rassenti, L.Z.; Widhopf, G.F., II; Kipps, T.J. Wnt5a enhances proliferation of chronic lymphocytic leukemia and ERK1/2 phosphorylation via a ROR1/DOCK2-dependent mechanism. Leukemia 2021, 35, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.; Sinha, N.; Girgis, C.; Oh, C.S.; Cring, M.R.; Widhopf, G.F., II; Kipps, T.J. Variant Transcript of ROR1 ENST00000545203 Does Not Encode ROR1 Protein. Biomedicines 2024, 12, 1573. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Widhopf, G.F., II; Kipps, T.J. Combined Therapy of Zanubrutinib and Zilovertamab in the Inhibition of Invasive Capability of Chronic Lymphocytic Leukemia Cells. Blood 2023, 142, 6517. [Google Scholar] [CrossRef]
- Wang, M.L.; Barrientos, J.C.; Furman, R.R.; Mei, M.; Barr, P.M.; Choi, M.Y.; de Vos, S.; Kallam, A.; Patel, K.; Kipps, T.J.; et al. Zilovertamab Vedotin targeting of ROR1 as therapy for lymphoid cancers. NEJM Evid. 2022, 1, EVIDoa2100001. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Y.; Ma, J.; Zhang, Y. Immunotherapy in chronic lymphocytic leukemia: Advances and challenges. Exp. Hematol. Oncol. 2025, 14, 53. [Google Scholar] [CrossRef]
- Danecki, M.; Jurczak, W. Bispecific antibodies in relapsed/refractory diffuse large B-cell lymphoma. Acta Haematol. Pol. 2024, 55, 186–191. [Google Scholar] [CrossRef]
- Visentin, A.; Frazzetto, S.; Trentin, L.; Chiarenza, A. Innovative combinations, cellular therapies and bispecific antibodies for chronic lymphocytic leukemia: A narrative review. Cancers 2024, 16, 1290. [Google Scholar] [CrossRef]
- Shahzad, M.; Basharat, A.; Irfan, S.; Sadiq, M.H.; Amin, M.K.; Jaglal, M.V. Future landscapes of bispecific antibodies in chronic lymphocytic leukemia (CLL). Systematic review of ongoing trials. Blood 2024, 144, 6804. [Google Scholar] [CrossRef]
- Danilov, A.; Fakhri, B.; Awan, F.T.; Bentzen, H.H.; Eradat, H.A.; Niemann, C.U.; Offner, F.; Poulsen, C.B.; Hoeyer, T.; Bellido, M.; et al. Epcoritamab monotherapy in patients (Pts) with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL): Results from CLL expansion and optimization cohorts of Epcore CLL-1. Blood 2024, 144, 883. [Google Scholar] [CrossRef]
- Kater, A.P.; Christensen, J.H.; Bentzen, H.H.; Niemann, C.U.; Hutchings, M.; Chen, J.; Rios, M.; Palenski, T.; Li, T.; Mato, A.R. Subcutaneous epcoritamab in patients with relapsed/refractory chronic lymphocytic leukemia: Preliminary results from the Epcore CLL-1 Trial. Blood 2021, 138, 2627. [Google Scholar] [CrossRef]
- Mhibik, M.; Gaglione, E.M.; Eik, D.; Herrick, J.; Le, J.; Ahn, I.E.; Chiu, C.; Wielgos-Bonvallet, M.; Hiemstra, I.H.; Breij, E.C.W.; et al. Cytotoxicity of the CD3×CD20 bispecific antibody epcoritamab in CLL is increased by concurrent BTK or BCL-2 targeting. Blood Adv. 2023, 7, 4089–4101. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Assouline, S.; Baker, R.; Bartlett, N.L.; El-Sharkawi, D.; Giri, P.; Ku, M.; Schuster, S.J.; Matasar, M.; Radford, J.; et al. Mosunetuzumab Monotherapy Demonstrates Activity and a Manageable Safety Profile in Patients with Relapsed or Refractory Richter’s Transformation. Blood 2023, 142, 614. [Google Scholar] [CrossRef]
- Song, Y.Q.; Zhang, H.L.; Huang, H.Q.; Zhang, Q.Y.; Jing, H.M.; Wang, C.; Wu, C.; Li, D.H.; Dai, Y.; Humphrey, K.; et al. Glofitamab monotherapy induces high complete response rates and manageable safety in Chinese patients with heavily pretreated relapsed or refractory diffuse large B-cell lymphoma. Haematologica 2024, 109, 1269–1273. [Google Scholar] [CrossRef]
- Carlo-Stella, C.; Hutchings, M.; Offner, F.; Mulvihill, E.; Relf, J.; Byrne, B.; Lundberg, L.; Dickinson, M. Glofitamab monotherapy induces durable complete remissions and has a manageable safety profile in patients with Richter’s transformation. Hematol. Oncol. 2023, 41, 63–65. [Google Scholar] [CrossRef]
- Patel, K.; Riedell, P.A.; Tilly, H.; Ahmed, S.; Michot, J.-M.; Ghesquieres, H.; Schiano de Collela, J.M.; Chanan-Khan, A.; Bouabdallah, K.; Tessoulin, B.; et al. A Phase 1 study of plamotamab, an anti-CD20 x anti-CD3 bispecific antibody, in patients with relapsed/refractory non-Hodgkin’s lymphoma: Recommended dose safety/efficacy update and escalation exposure-response analysis. Blood 2022, 140, 9470–9472. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Li, L.; Qian, Z.; Zhou, K.; Fan, L.; Tan, P.; Giri, P.; Li, Z.; Kenealy, M.; et al. GB261, an Fc-function enabled and CD3 affinity De-Tuned CD20/CD3 bispecific antibody, demonstrated a highly advantageous safety/efficacy balance in an ongoing first-in-human dose-escalation study in patients with relapsed/refractory non-Hodgkin lymphoma. Blood 2023, 142, 1719. [Google Scholar] [CrossRef]
- Townsend, W.; Leong, S.; Tucker, D.; Pottinger, B.; Paneesha, S.; El-Sharkawi, D.; Eyre, T.A.; Batten, T.; Shah, M.; Cook, S.; et al. First-in-human phase I trial of a ROR1 targeting bispecific t cell engager (NVG-111) in combination with ibrutinib or as monotherapy in subjects with relapsed refractory chronic lymphocytic leukaemia (CLL) and mantle cell lymphoma (MCL). Blood 2022, 140, 4162–4163. [Google Scholar] [CrossRef]
- Shin, H.G.; Yang, H.R.; Yoon, A.; Lee, S. Bispecific antibody-based immune-cell engagers and their emerging therapeutic targets in cancer immunotherapy. Int. J. Mol. Sci. 2022, 23, 5686. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M.; Thompson, P.; Heyman, B.; Hutchings, M.; Lori, L.A.; Yuda, J.; Zinzani, P.L.; Colton, D.; Hoehn, D.; Izuzquiza, M.; et al. TITANium: An open-label, global multicenter phase 1/2 study of AZD5492, a first-in-class subcutaneous CD8-guided tri-specific T-cell engager (TCE), in patients (pts) with relapsed or refractory (r/r) B-cell malignancies. J. Clin. Oncol. 2025, 43, TPS7091. [Google Scholar] [CrossRef]
- Khalili, J.S.; Xiao, S.; Zhu, Y. Abstract 5679: Tetra-specific antibody GNC-035: Guidance and navigation control (GNC) molecule development for treatment of ROR1+ malignancies. Cancer Res. 2023, 83, 5679. [Google Scholar] [CrossRef]
- Cai, W.; Dong, J.; Gallolu Kankanamalage, S.; Titong, A.; Shi, J.; Jia, Z.; Wang, B.; Huang, C.; Zhang, J.; Lin, J.; et al. Biological activity validation of a computationally designed Rituximab/CD3 T cell engager targeting CD20+ cancers with multiple mechanisms of action. Antib. Ther. 2021, 4, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.; Cayatte, C.; Izuzquiza Fernandez, M.; Rata, S.; Ciucci, T.; Lin, W.; Lara, A.; Jafarzadeh, N.; Foreman, T.; Payne, S.; et al. Pre-clinical evaluation of AZD5492, a novel CD8-guided T cell engager, for B-non Hodgkin lymphoma indications. Blood 2024, 144, 959. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, R.; Zhang, X.; Jing, Z.; Zhao, C.; Pan, F.; Zheng, B.; Dai, R.; Zeng, L. Abstract 3513: CC312, a trispecific CD19-targeting co-stimulatory T cell engager, for the treatment of B cell malignancies and autoimmune diseases. Cancer Res. 2025, 85, 3513. [Google Scholar] [CrossRef]
- Abbasi, S.; Totmaj, M.A.; Abbasi, M.; Hajazimian, S.; Goleij, P.; Behroozi, J.; Shademan, B.; Isazadeh, A.; Baradaran, B. Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies. Cancer Med. 2023, 12, 7844–7858. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, Z.; Todorovic, D.; Markovic, V.; Ladjevac, N.; Zdravkovic, N.; Djurdjevic, P.; Arsenijevic, N.; Milovanovic, M.; Arsenijevic, A.; Milovanovic, J. CAR T cell therapy for chronic lymphocytic leukemia: Successes and shortcomings. Curr. Oncol. 2022, 29, 3647–3657. [Google Scholar] [CrossRef]
- Bogacz, A.; Bukowska, A.; Bukowska, M.; Olbromski, K.; Łaba, A.; Klupieć, R.; Jopek, K. Modern immunotherapy using CAR-T cells in haemato-oncology and solid tumors. Acta Haematol. Pol. 2024, 55, 34–41. [Google Scholar] [CrossRef]
- Bock, T.J.; Colonne, C.K.; Fiorenza, S.; Turtle, C.J. Outcome correlates of approved CD19-targeted CAR T cells for large B cell lymphoma. Nat. Rev. Clin. Oncol. 2025, 22, 241–261. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Chavez, J.C.; Sehgal, A.R.; Epperla, N.; Ulrickson, M.; Bachy, E.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Three-year follow-up analysis of axicabtagene ciloleucel in relapsed/refractory indolent non-Hodgkin lymphoma (ZUMA-5). Blood 2024, 143, 496–506. [Google Scholar] [CrossRef]
- Siddiqi, T.; Maloney, D.G.; Kenderian, S.S.; Brander, D.M.; Dorritie, K.; Soumerai, J.; Riedell, P.A.; Shah, N.N.; Nath, R.; Fakhri, B.; et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): A multicentre, open-label, single-arm, phase 1-2 study. Lancet 2023, 402, 641–654. [Google Scholar] [CrossRef]
- Siddiqi, T.; Soumerai, J.D.; Dorritie, K.A.; Stephens, D.M.; Riedell, P.A.; Arnason, J.E.; Kipps, T.J.; Gillenwater, H.H.; Gong, L.; Yang, L.; et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood 2021, 139, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Wierda, W.G.; Maloney, D.G.; Kenderian, S.S.; Brander, D.M.; Papp, E.; Ray, P.; Okal, A.; Perna, S.K.; Tuazon, S.A.; Ou, S.-S.; et al. CLL-184 characteristics associated with response to lisocabtagene maraleucel (liso-cel) in patients with R/R CLL/SLL: Exploratory analyses from the phase 1/2 TRANSCEND CLL 004 Study. Clin. Lymphoma Myeloma Leuk. 2024, 24, S346. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef]
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.M.; et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020, 135, 1650–1660. [Google Scholar] [CrossRef]
- Gill, S.; Vides, V.; Frey, N.V.; Hexner, E.O.; Metzger, S.; O’Brien, M.; Hwang, W.T.; Brogdon, J.L.; Davis, M.M.; Fraietta, J.A.; et al. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 2022, 6, 5774–5785. [Google Scholar] [CrossRef]
- Davids, M.S.; Kenderian, S.S.; Flinn, I.; Hill, B.T.; Maris, M.; Ghia, P.; Byrne, M.; Bartlett, N.L.; Pagel, J.M.; Zheng, Y.; et al. ZUMA-8: A phase 1 study of brexucabtagene autoleucel in patients with relapsed/refractory chronic lymphocytic leukemia. Blood 2025, 146, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kenderian, S.S.; Flinn, I.W.; Hill, B.T.; Maris, M.; Ghia, P.; Byrne, M.; Bartlett, N.L.; Pagel, J.M.; Zheng, Y.; et al. ZUMA-8: A phase 1 study of KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia. Blood 2022, 140, 7454–7456. [Google Scholar] [CrossRef]
- Liang, E.C.; Albittar, A.; Huang, J.J.; Hirayama, A.V.; Kimble, E.L.; Portuguese, A.J.; Chapuis, A.; Shadman, M.; Till, B.G.; Cassaday, R.D.; et al. Factors associated with long-term outcomes of CD19 CAR T-cell therapy for relapsed/refractory CLL. Blood Adv. 2023, 7, 6990–7005. [Google Scholar] [CrossRef] [PubMed]
- Derigs, P.; Schubert, M.L.; Dreger, P.; Schmitt, A.; Yousefian, S.; Haas, S.; Röthemeier, C.; Neuber, B.; Hückelhoven-Krauss, A.; Brüggemann, M.; et al. Third-generation anti-CD19 CAR T cells for relapsed/refractory chronic lymphocytic leukemia: A phase 1/2 study. Leukemia 2024, 38, 2419–2428. [Google Scholar] [CrossRef]
- Schubert, M.L.; Schmitt, A.; Sellner, L.; Neuber, B.; Kunz, J.; Wuchter, P.; Kunz, A.; Gern, U.; Michels, B.; Hofmann, S.; et al. Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: A unicentre phase I/II clinical trial protocol. BMJ Open 2019, 9, e026644. [Google Scholar] [CrossRef]
- Braun, T.; Kuschel, F.; Reiche, K.; Merz, M.; Herling, M. Emerging T-cell lymphomas after CAR T-cell therapy. Leukemia 2025, 39, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, T.; Puła, A.; Gołos, A.; Bołkun, Ł.; Puła, B. Indicators of an increased risk of therapy-related myeloid neoplasms in lymphoma patients: How can we best evaluate severe impairment of bone marrow function? Expert Rev. Hematol. 2025, 18, 923–934. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).