Progressive Spastic Paraparesis as the Dominant Manifestation of Adolescent-Onset Alexander Disease: Case Report and Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Case Presentation
3.2. Literature Review
- Clinical spectrum and diagnostic criteria
- Genetic findings
- Neuroimaging characteristics
- Clinical manifestations and disease course
- Cognitive and experimental data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, J.; Cascella, M. Alexander Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562242/ (accessed on 10 September 2025).
- Li, R.; Johnson, A.B.; Salomons, G.; Goldman, J.E.; Naidu, S.; Quinlan, R.; Cree, B.; Ruyle, S.Z.; Banwell, B.; D’Hooghe, M.; et al. Glial fibrillary acidic protein mutations in infantile, juvenile, and adult forms of Alexander disease. Ann. Neurol. 2005, 57, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sasaki, M.; Yoshida, M.; Namekawa, M.; Okamoto, Y.; Tsujino, S.; Sasayama, H.; Mizuta, I.; Nakagawa, M.; Alexander Disease Study Group in Japan. Nationwide survey of Alexander disease in Japan and proposed new guidelines for diagnosis. J. Neurol. 2011, 258, 1998–2008. [Google Scholar] [CrossRef]
- Hagemann, T.L.; Connor, J.X.; Messing, A. Alexander disease–associated glial fibrillary acidic protein mutations in mice induce Rosenthal fiber formation and a white matter stress response. J. Neurosci. 2006, 26, 11162–11173. [Google Scholar] [CrossRef]
- Sosunov, A.A.; Olabarria, M.; Goldman, J.E. Alexander disease: An astrocytopathy that produces a leukodystrophy. Brain Pathol. 2018, 28, 388–398. [Google Scholar] [CrossRef]
- Springer, S.; Erdem, H.; Ohlenbusch, A.; Kohlschütter, A.; Hanefeld, F. Alexander disease—Classification revisited and isolation of a neonatal form. Neuropediatrics 2000, 31, 86–92. [Google Scholar] [CrossRef]
- Paprocka, J.; Płoski, R.; Nowak, M.; Machnikowska-Sokołowska, M.; Rutkowska, K. Leukodystrophy with macrocephaly, refractory epilepsy, and severe hyponatremia—The neonatal type of Alexander disease. Genes 2024, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Knuutinen, O.; Kousi, M.; Suo-Palosaari, M.; Moilanen, J.S.; Tuominen, H.; Vainionpää, L.; Joensuu, T.; Anttonen, A.-K.; Uusimaa, J.; Lehesjoki, A.-E.; et al. Neonatal Alexander disease: Novel GFAP mutation and comparison to previously published cases. Neuropediatrics 2018, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Tonduti, D.; Ardissone, A.; Ceccherini, I.; Giaccone, G.; Farina, L.; Moroni, I. Unusual presentations and intrafamilial phenotypic variability in infantile onset Alexander disease. Neurol. Sci. 2016, 37, 973–977. [Google Scholar] [CrossRef]
- Romano, S.; Salvetti, M.; Ceccherini, I.; De Simone, T.; Savoiardo, M. Brainstem signs with progressing atrophy of medulla oblongata and upper cervical spinal cord. Lancet Neurol. 2007, 6, 562–570. [Google Scholar] [CrossRef]
- van der Knaap, M.S.; Naidu, S.; Breiter, S.N.; Blaser, S.; Stroink, H.; Springer, S.; Begeer, J.C.; van Coster, R.; Barth, P.G.; Thomas, N.H.; et al. Alexander disease: Diagnosis with MR imaging. AJNR Am. J. Neuroradiol. 2001, 22, 541–552. [Google Scholar]
- Vaia, Y.; Mura, E.; Tonduti, D. Type I Alexander Disease: Update and Validation of the Clinical Evolution-Based Classification. Mol. Genet. Metab. 2023, 138, 107540. [Google Scholar] [CrossRef]
- Prust, M.; Wang, J.; Morizono, H.; Messing, A.; Brenner, M.; Gordon, E.; Hartka, T.; Sokohl, A.; Schiffmann, R.; Gordish-Dressman, H.; et al. GFAP mutations, age at onset, and clinical subtypes in Alexander disease. Neurology 2011, 77, 1287–1294. [Google Scholar] [CrossRef]
- Johnson, A.B.; Brenner, M. Alexander disease: A review and the gene. Int. J. Dev. Neurosci. 2002, 20, 391–394. [Google Scholar] [CrossRef]
- Palczewska, I.; Niedźwiecka, Z. Wskaźniki rozwoju somatycznego dzieci i młodzieży warszawskiej. Med. Wieku Rozw. 2001, 5 (Suppl. S1), 18–118. [Google Scholar]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, C.; Verdura, E.; Vélez, V.; Schlüter, A.; Pons-Escoda, A.; Homedes, C.; Ruiz, M.; Fourcade, S.; Launay, N.; Pujol, A. A novel mutation in the GFAP gene expands the phenotype of Alexander disease. J. Med. Genet. 2019, 56, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mizuta, I.; Yasuda, R.; Nakagawa, M.; Mizuno, T. Characteristics of cerebral lesions in adult-onset Alexander disease. Neurol. Sci. 2020, 41, 225–227. [Google Scholar] [CrossRef]
- Yoshida, T.; Yasuda, R.; Mizuta, I.; Nakagawa, M.; Mizuno, T. Quantitative Evaluation of Brain Stem Atrophy Using Magnetic Resonance Imaging in Adult Patients with Alexander Disease. Eur. Neurol. 2017, 77, 296–302. [Google Scholar] [CrossRef]
- Yoshida, T.; Mizuta, I.; Yasuda, R.; Mizuno, T. Clinical and radiological characteristics of older-adult-onset Alexander disease. Eur. J. Neurol. 2021, 28, 3760–3767. [Google Scholar] [CrossRef]
- Kirsch, A.C.; McCall, D.M.; Lange, H.; Renaud, D.; Brown, T.; Zaccariello, M.J. Neuropsychological Functioning in Alexander Disease: A Case Series. Child. Neurol. Open. 2021, 8, 2329048X211048614. [Google Scholar] [CrossRef]
- Berman, R.F.; Matson, M.R.; Bachman, A.M.; Lin, N.-H.; Coyne, S.; Frelka, A.; Pearce, R.A.; Messing, A.; Hagemann, T.L. GFAP mutation and astrocyte dysfunction lead to a neurodegenerative profile with impaired synaptic plasticity and cognitive deficits in a rat model of Alexander disease. eNeuro 2025, 12, ENEURO.0504-24.2025. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Waldman, A.; Naidu, S. Alexander Disease. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2025; Last Update: 12 November 2020. [Google Scholar]
- van der Knaap, M.S.; Ramesh, V.; Schiffmann, R.; Blaser, S.; Kyllerman, M.; Gholkar, A.; Ellison, D.W.; van der Voorn, J.P.; van Dooren, S.J.; Jakobs, C.; et al. Alexander disease: Ventricular garlands and abnormalities of the medulla and spinal cord. Neurology 2006, 66, 494–498. [Google Scholar] [CrossRef]
- van der Knaap, M.S.; Salomons, G.S.; Li, R.; Franzoni, E.; Gutiérrez-Solana, L.G.; Smit, L.M.; Robinson, R.; Ferrie, C.D.; Cree, B.; Reddy, A.; et al. Unusual variants of Alexander’s disease. Ann. Neurol. 2005, 57, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Peer, S.; Wander, A.; Singh, R.; Lakhanpal, V. Frog face and strangulated medulla: Neuroimaging phenotype in a novel mutation in GFAP gene causing adult-onset Alexander disease. Neurol. Sci. 2025, 46, 4065–4068. [Google Scholar] [CrossRef]
- Sundblom, J.; Melberg, A.; Kalimo, H.; Smits, A.; Raininko, R. MR imaging characteristics and neuropathology of the spinal cord in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. AJNR Am. J. Neuroradiol. 2009, 30, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ionis Pharmaceuticals. Ionis Announces Positive Topline Results from Pivotal Study of Zilganersen in Alexander Disease. Business Wire. Available online: https://www.businesswire.com/news/home/20250922674696/en/Ionis-announces-positive-topline-results-from-pivotal-study-of-zilganersen-in-Alexander-disease (accessed on 9 October 2025).
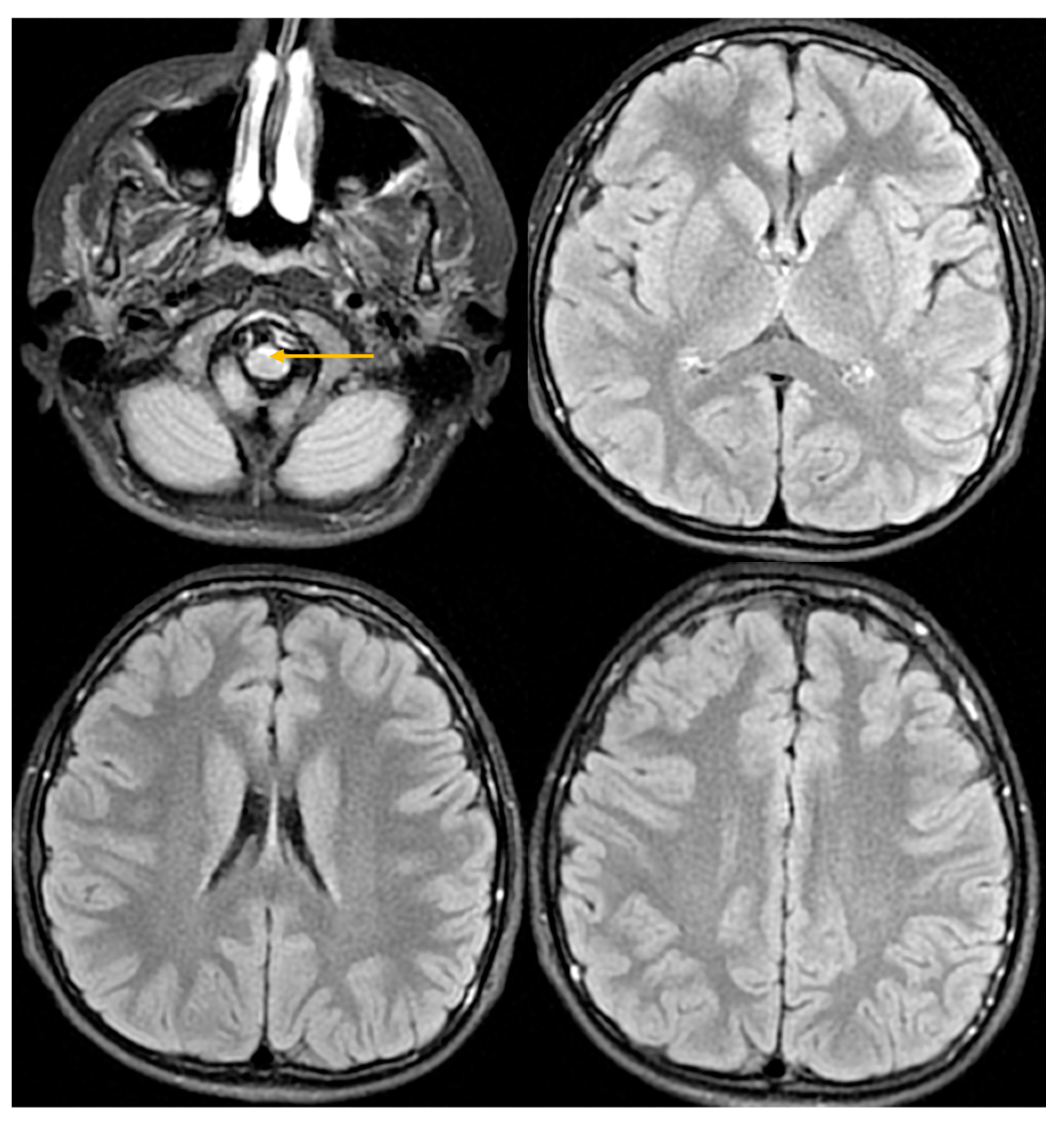
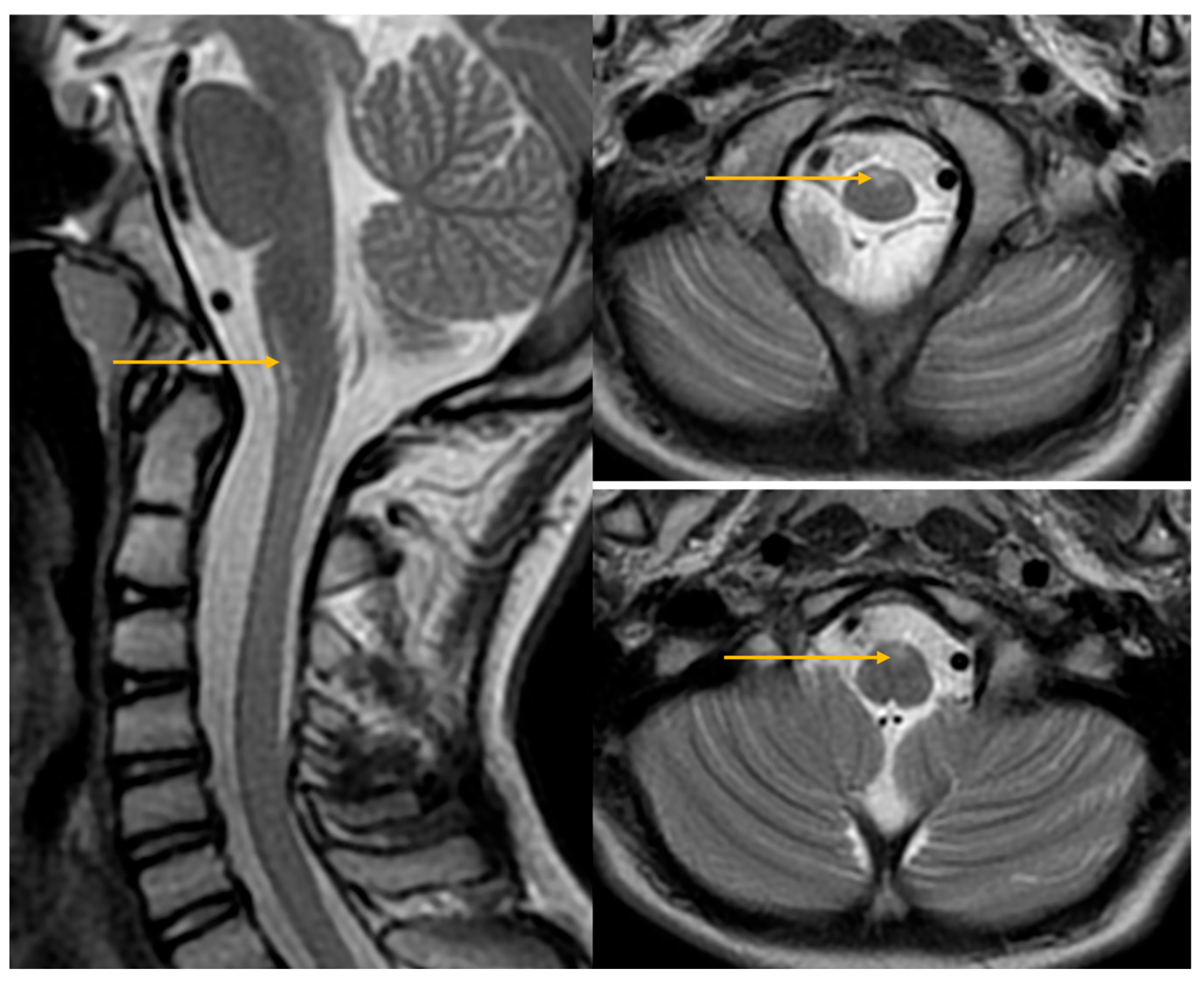
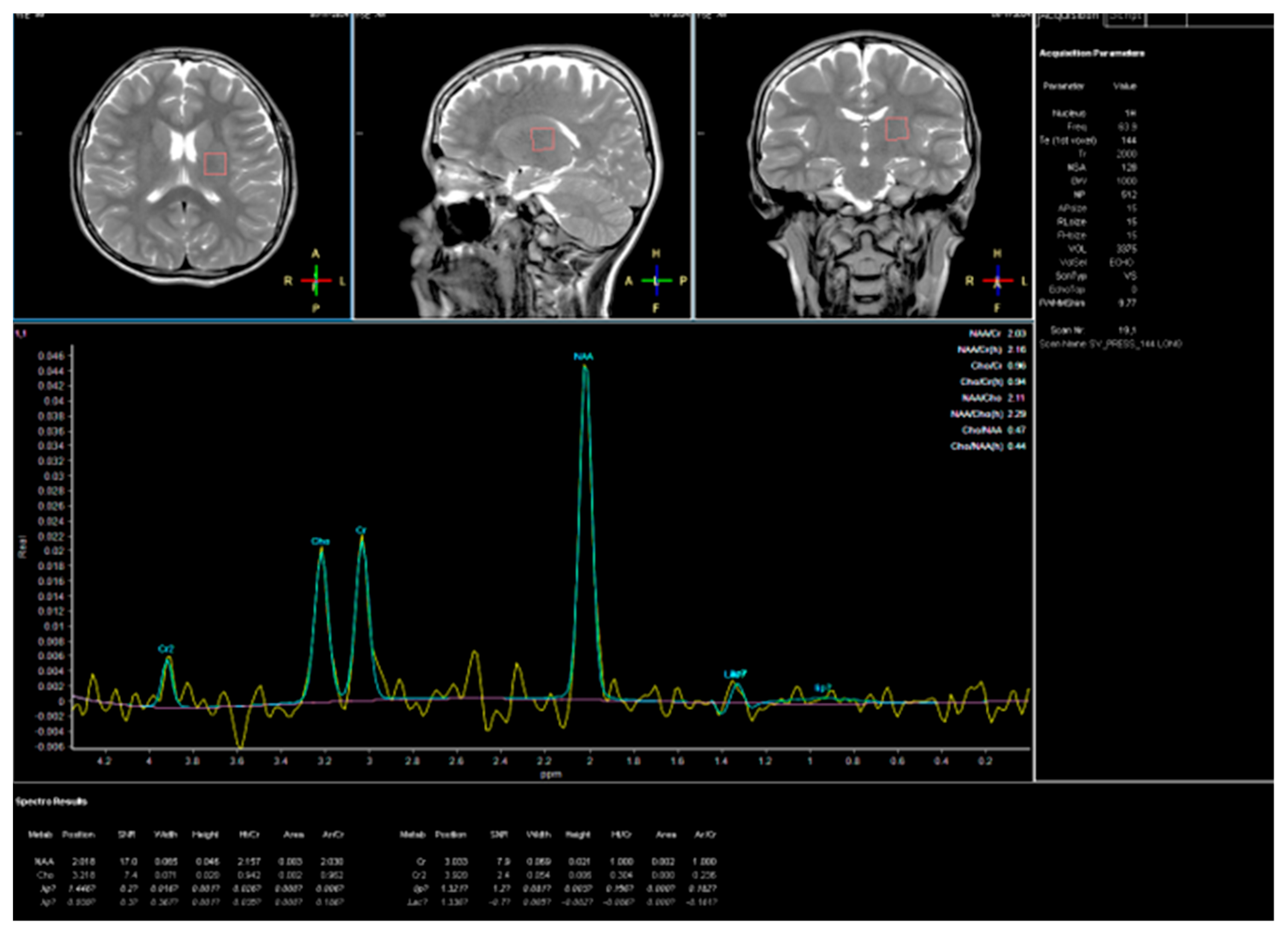
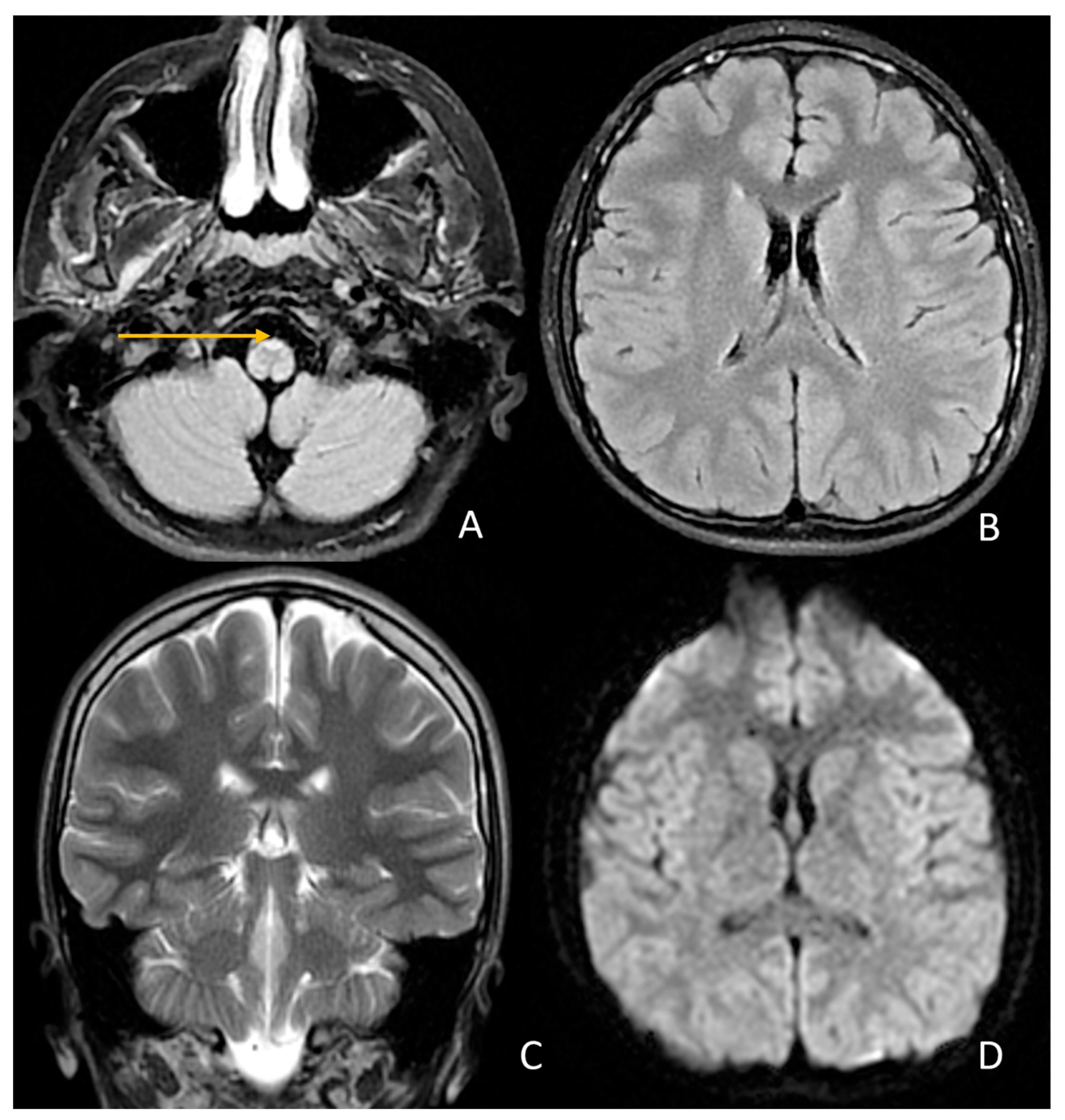

| Domain | July 2024 (Age 14 Year 10 Month | September 2025 (Age 16 Year) | Change/Observation |
|---|---|---|---|
| Contact, Orientation, Affect | Normal contact and orientation; affect appropriate; patient tense | Normal contact and orientation; affect appropriate; emotional tension and sadness noted | Persistent emotional tension, increased sadness |
| School and Social Functioning | Grade 8 student, learning fairly well; positive peer relations; anxiety in social situations (somatization) | Limited data; emotional tension and sadness possibly affecting functioning | Possible impact of emotional state on functioning |
| Emotional Assessment | STAIC and CES-DC: anxiety and depressive symptoms (no suicidal thoughts) | Observation: emotional tension, sad mood (patient denies low mood) | Persistent affective symptoms |
| Visual Memory (Benton) | Impaired (sten 3, 6 errors; population mean 6) | No data available | — |
| Short-term Auditory Memory (IDS-2) | WP = 6 (below average) | WP = 7 (average) | Improvement |
| Long-term Auditory Memory (IDS-2) | WP = 6 (below average) | WP = 6 (low) | No change |
| Abstract Reasoning (Matrices) | WP = 9 (average) | WP = 7 (average) | Slight decline |
| Conceptual Reasoning (Categories) | WP = 10 (average) | WP = 8 (average) | Slight decline |
| Intelligence Quotient (IDS-2) | IQ = 98 (average) | IQ = 85 (lower end of average) | Decrease in overall cognitive performance |
| Symptom/Feature | Our Patient | Patient I:2 | Patient II:2 | Patient II:3 | Patient II:5 | Patient III:4 | Patient III:5 |
|---|---|---|---|---|---|---|---|
| Sex | M | M | F | F | F | M | M |
| Age at onset | 13 | NA | NA | 46 | 17 | 10 | 9 |
| Age of diagnosis | 15 | 73 | 49 | 50 | 40 | 40 | 16 |
| Spastic paraparesis | Yes | No | No | Yes | Yes | Yes | Yes |
| Pyramidal signs | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ataxia | Mild | No | No | Mild | Mild | Mild | Mild |
| Bulbar symptoms | No | Subtle | Subtle | Yes | Yes | Yes | Yes |
| Gait abnormalities | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Scoliosis | Yes | No | Yes | Yes | No | Yes | Yes |
| Neurocognitive deficits | Yes | No | No | No | No | No | No |
| Ocular movement abnormalities | No | Not reported | Yes (nystagmus, diplopia) | Yes (nystagmus) | Yes | Yes | Yes |
| Dysarthia/dysphagia | No | No | No | No | No | No | No |
| Bladder dysfunction | No | No | No | Yes | Yes | No | No |
| Palatal tremor | No | No | No | No | No | No | No |
| Dysautonomia | No | No | No | No | No | No | No |
| References | this paper | [17] | |||||
| Symptom/Feature | Our Patient | Patient I:2 | Patient II:2 | Patient II:3 | Patient II:5 | Patient III:4 | Patient III:5 |
|---|---|---|---|---|---|---|---|
| Abnormal signal intensity of the anterior portion of the medulla oblongata | Yes | NA | Yes | Yes | Yes | Yes | Yes |
| Atrophy of the medulla | No | NA | No | Yes | Yes | Yes | Yes |
| Atrophy of the cervical spinal cord | Yes | NA | No | Yes | Yes | Yes | Yes |
| Signal abnormalities in the cerebellar white matter or hilus of the dentate nucleus | No | NA | No | No | No | No | No |
| Cyst formation in white matter around the anterior horn of the lateral ventricles | No | NA | No | No | No | No | No |
| Ventricular garlands | No | NA | NA | NA | NA | NA | NA |
| References | This paper | [17] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smółka, K.A.; Smółka, L.; Guz, W.; Chaber, E.; Perenc, L. Progressive Spastic Paraparesis as the Dominant Manifestation of Adolescent-Onset Alexander Disease: Case Report and Literature Review. J. Clin. Med. 2025, 14, 8232. https://doi.org/10.3390/jcm14228232
Smółka KA, Smółka L, Guz W, Chaber E, Perenc L. Progressive Spastic Paraparesis as the Dominant Manifestation of Adolescent-Onset Alexander Disease: Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(22):8232. https://doi.org/10.3390/jcm14228232
Chicago/Turabian StyleSmółka, Katarzyna Anna, Leon Smółka, Wiesław Guz, Emilia Chaber, and Lidia Perenc. 2025. "Progressive Spastic Paraparesis as the Dominant Manifestation of Adolescent-Onset Alexander Disease: Case Report and Literature Review" Journal of Clinical Medicine 14, no. 22: 8232. https://doi.org/10.3390/jcm14228232
APA StyleSmółka, K. A., Smółka, L., Guz, W., Chaber, E., & Perenc, L. (2025). Progressive Spastic Paraparesis as the Dominant Manifestation of Adolescent-Onset Alexander Disease: Case Report and Literature Review. Journal of Clinical Medicine, 14(22), 8232. https://doi.org/10.3390/jcm14228232





