1. Introduction
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is one of the surgical methods used in patients with peritoneal carcinomatosis (PC). Its benefits have been demonstrated particularly in appendiceal PC cases [
1] while its efficacy in other origins remains a topic of debate [
2]. Moreover, although no randomized trial has been performed for diffuse malignant peritoneal mesothelioma, the beneficial effect of CRS + HIPEC is widely accepted. Its applications have further expanded to include various malignancies with peritoneal spread, including colorectal, gastric and pseudomyxoma peritonei. However, given the life-threatening complications of this procedure, patients’ peculiarities should be considered in the selection of this procedure [
3,
4,
5,
6,
7]. In recent studies, the morbidity rates in patients undergoing CRS + HIPEC ranged from approximately 12% to 60%, while mortality rates ranged from approximately 0.9% to 5.8% [
8].
Splenectomy may be necessary in peritoneal carcinomatosis (PC) due to hematologic, direct, or peritoneal dissemination, as well as following iatrogenic injuries. A recent study involving patients with epithelial ovarian cancer undergoing CRS with concurrent splenectomy due to splenic invasion found no difference in postoperative prognosis compared to other patient populations [
9]. Another study evaluating patients with advanced-stage epithelial ovarian cancer reported that splenectomy was associated with more aggressive surgical interventions, higher rates of reoperation, blood transfusions, postoperative infections, and prolonged ICU stays, although disease-free survival (DFS) and overall survival (OS) rates were comparable between groups [
10].
Among patients undergoing CRS, splenectomy was associated with increased minor complications, leading to recommendations for spleen preservation when feasible [
11]. Similarly, data from PC patients revealed higher rates of infectious complications, pancreatic fistula, and intestinal perforation in those undergoing splenectomy, identifying it as a poor perioperative risk indicator [
12]. Moreover, it has been shown that splenectomy performed during CRS + HIPEC may induce hematotoxic effects, and that granulocyte counts may increase, particularly following the use of oxaliplatin [
13,
14]. Another analysis highlighted that splenectomy was more frequent in patients with higher peritoneal cancer index (PCI) scores and elevated BMI undergoing CRS + HIPEC [
15].
The impact of splenectomy on survival remains more controversial. Several studies have reported that cancer patients exhibit altered immune responses following splenectomy [
16]. The most prominent theories supporting this observation primarily emphasize the spleen’s central role in the immune system, and secondarily, the potential delay of adjuvant therapy in already vulnerable patients due to major morbidity associated with splenectomy [
17].
Two main mechanisms may explain the potential impact of splenectomy on survival. The spleen exerts a dual role in tumor immunology, influencing both immune activation and suppression depending on the host status [
16,
18]. While some studies suggest reduced immunosuppression and enhanced cytotoxic activity after splenectomy, others report increased regulatory T cell activity and metastasis [
19,
20,
21,
22,
23]. Clinically and experimentally, inferior survival outcomes or immunosuppression have been observed in gastric and pancreatic cancers, suggesting that immune dysregulation after splenectomy may contribute to poorer prognosis, particularly in conditions like peritoneal carcinomatosis [
24]. As another example in patients with ovarian cancer undergoing CRS + HIPEC, splenectomy performed due to invasion or hematologic dissemination did not influence long-term survival outcomes, which were instead dependent on chemotherapy sensitivity [
25]. The specific impact of splenectomy during CRS + HIPEC procedures in malignant peritoneal mesothelioma and gastric cancers has not yet been specifically evaluated in the literature.
While prior studies have explored the implications of splenectomy in CRS + HIPEC, its effect on postoperative morbidity in PC patients remains unclear [
26]. This study aims to evaluate the impact of splenectomy on postoperative morbidity in patients with PC undergoing CRS + HIPEC.
3. Results
Among the 149 patients included in the study, 78 (52.3%) had colorectal cancer, 24 (16.1%) had pseudomyxoma peritonei, 20 (13.4%) had gastric cancer, 18 (12.1%) had ovarian cancer, and 9 (6.0%) had malignant peritoneal mesothelioma. Splenectomy was performed in 52 (34.9%) patients, while 97 (65.1%) patients did not undergo splenectomy.
When comparing demographic and clinical parameters, gender distribution showed no significant difference between splenectomy and non-splenectomy groups (p = 0.376).
Primary malignancy distribution differed significantly between groups (p < 0.001), with colorectal cancer predominant in the non-splenectomy group (64.0%) compared to the splenectomy group (30.8%). Conversely, pseudomyxoma peritonei (26.9% vs. 10.3%) and malignant mesothelioma (13.4% vs. 2.1%) were more frequent in the splenectomy group. Comorbidities did not affect splenectomy rates. The median Peritoneal Cancer Index was significantly higher in the splenectomy group (9 vs. 3, p = 0.010). HIPEC regimen differed between groups, with oxaliplatin more commonly used in the non-splenectomy group (74.9% vs. 57.6%) and cisplatin more frequent in the splenectomy group (42.4% vs. 25.1%, p = 0.038).
Splenectomy group patients underwent more extensive surgical procedures, including peritonectomy (86.6% vs. 38.2%, p < 0.001), diaphragmatic peritonectomy (80.8% vs. 24.7%, p < 0.001), pelvic peritonectomy (59.7% vs. 28.9%, p < 0.001), total colectomy (26.9% vs. 2.1%, p < 0.001), colon resection (59.7% vs. 50.5%, p = 0.009), gastrectomy (25.0% vs. 12.4%, p = 0.049), Glisson capsule resection (36.5% vs. 7.3%, p < 0.001), distal pancreatectomy (7.7% vs. 0%, p = 0.014), and small intestine resection (30.7% vs. 14.4%, p = 0.017). Ostomy formation was more common in the splenectomy group, with ileostomy being the predominant type (88.4% ileostomy vs. 11.6% colostomy in splenectomy group, p = 0.041).
Intraoperative complications were more frequent in the splenectomy group (9.6% vs. 1.1%, p = 0.048), primarily bladder injury. Splenectomy patients had longer operations (10 [5–16] vs. 6 [3–14] h, p < 0.001), higher mean albumin transfusion (0.32 ± 0.10 vs. 0.17 ± 0.04 vials, p < 0.002), and extended ICU (1.5 [1–21] vs. 1 [1–20] days, p < 0.001) and hospital stays (12 [5–43] vs. 9 [4–77] days, p = 0.005).
Postoperatively, splenectomy patients experienced significantly higher rates of hepatotoxicity (27.0% vs. 4.2%,
p < 0.001), pleural effusion (25.0% vs. 4.1%,
p < 0.001), pancreatic fistula (15.4% vs. 1.1%,
p = 0.001), nephrotoxicity (21.2% vs. 7.2%,
p = 0.012), and pneumothorax (7.7% vs. 0%,
p = 0.013). Disease recurrence and progression for CCS1 patients was significantly more common in the splenectomy group (44.2% vs. 26.8%,
p = 0.031) (
Table 1).
During OS and DFS analysis, 3 patients with early postoperative mortality were excluded and remaining 146 patients were analyzed. OS analysis showed no gender differences between survivors and non-survivors (p = 0.510). Primary tumor distribution showed no significant difference (p = 0.063), though gastric cancer was more prevalent among non-survivors (26.1% vs. 10.6%). Comorbidities showed no impact on survival. OS was lower in patients who underwent ostomy formation. (p = 0.014), with more colostomies among non-survivors (26.1% vs. 4.9%). Non-survivors had significantly longer operations (8.25 [6.00–11.50] vs. 6.00 [5.00–9.00] h, p = 0.030) and hospital stays (13.00 [9.00–22.00] vs. 10.00 [7.00–13.00] days, p = 0.015).
Specific complications were significantly more common in non-survivors, including encephalopathy (8.7% vs. 0.0%,
p = 0.001), paraplegia (4.3% vs. 0.0%,
p = 0.020), and cellulitis (8.7% vs. 0.8%,
p = 0.014) (
Table 2).
During DFS analysis, 146 patients were evaluated (101 with recurrence, 45 without recurrence). Primary tumor distribution and demographics showed no differences between groups. Smoking history was unexpectedly more common in the non-recurrence group (17.8% vs. 5.9%, p = 0.025).
Patients without recurrence underwent significantly more extensive surgical procedures, including peritonectomy (68.9% vs. 48.5%, p = 0.022), total colectomy (24.4% vs. 5.0%, p < 0.001), total gastrectomy (22.2% vs. 9.9%, p = 0.046), and splenectomy (46.7% vs. 29.7%, p = 0.047). Correspondingly, these patients had longer operative times (8.25 [6.00–12.00] vs. 6.00 [5.00–8.00] h, p < 0.001) and hospital stays (12.00 [9.00–15.00] vs. 9.00 [7.00–13.00] days, p = 0.002), reflecting more aggressive cytoreductive efforts.
Patients with recurrence had higher PCI scores (5 vs. 3,
p = 0.002) and greater transfusion requirements, including albumin (0.40 ± 0.08 vs. 0.12 ± 0.04 vials,
p < 0.001), packed red blood cells (1.31 ± 0.22 vs. 0.61 ± 0.11 units,
p = 0.008), and fresh frozen plasma (2.04 ± 0.30 vs. 0.67 ± 0.12 units,
p = 0.002). CCS 0 rates were higher in those without recurrence (86.7% vs. 96.0%,
p = 0.038) (
Table 3).
Cox regression analyses for OS showed that demographic factors including age, gender, BMI and complications associated with mortality were not significant predictors. In univariate analysis, splenectomy indication was significant (overall p = 0.117), with peritoneal implant splenectomy associated with increased mortality risk (OR = 3.679, 95% CI: 1.224–11.060, p = 0.020), while hilar invasion and iatrogenic splenectomy showed no significant associations. Postoperative complications (nephrotoxicity, hepatotoxicity, pneumothorax, pleural effusion, pancreatic fistula) showed no significant associations. Hospitalization duration was significant in univariate analysis (OR = 1.055, 95% CI: 1.014–1.098, p = 0.008).
Multivariate Cox regression analysis for OS included primary tumor type, complete cytoreduction score, PCI, splenectomy indication, and hospital length of stay. Primary tumor type showed no significant association with OS (overall p = 0.345), with all individual diagnostic categories remaining non-significant in multivariate modeling. Pseudomyxoma peritonei could not be evaluated due to zero mortality events in this group.
PCI emerged as a significant independent predictor of OS when modeled continuously (OR = 1.150 per 1-point increase, 95% CI: 1.041–1.270, p = 0.006), indicating that each unit increase in PCI was associated with a 15% increase in mortality risk. Hospital length of stay remained an independent predictor (OR = 1.058 per day, 95% CI: 1.005–1.112, p = 0.030), with each additional day increasing mortality risk by approximately 5.8%.
Splenectomy indication showed no overall significant association with OS in multivariate analysis (
p = 0.121). However, when examined by specific indication, peritoneal implant splenectomy demonstrated a borderline association with increased mortality risk (OR = 4.505, 95% CI: 0.939–21.620,
p = 0.060), though this did not reach statistical significance. Hilar invasion splenectomy showed no association with OS (OR = 0.448, 95% CI: 0.078–2.582,
p = 0.369), and iatrogenic splenectomy could not be estimated due to zero events. Complete cytoreduction score showed no significant association with OS in multivariate analysis (
Table 4).
Cox regression analyses for DFS showed no significant associations with age, gender, BMI, complications or primary tumor type. Also HIPEC-related and splenectomy-associated complications showed no association. Splenectomy indication was significant in both univariate and multivariate analyses (p < 0.001). In univariate analysis, splenectomy for peritoneal implants was associated with significantly increased recurrence risk (OR = 25.146, 95% CI: 5.410–116.886, p < 0.001), while iatrogenic splenectomy and hilar invasion showed no significant associations. PCI analyzed as a continuous variable was an independent predictor of recurrence in univariate (OR = 1.080, p = 0.003) analysis. Complete cytoreduction score showed borderline significance in univariate analysis (p = 0.050).
Multivariate Cox regression analysis for DFS included primary tumor type, splenectomy indication, complete cytoreduction score, and PCI (continuous). Primary tumor type showed no overall significant association with DFS (p = 0.163), though gastric cancer demonstrated a borderline trend toward increased recurrence risk (OR = 3.081, 95% CI: 0.845–11.230, p = 0.088).
Splenectomy indication emerged as a highly significant independent predictor of DFS (overall p < 0.001). Peritoneal implant-related splenectomy was strongly associated with increased recurrence risk (OR = 17.814, 95% CI: 3.025–104.894, p = 0.001), indicating an approximately 18-fold increase in recurrence compared to patients without splenectomy. In contrast, hilar dissection-related splenectomy was independently associated with improved DFS (OR = 0.136, 95% CI: 0.025–0.736, p = 0.021), representing an 86% reduction in recurrence risk.
PCI analyzed as a continuous variable was a significant independent predictor of recurrence (OR = 1.166 per 1-point increase, 95% CI: 1.066–1.276,
p = 0.001), with each unit increase in PCI associated with a 16.6% increase in recurrence risk. Complete cytoreduction score showed no significant association with DFS in multivariate analysis (
Table 5).
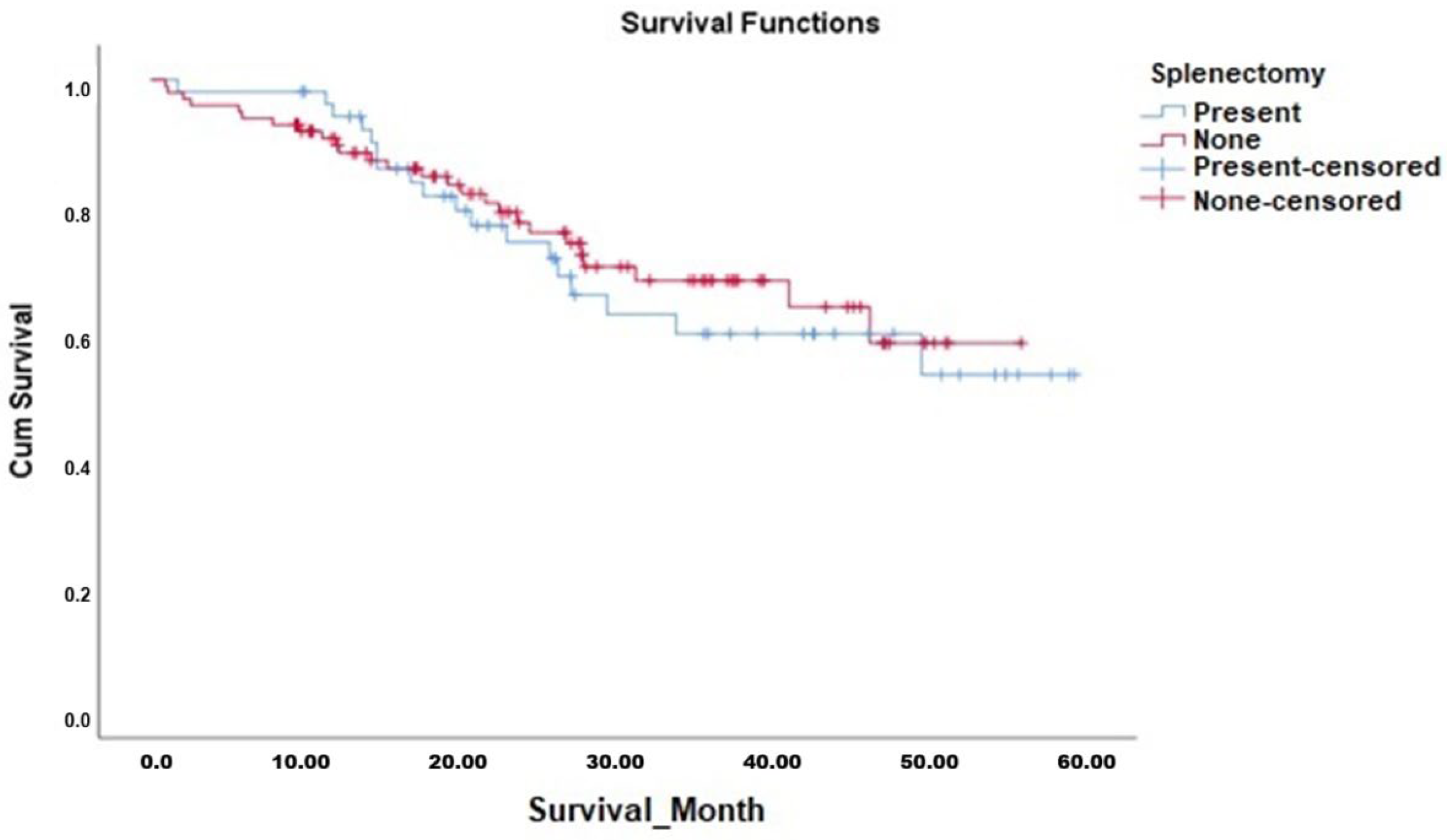
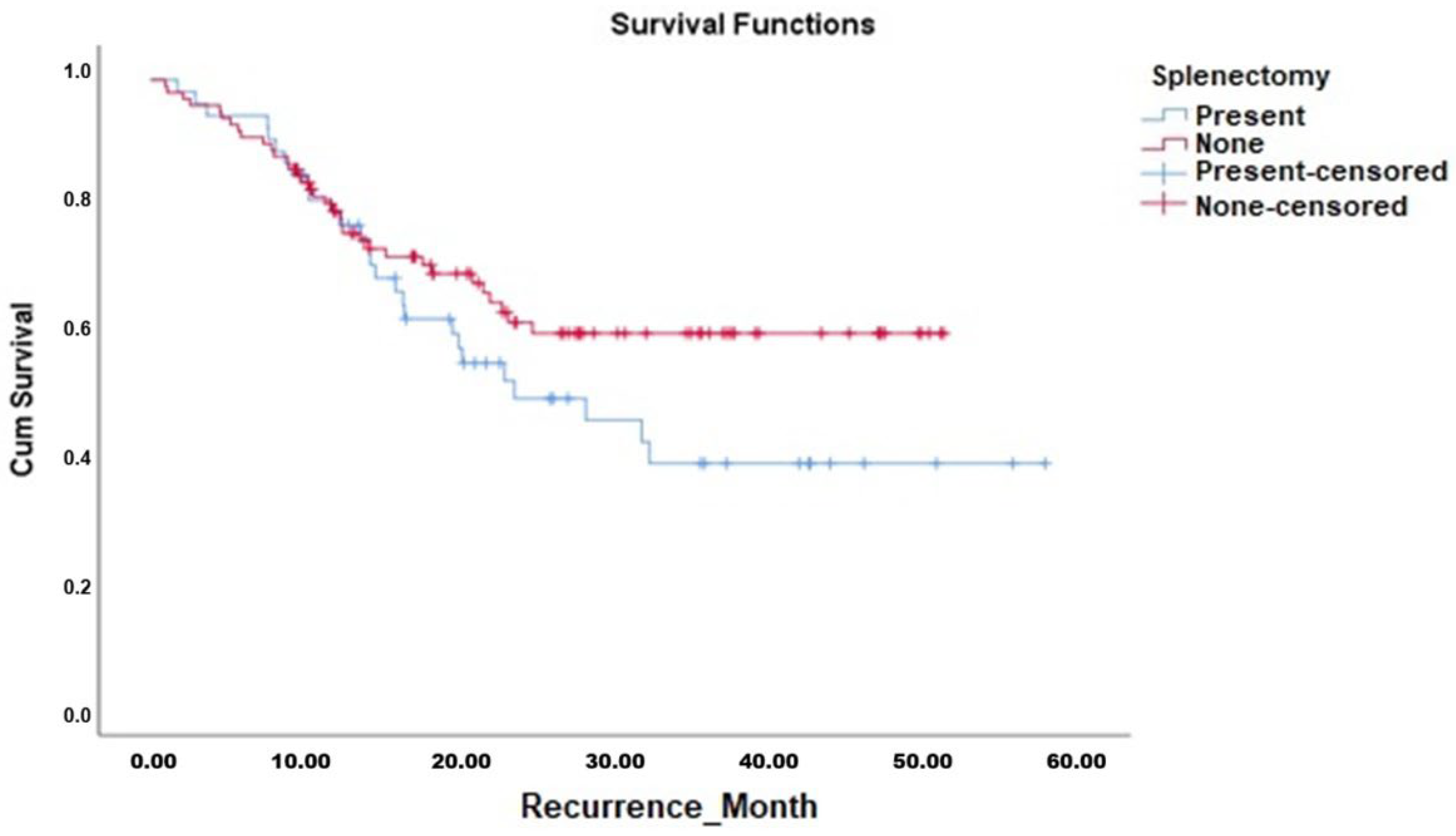
Kaplan–Meier survival analyses demonstrated that the OS duration in patients who underwent splenectomy (median 42.7 months, 95% CI 36.7–48.7 months) was comparable to those who did not undergo splenectomy (median 42.2 months, 95% CI 37.9–46.5 months). The Log-Rank test confirmed no significant difference between the survival curves (χ
2 = 0.187,
p = 0.665). Similarly, DFS analysis showed no significant difference between patients who underwent splenectomy (median 32.1 months, 95% CI 25.8–38.5 months) and those who did not (median 35.4 months, 95% CI 31.2–39.6 months; Log-Rank χ
2 = 2.202,
p = 0.138). The Kaplan–Meier curves for OS and DFS are presented in
Figure 1 and
Figure 2, respectively, with corresponding survival data detailed in
Table 6.
4. Discussion
This study aimed to analyze the morbidity and survival outcomes following splenectomy in patients undergoing CRS + HIPEC. Our findings demonstrated that splenectomy was significantly associated with prolonged ICU and hospital stay durations. While complications such as nephrotoxicity, hepatotoxicity, pneumothorax, pleural effusion, and pancreatic fistula were more frequently observed in patients who underwent splenectomy, the rates of major complications remained statistically similar between the splenectomy and non-splenectomy groups. Correspondingly, OS rates showed no significant differences between the two groups. However, when stratified by surgical indication, splenectomy demonstrated divergent prognostic impacts on DFS. In patients who underwent splenectomy due to the presence of peritoneal implants, DFS was independently and strongly associated with recurrence (OR = 17.814, p = 0.001), representing an approximately 18-fold increased risk. Conversely, splenectomy for hilar tumor invasion was independently associated with improved DFS (OR = 0.136, p = 0.021), likely reflecting the more extensive perisplenic lymphadenectomy required in these cases. This association was not observed in cases where the indication was iatrogenic injury, which showed a protective trend without reaching statistical significance. Additionally, peritoneal disease burden quantified as continuous PCI emerged as an independent predictor of both OS (OR = 1.150, p = 0.006) and DFS (OR = 1.166, p = 0.001), while primary tumor type showed no independent prognostic significance after adjusting for PCI and surgical factors (OS p = 0.345, DFS p = 0.163).
Non-hematological cancer cases have been associated with splenectomy as an additional surgical procedure, which has been shown to be associated with additional surgical complications and a poor prognostic effect. Studies evaluating the relationship between splenectomy and complications in patients with PC reveal that splenectomy in CRS + HIPEC procedures is associated with an increased frequency of pulmonary complications and a higher incidence of Grade 3–4 complications. In our study, although individual pulmonary complications were observed more frequently in the splenectomy group (pneumothorax, pleural effusion), multivariate analyses showed that major complications (Clavien–Dindo Grade ≥ 3) were not independently associated with splenectomy. Splenectomy was independently associated with prolonged hospitalization (OR = 1.058 per day, p = 0.030), likely reflecting overall surgical complexity and disease burden rather than representing a direct causal effect.
Saxena et al. conducted a comprehensive study including 936 patients to investigate the outcomes of splenectomy in all patients undergoing CRS + HIPEC. It was observed that patients who underwent splenectomy, approximately 418 patients, had higher PCIs, and underwent more aggressive surgical procedures such as major peritonectomy, partial gastrectomy, or colectomy. It was shown that patients who underwent splenectomy had a higher incidence of pancreatic fistula, infectious complications, intra-abdominal collection development, bleeding, intestinal fistula, and sepsis. An increase in all grade 3 and 4 complications, as well as prolonged hospital and ICU stays, were observed. Similarly, in the present study, similar to the report by Saxena et al., the need for splenectomy increased when the PCI was ≥6. Additionally, longer hospital and ICU stays in splenectomy patients were also demonstrated in our study. However, the isolated or overall increase in complications mentioned in the study by Saxena et al. was not observed to be associated with splenectomy in our study [
12].
In a study by Angeles et al. involving 992 patients who underwent HIPEC, splenectomy requirement was found to be associated with the development of gastric perforation, high BMI, and high PCI in 533 patients who underwent CRS. However, in our study group, no patients with gastric perforation were observed. Similarly to the study by Angeles et al., our study also showed a significant association between higher peritoneal disease burden and increased need for splenectomy [
15].
Regarding studies on patient survival, only studies associated with CRS + HIPEC in ovarian cancers have been identified in the literature. In a study involving 28 patients with ovarian cancer who underwent CRS + HIPEC, it was shown that there was no difference between patients who underwent splenectomy and those who did not [
9]. However, this study included only patients with splenectomized ovarian cancers and was primarily designed to evaluate hematological complications. Therefore, it would not be appropriate to make comparisons between the study and our study. In a study by Said et al., splenectomy was performed in 99 patients with advanced ovarian cancer, and their data were compared with those of patients who did not undergo splenectomy. Although patients who underwent splenectomy required more aggressive surgical interventions, surgical procedure reoperations, and blood transfusions and had more postoperative infections and longer ICU stays, similar rates of DFS and OS were found in these patients [
10]. In our study, no increased need for splenectomy, specifically in patients with ovarian cancers, was observed. When all patient groups were examined, it was shown that patients who underwent splenectomy had higher PCI scores, required more transfusions, and had longer ICU and hospital stays; this is similar to the findings in the study by Said et al. [
10]. However, in our study, the rates of postoperative surgical site infections and reoperation were similarly distributed between the groups. Disease-specific subgroup analysis for ovarian cancer was not feasible due to limited sample size (
n = 18 with only 2 deaths), precluding reliable survival comparisons stratified by splenectomy status. Nevertheless, in the overall cohort analysis, splenectomy indication emerged as a critical prognostic determinant: splenectomy performed for peritoneal implants was independently associated with worse DFS (OR = 17.814,
p = 0.001), while splenectomy for hilar invasion requiring extensive lymphadenectomy was protective (OR = 0.136,
p = 0.021).
In our study, no relationship was found between early postoperative mortality (<90 days) and splenectomy. When analyzed without stratification by indication, splenectomy showed no independent association with OS or DFS. However, stratification by surgical indication revealed critical prognostic differences: splenectomy for peritoneal implants independently predicted markedly increased recurrence (OR = 17.814, p = 0.001), while splenectomy for hilar tumor invasion was protective (OR = 0.136, p = 0.021). The association between peritoneal implant splenectomy and recurrence primarily reflects the extent of peritoneal disease burden rather than the splenectomy procedure itself. Patients requiring splenectomy for peritoneal implants had significantly higher PCI scores (median 9 vs. 3, p = 0.010) and more extensive peritoneal involvement, which independently drives recurrence risk.
Several limitations should be considered when interpreting our findings. The retrospective design limits our ability to control for all confounding factors. Patient selection for CRS + HIPEC is individualized based on tumor characteristics and clinical condition. Our cohort included patients with different primary malignancies, each with different biological behavior and prognosis. This heterogeneity makes it difficult to separate the effects of splenectomy from the effects of underlying disease.
The rarity of some conditions prevented reliable disease-specific analyses (
Supplementary Materials). Therefore, survival outcomes may reflect tumor biology and disease burden rather than splenectomy itself. For this reason, we analyzed the entire cohort to maintain statistical power while acknowledging this limitation. In view of these limitations, we analyzed the widest possible range of parameters to achieve the most comprehensive evaluation of patient survival and postoperative complications. Further evaluations with more homogenous and disease specific studies are needed.
While our stratified analysis by splenectomy indication revealed divergent prognostic effects (implant indicated splenectomy predicting worse DFS vs. hilar invasion-related splenectomy being protective), we cannot definitively establish causality. The finding that hilar invasion splenectomy improves DFS while peritoneal implant splenectomy worsens outcomes cannot fully separate whether benefits derive from completeness of lymphadenectomy, en bloc resection technique, or unmeasured confounders such as intrinsic tumor biology. Prospective studies with standardized lymph node dissection protocols, pathologic assessment of lymph node yields and metastatic involvement, and ideally propensity score matching or randomized comparison would be required to definitively establish whether lymphadenectomy completeness causally determines outcomes.
Even when analyzing the overall cohort without disease-specific stratification, sample sizes within splenectomy indication subgroups were limited, resulting in wide confidence intervals and limited statistical power for detecting small effect sizes. The protective trend observed with iatrogenic splenectomy (OR = 0.133, p = 0.120), while reassuring, did not reach statistical significance and should be interpreted cautiously given the small sample size.
Also exploring complications for CRS + HIPEC in a heterogenous cohorts should be carefully evaluated. As for disease specific regimens used in protocols, tendency for complications may cause different results. Our cohort consists of two different hipec protocols. OS and DFS analysis stratified by tumor type was not feasible due to small sample sizes in most diagnostic categories (gastric n = 20, ovarian n = 18, mesothelioma n = 9) and insufficient outcome events. Notably, pseudomyxoma peritonei demonstrated zero mortality events (excellent prognosis), precluding any statistical comparison. This limitation also depends on both temporal factors (patients operated in 2021–2022 with <2 years follow-up) and the favorable oncologic outcomes achieved in certain diagnostic subgroups.
Also these limitations reflect the natural challenges of studying rare peritoneal surface malignancies, where even multi-year single-center cohorts struggle to achieve adequate sample sizes for disease-specific analyses. Multicenter collaborative studies or international registry data would be required to adequately power subgroup analyses by tumor type.
This study has several notable strengths. The stratified analysis by splenectomy indication represents a novel approach not previously reported in peritoneal surface malignancy literature, revealing prognostic differences obscured in aggregate analyses. Complete perioperative data collection, standardized HIPEC protocols, and detailed documentation of splenectomy indications strengthen the validity of our findings. The analysis of PCI as a continuous variable rather than dichotomized cutoffs provides superior prognostic stratification and validates the Sugarbaker index as a quantitative biomarker. Finally, inclusion of both morbidity and survival outcomes provides comprehensive evaluation of splenectomy’s impact on perioperative and oncologic endpoints.