Middle Meningeal Artery Embolization in the Treatment of Chronic Subdural Hematoma: A Two-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
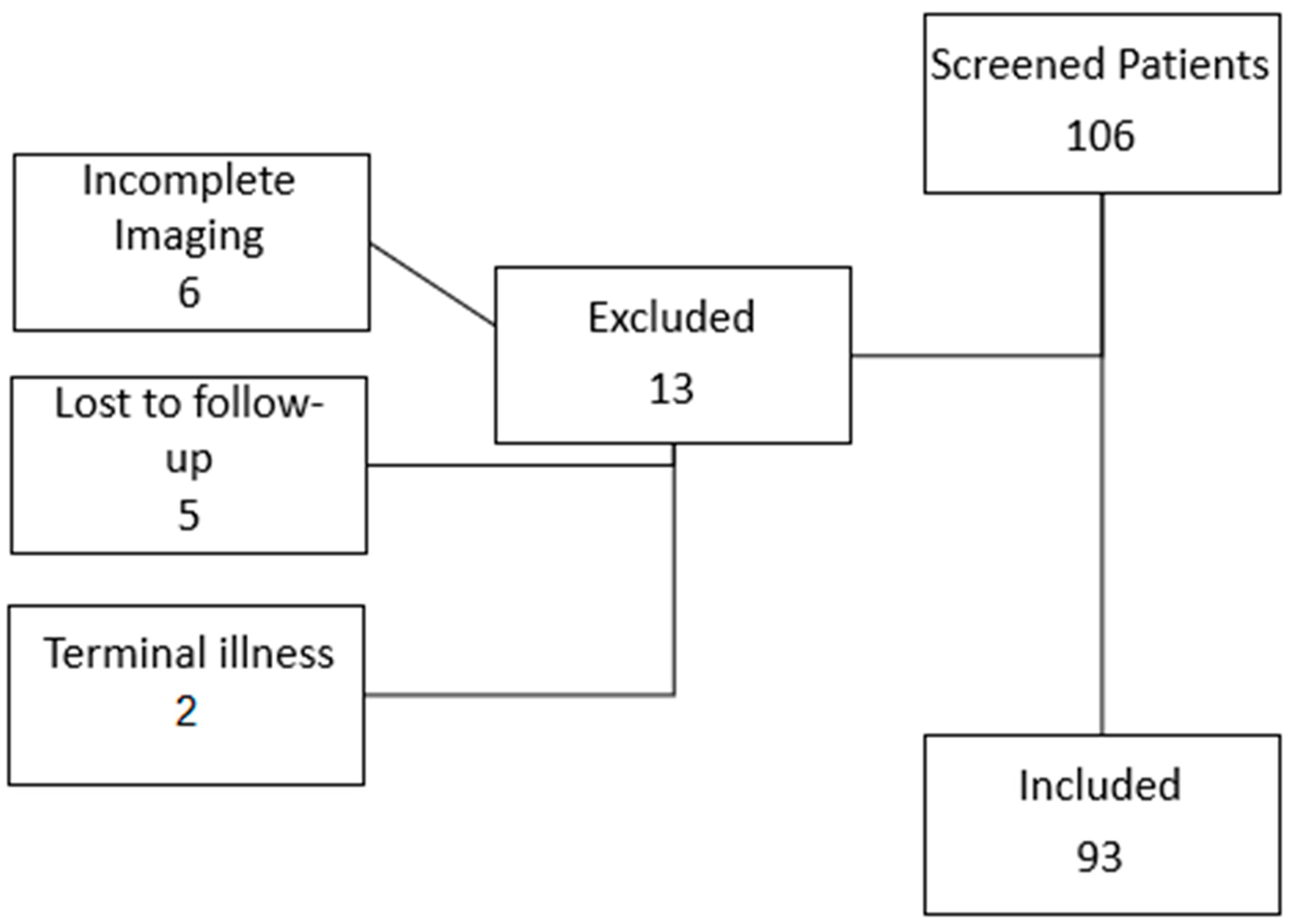
2.1. Study Design and Patient Selection
2.2. Embolization Procedure
2.3. Data Collection and Analysis
2.4. Statistical Analysis
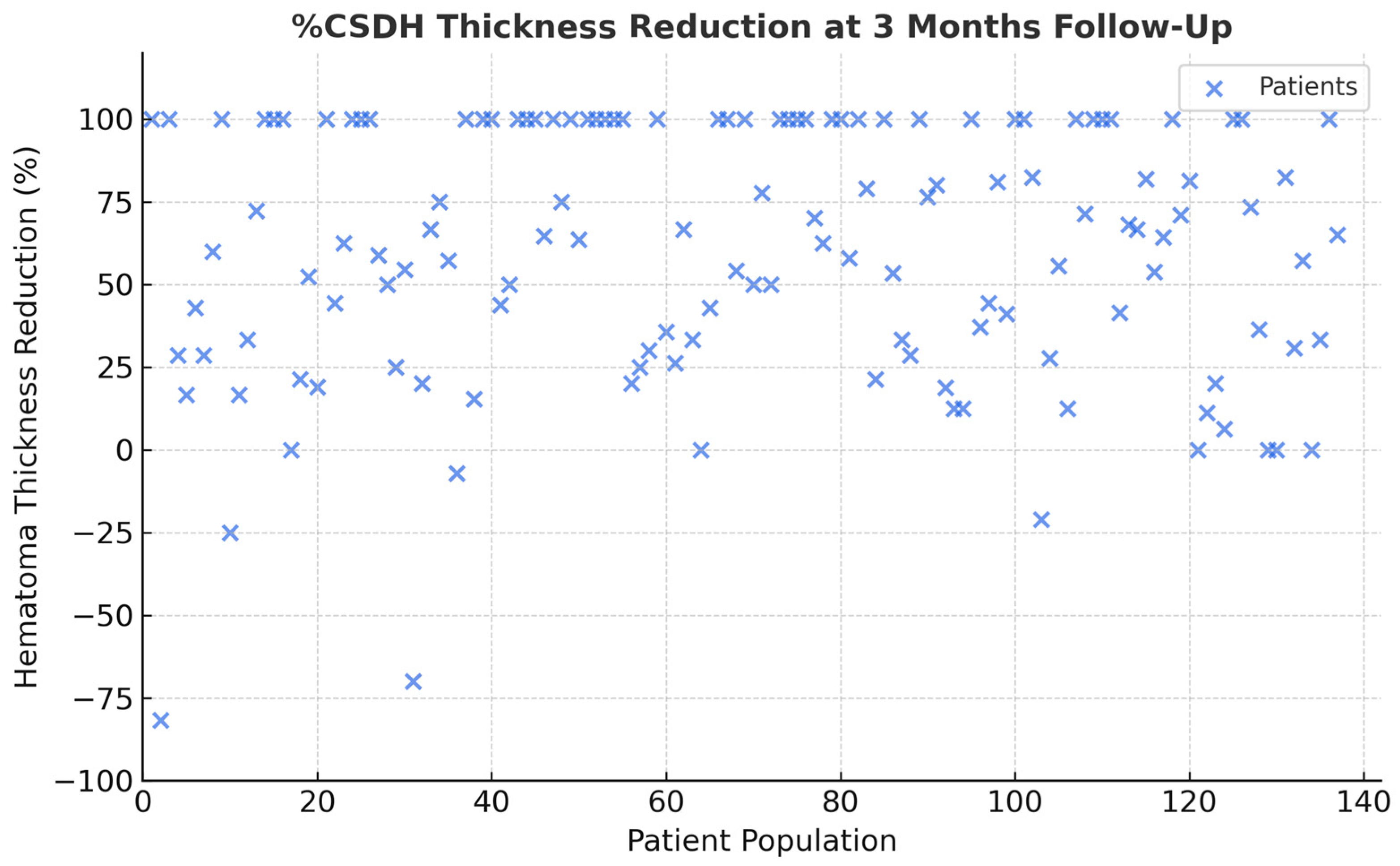
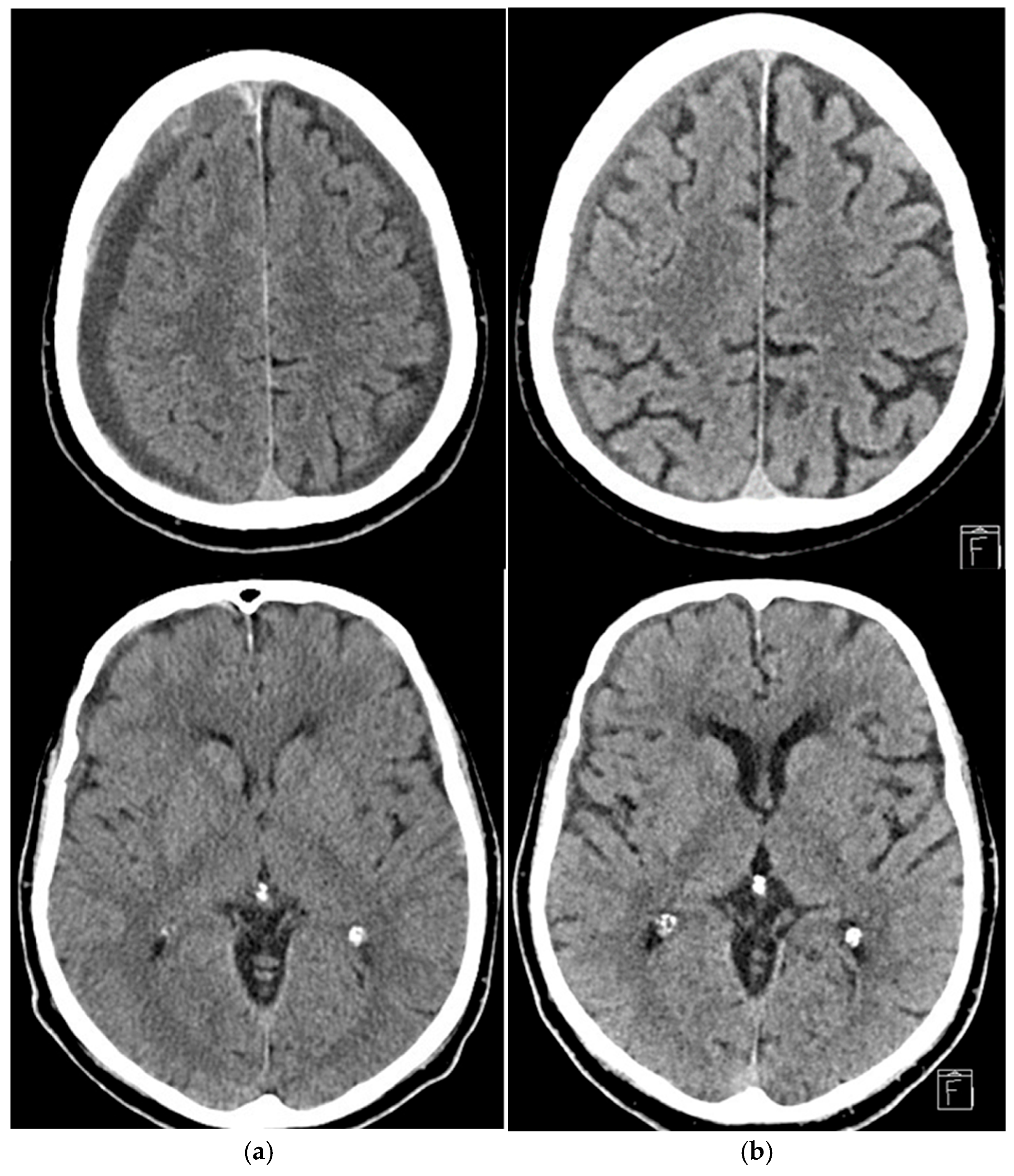
3. Results
4. Discussion
4.1. Treatment Effectiveness
4.2. Embolic Materials: Advantages and Limitations
4.3. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cSDH | Chronic Subdural Hematoma |
| MMA | Middle Meningeal Artery |
| PVA | Polyvinyl Acetate |
| EVOH | Ethylene Vinyl Alcohol Copolymer-Based Liquid Embolic Agent |
References
- Omura, Y.; Ishiguro, T. Middle meningeal artery embolization for chronic subdural hematoma: A systematic review. Front. Neurol. 2023, 14, 1259647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmolling, Á.H.; Pérez-García, C. Middle Meningeal Artery Embolization for Management of Chronic Subdural Hematoma. Radiographics 2024, 44, e230158. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L. Youmans & Winn Neurological Surgery, 7th ed.; Pathophysiology of chronic subdural hematoma; Winn, R.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Moshayedi, P.; Liebeskind, D.S. Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: Implications of Pathophysiology in Trial Design. Front. Neurol. 2020, 11, 923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudy, R.F.; Catapano, J.S.; Jadhav, A.P.; Albuquerque, F.C.; Ducruet, A.F. Middle Meningeal Artery Embolization to Treat Chronic Subdural Hematoma. Stroke Vasc. Interv. Neurol. 2022, 3, e000490. [Google Scholar] [CrossRef]
- Nakaguchi, H.; Tanishima, T.; Yoshimasu, N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J. Neurosurg. 2001, 95, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Orscelik, A.; Senol, Y.C. Middle meningeal artery embolization without surgical evacuation for chronic subdural hematoma: A single-center experience of 209 cases. Front. Neurol. 2023, 14, 1222131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, I.; Shakir, M.; Hika, B.; Khan, M.; Bhatti, I.A.; Qureshi, A.I.; Thomas, A.; Kan, P.; Siddiq, F. Failure Rates of Conservative Management of Minimally Symptomatic Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. World Neurosurg. 2024, 191, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Kan, P.; Maragkos, G.A.; Srivatsan, A.; Srinivasan, V.; Johnson, J.; Burkhardt, J.K.; Robinson, T.M.; Salem, M.M.; Chen, S.; Riina, H.A. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: A Multi-Center Experience of 154 Consecutive Embolizations. Neurosurgery 2021, 88, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Link, T.W.; Boddu, S. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: A Series of 60 Cases. Neurosurgery 2019, 85, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Catapano, J.S.; Koester, S.W. Middle meningeal artery embolization associated with reduced chronic subdural hematoma volume and midline shift in the acute postoperative period. J. Neurointerv. Surg. 2024, 16, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, A.; Mohanty, A.; Nascimento, F.A.; Hafeez, M.U.; Srinivasan, V.M.; Thomas, A.; Chen, S.R.; Johnson, J.N.; Kan, P. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Meta-Analysis and Systematic Review. World Neurosurg. 2019, 122, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.P.; Hwang, G.; Byoun, H.S.; Kim, T.; Lee, S.U.; Bang, J.S.; Han, J.H.; Kim, C.Y.; Kwon, O.K.; Oh, C.W. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma. Radiology 2018, 286, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Fiorella, D.; Monteith, S.J.; Hanel, R.; Atchie, B.; Boo, S.; McTaggart, R.A.; Zauner, A.; Tjoumakaris, S.; Barbier, C.; Benitez, R.; et al. Embolization of the Middle Meningeal Artery for Chronic Subdural Hematoma. N. Engl. J. Med. 2025, 392, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lim, J.X.; Wen, D.; Wong, C.P.; Lim, W.E.H.; Chia, G.S. Adjunct Middle Meningeal Artery Embolization Versus Surgery for Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2024, 47, 876. [Google Scholar] [CrossRef] [PubMed]
- Dofuku, S.; Sato, D.; Nakamura, R.; Ogawa, S.; Torazawa, S.; Sato, M.; Ota, T. Sequential Middle Meningeal Artery Embolization after Burr Hole Surgery for Recurrent Chronic Subdural Hematoma. Neurol. Med. Chir. 2023, 63, 17–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumoto, H.; Hanayama, H.; Okada, T.; Sakurai, Y.; Minami, H.; Masuda, A.; Tominaga, S.; Miyaji, K.; Yamaura, I.; Yoshida, Y. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J. Clin. Neurosci. 2018, 49, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.; Morgan, I.; Young, T.; Vlastos, J.; Williams, T.; Hrabarchuk, E.I.; Tepper, J.; Baker, T.; Kellner, C.P.; Bederson, J.; et al. Surgical techniques for evacuation of chronic subdural hematoma: A mini-review. Front. Neurol. 2023, 14, 1086645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onyinzo, C.; Berlis, A.; Abel, M.; Kudernatsch, M.; Maurer, C.J. Efficacy and mid-term outcome of middle meningeal artery embolization with or without burr hole evacuation for chronic subdural hematoma compared with burr hole evacuation alone. J. Neurointerv. Surg. 2022, 14, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Marulanda, A.; Gomez-Paz, S.; Salem, M.M.; Mallick, A.; Motiei-Langroudi, R.; Arle, J.E.; Stippler, M.; Papavassiliou, E.; Alterman, R.L.; Ogilvy, C.S.; et al. Middle Meningeal Artery Embolization Versus Conventional Treatment of Chronic Subdural Hematomas. Neurosurgery 2021, 89, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Shotar, E.; Mathon, B.; Salle, H.; Rouchaud, A.; Mounayer, C.; Bricout, N.; Lejeune, J.P.; Janot, K.; Amelot, A.; Naggara, O.; et al. Meningeal embolization for preventing chronic subdural hematoma recurrence after surgery: The EMPROTECT randomized clinical trial. JAMA 2025, 334, 127–135. [Google Scholar] [CrossRef]
- Liu, J.; Ni, W.; Zuo, Q.; Yang, H.; Peng, Y.; Lin, Z.; Li, Z.; Wang, J.; Zhen, Y.; Luo, J.; et al. Middle Meningeal Artery Embolization for Nonacute Subdural Hematoma. N. Engl. J. Med. 2024, 391, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.H.; Akhter, A.; Zakeri, A.; Tanweer, O.; Zyck, S.; Tjoumakaris, S.; Jabbour, P.; Kan, P.; Peng, J.; Youssef, P. Middle Meningeal Artery Embolization for Membranous Versus Nonmembranous Subdural Hematomas: A Retrospective and Multicenter Cohort Study. World Neurosurg. 2023, 177, e680–e685. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Derraz, I.; Marnat, G.; Jecko, V.; Papaxanthos, J.; Bellanger, G.; Cognard, C.; Sol, J.C.; Le Van, T.; Berhouma, M.; et al. Embolization of the middle meningeal artery for chronic subdural hematoma: The OTEMACS multicenter, randomized, clinical trial protocol. Interv. Neuroradiol. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffman, H.; Sims, J.J.; Nickele, C.; Inoa, V.; Elijovich, L.; Goyal, N. Middle meningeal artery embolization with standalone or adjunctive coiling for treatment of chronic subdural hematoma: Systematic review and meta-analysis. Interv. Neuroradiol. 2024, 15910199241304852, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shehabeldin, M.; Amllay, A.; Jabre, R.; Chen, C.J.; Schunemann, V.; Herial, N.A.; Gooch, M.R.; Mackenzie, L.; Choe, H.; Tjoumakaris, S.; et al. Onyx Versus Particles for Middle Meningeal Artery Embolization in Chronic Subdural Hematoma. Neurosurgery 2023, 92, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, A.A.; Naqvi, A.; Chen, J.Y.; Custozzo, A.; Boulos, A.S.; Dalfino, J.C.; Field, N.C.; Paul, A.R. 2024 middle meningeal artery embolization trials: A comprehensive review of past, recent, and ongoing trials. Interv. Neuroradiol. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, X.; Xue, X.; Liu, A.; Qi, P. Comparative Study on Clinical Outcomes and Cost-Effectiveness of Chronic Subdural Hematomas Treated by Middle Meningeal Artery Embolization and Conventional Treatment: A National Cross-Sectional Study. Int. J. Surg. 2023, 109, 3836–3847. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Mean age (years) | 75.5 ± 8.9 |
| Sex (male/female) | 61/32 |
| Hypertension | 58 (62.3%) |
| Diabetes | 17 (18.3%) |
| Cardiovascular disease | 22 (23.6%) |
| Antiplatelet therapy | 35 (37.6%) |
| Anticoagulant therapy | 22 (23.5%) |
| None | 36 (38.9%) |
| First-line embolization | 60 (64.5%) |
| Post-surgical embolization | 33 (35.5%) |
| Bilateral/unilateral | 44/49 |
| Midline shift present | 27 (29%) |
| Markwalder 1 | 63 (67.7%) |
| Embolization Materials | cSDH (%) | Mean Thickness Reduction (%) ± SD (p > 0.05) | Clinical Complications | Rebleeding |
|---|---|---|---|---|
| EVOH | 44 (33.9) | 59.2 ± 33.1 | 0 | 1 |
| PVA | 27 (20.8) | 58.6 ± 31.7 | 1 (transient deficit) | 1 |
| PVA + EVOH | 31 (23.8) | 61.3 ± 32.9 | 0 | 0 |
| PVA + COIL | 27 (20–8) | 57.9 ± 34.2 | 0 | 1 |
| GLUBRAN | 1 (0.7) | 0 | 0 | 0 |
| Material | Type | Main Advantage | Main Limitation |
|---|---|---|---|
| EVOH | Liquid | Deep distal penetration | Cost, DMSO irritation |
| PVA | Particulate | Widely available | Less durable occlusion |
| Coils | Mechanical | Stable occlusion | Proximal only, incomplete distal flow block |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adduci, F.; Del Sette, B.; Salsano, G.; Venturi, G.; Sturiale, C.; Dall’Olio, M.; Rolla Bigliani, C.; Fiaschi, P.; Cirillo, L.; Castellan, L. Middle Meningeal Artery Embolization in the Treatment of Chronic Subdural Hematoma: A Two-Center Retrospective Study. J. Clin. Med. 2025, 14, 8226. https://doi.org/10.3390/jcm14228226
Adduci F, Del Sette B, Salsano G, Venturi G, Sturiale C, Dall’Olio M, Rolla Bigliani C, Fiaschi P, Cirillo L, Castellan L. Middle Meningeal Artery Embolization in the Treatment of Chronic Subdural Hematoma: A Two-Center Retrospective Study. Journal of Clinical Medicine. 2025; 14(22):8226. https://doi.org/10.3390/jcm14228226
Chicago/Turabian StyleAdduci, Francesco, Bruno Del Sette, Giancarlo Salsano, Greta Venturi, Carmelo Sturiale, Massimo Dall’Olio, Claudia Rolla Bigliani, Pietro Fiaschi, Luigi Cirillo, and Lucio Castellan. 2025. "Middle Meningeal Artery Embolization in the Treatment of Chronic Subdural Hematoma: A Two-Center Retrospective Study" Journal of Clinical Medicine 14, no. 22: 8226. https://doi.org/10.3390/jcm14228226
APA StyleAdduci, F., Del Sette, B., Salsano, G., Venturi, G., Sturiale, C., Dall’Olio, M., Rolla Bigliani, C., Fiaschi, P., Cirillo, L., & Castellan, L. (2025). Middle Meningeal Artery Embolization in the Treatment of Chronic Subdural Hematoma: A Two-Center Retrospective Study. Journal of Clinical Medicine, 14(22), 8226. https://doi.org/10.3390/jcm14228226






