Global Trends in Joint Arthroplasty: A Systematic Review and Future Projections
Abstract
1. Introduction
2. Objectives
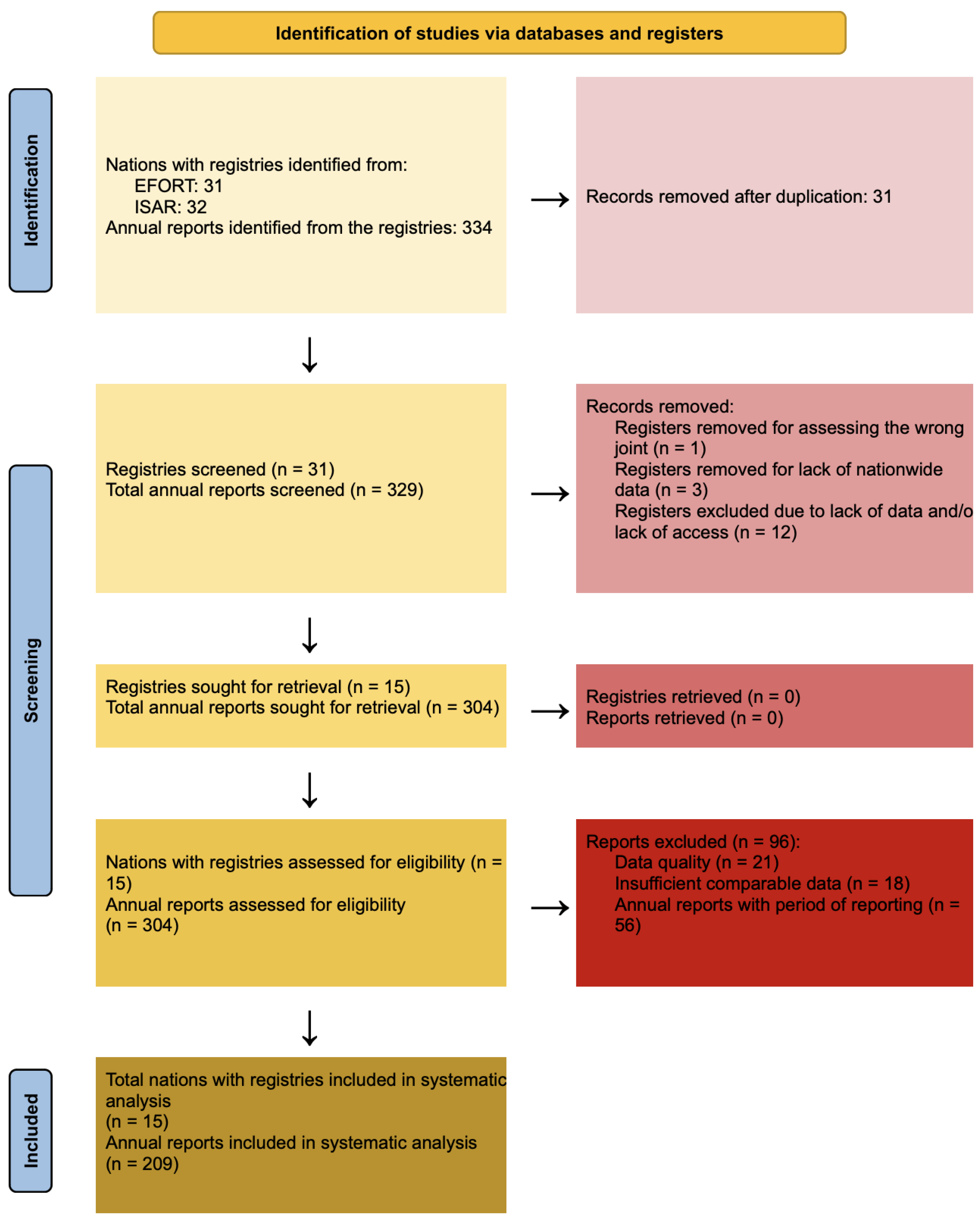
3. Methods
3.1. Eligibility Criteria
- National scope and coverage: nationwide capture (or a national mandate) with documented completeness sufficient for incidence/trend analysis (e.g., audit, statutory requirement, or published completeness estimates).
- Continuity of reporting: a clearly specified stretch of consecutive annual reports prior to 2020 adequate for modelling (recorded in years for each registry). No more than one missing year within any rolling five-year window was acceptable.
- Data granularity and consistency: availability of annual counts for primary and, where applicable, revision THA/TKA with stable procedure definitions over time or documented definitional changes amenable to harmonisation.
- Validation and quality controls: evidence of internal or external data quality procedures (e.g., cross-linkage, audit summaries, or formal data quality statements) described in registry documentation.
3.2. Information Sources
3.3. Search Strategy
3.4. Selection Process
3.5. Data Collection Process
3.6. Data Items
3.7. Study Risk of Bias Assessment
3.8. Synthesis Methods
4. Results
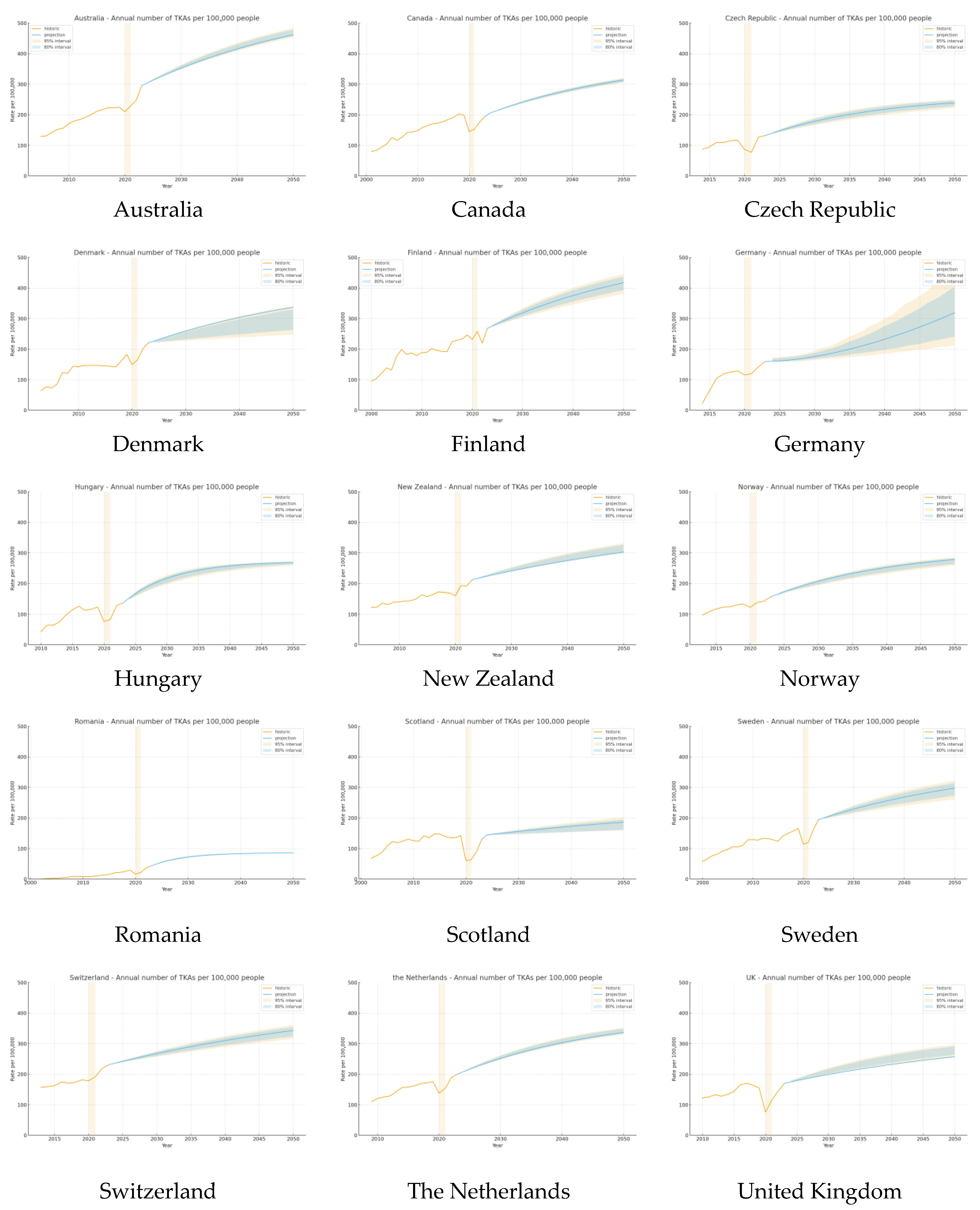
4.1. Total Knee Arthroplasty
4.2. Total Hip Arthroplasty
5. Discussion
5.1. Total Knee Arthroplasty
5.2. Total Hip Arthroplasty
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUD | Australian dollars (AUD) at first use. |
| CI | Confidence interval (CI). |
| COVID-19 | Coronavirus disease 2019 (COVID-19). |
| EPRD | German Arthroplasty Registry (Endoprothesenregister Deutschland, EPRD) at first mention. |
| OECD | Organisation for Economic Co-operation and Development (OECD). |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). |
| PROSPERO | International Prospective Register of Systematic Reviews (PROSPERO). |
| THA | Total hip arthroplasty (THA). |
| TJA | Total joint arthroplasty (TJA). |
| TKA | Total knee arthroplasty (THA). |
| UK | United Kingdom (UK). |
| USD | US dollars (USD). |
| WHO | World Health Organization (WHO). |
References
- Evans, J.T.; Walker, R.W.; Evans, J.P.; Blom, A.W.; Sayers, A.; Whitehouse, M.R. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Blümel, S.; Hanauer, M.; Heimann, A.; Tannast, M.; Schwab, J.M. Cost and resource comparison analysis for THA in Switzerland and Austria. Int. J. Technol. Assess Health Care 2024, 40, e36. [Google Scholar] [CrossRef] [PubMed]
- Daigle, M.E.; Weinstein, A.M.; Katz, J.N.; Losina, E. The cost-effectiveness of total joint arthroplasty: A systematic review of published literature. Best Pract. Res. Clin. Rheumatol. 2012, 26, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.F.; Lee, Y.y.; Su, E.P.; McLawhorn, A.S. Quality-Adjusted Life Years After Hip and Knee Arthroplasty: Health-Related Quality of Life After 12,782 Joint Replacements. JBJS Open Access 2018, 3, e0007. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; de Steiger, R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Klug, A.; Gramlich, Y.; Rudert, M.; Drees, P.; Hoffmann, R.; Weißenberger, M.; Kutzner, K.P. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2021, 29, 3287–3298. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplasty 2012, 27, 61–65.e1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.S.; McKenzie, J.E.; Welch, V.A.; Brennan, S.E. Strengthening systematic reviews in public health: Guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J. Public Health 2022, 44, e588–e592. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane, 2022. Available online: https://www.training.cochrane.org/handbook (accessed on 8 November 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation. GBD Results. IHME: Seattle, WA, USA. Available online: https://vizhub.healthdata.org/gbd-results (accessed on 23 May 2025).
- World Health Organization. Musculoskeletal Health. World Health Organization: Geneva, Switzerland. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 23 May 2025).
- OECD. Health at a Glance 2023: OECD Indicators; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Lewis, P.L.; Gill, D.R.; McAuliffe, M.J.; McDougall, C.; Stoney, J.D.; Vertullo, C.J.; Wall, C.J.; Corfield, S.; Du, P.; Holder, C.; et al. Hip, Knee and Shoulder Arthroplasty: 2024 Annual Report, Australian Orthopaedic Association National Joint Replacement Registry; AOA: Adelaide, Australia, 2024. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information. Hip and Knee Replacements in Canada, 2014–2015: Canadian Joint Replacement Registry Annual Report; CIHI: Ottawa, ON, Canada, 2017. [Google Scholar]
- Institute of Health Information and Statistics of the Czech Republic. National Registry of Joint Replacement. Institute of Health Information and Statistics of the Czech Republic: Prague, Czech Republic. Available online: https://www.uzis.cz/index-en.php?pg=publications--library&id=275 (accessed on 24 May 2025).
- Danish Orthopaedic Society. Danish Hip Arthroplasty Register (DHR)—Annual Reports. Danish Orthopaedic Society: Aarhus, Denmark. Available online: https://danskhoftealloplastikregister.dk/en/publications/annual-reports/ (accessed on 24 May 2025).
- National Institute for Health and Welfare. Finnish Arthroplasty Register (FAR). National Institute for Health and Welfare: Helsinki, Finland. Available online: https://www2.thl.fi/endo/report/#index (accessed on 24 May 2025).
- Von Langenbeck, B. Zur Resection Des Kniegellenks. Verhandlungen Dtsch. Ges. Churg. 1878, 7, 23–30. [Google Scholar]
- Hungarian Orthopaedic Society. Hungarian Arthroplasty Register (MPR). Hungarian Orthopaedic Society: Budapest, Hungary. Available online: https://protreg.neak.gov.hu (accessed on 24 May 2025).
- New Zealand Orthopaedics Association. The New Zealand Joint Registry (NZJR). Wellington, New Zealand. Available online: https://www.nzoa.org.nz/annual-reports (accessed on 24 May 2025).
- Norwegian Orthopaedic Association. Norwegian Arthroplasty Register (NRL)—Annual Reports. Norwegian Orthopaedic Association: Bergen, Norway. Available online: https://www.helse-bergen.no/nasjonalt-kvalitets-og-kompetansenettverk-for-leddproteser-og-hoftebrudd/arsrapporter/#annual-reports (accessed on 24 May 2025).
- Romanian Ministry of Health and Family. Romanian Arthroplasty Register (RAR). Romanian Ministry of Health and Family: Bucharest, Romania. Available online: https://www.rne.ro/rne/informatii/ (accessed on 24 May 2025).
- Public Health Scotland. Scottish Arthroplasty Project (SAP). Public Health Scotland: Glasgow, Scotland. Available online: https://publichealthscotland.scot/publications/ (accessed on 24 May 2025).
- Swedish Arthroplasty Register. Swedish Arthroplasty Register (SAR)—Annual Reports. Swedish Arthroplasty Register: Gothenburg, Sweden. Available online: https://sar.registercentrum.se/about-the-register/annual-reports/p/SJW4-ZGyo (accessed on 24 May 2025).
- Swedish Hip Arthroplasty Register. Swedish Hip Arthroplasty Register (SHAR)—Annual Reports. Swedish Hip Arthroplasty Register: Gothenburg, Sweden. Available online: https://sar.registercentrum.se/about-the-register/annual-reports/swedish-hip-arthroplasty-register-annual-reports/p/S1Q-LDUBj (accessed on 24 May 2025).
- Swedish Knee Arthroplasty Register. Swedish Knee Arthroplasty Register (SKAR)—Annual Reports. Swedish Knee Arthroplasty Register: Lund, Sweden. Available online: https://www.myknee.se/en/publications/annual-reports (accessed on 24 May 2025).
- Swiss Federal Office of Public Health. Swiss National Joint Registry (SIRIS). Swiss Federal Office of Public Health: Bern, Switzerland. Available online: https://www.siris-implant.ch/en/Downloads&category=16 (accessed on 24 May 2025).
- Landelijke Registratie Orthopedische Interventies. Dutch Arthroplasty Register (LROI)—Annual Reports. Netherlands: Landelijke Registratie Orthopedische Interventies. Available online: https://www.lroi.nl/jaarrapportage/ (accessed on 16 November 2025).
- National Joint Registry UK (NJR). Available online: https://reports.njrcentre.org.uk/downloads (accessed on 24 May 2025).
- Canadian Institute for Health Information. Canadian Joint Replacement Registry (CJRR). Canadian Institute for Health Information: Ottawa, ON, Canada. Available online: https://www.cihi.ca/en/canadian-joint-replacement-registry-cjrr (accessed on 24 May 2025).
- World Health Organization. Years of Healthy Life Lost Due to Disability (YLD). World Health Organization: Geneva, Switzerland. Available online: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/mca/years-of-healthy-life-lost-due-to-disability-(yld) (accessed on 24 May 2025).
- Romanini, E.; Decarolis, F.; Luzi, I.; Zanoli, G.; Venosa, M.; Laricchiuta, P.; Carrani, E.; Torre, M. Total knee arthroplasty in Italy: Reflections from the last fifteen years and projections for the next thirty. Int. Orthop. 2019, 43, 133–138. [Google Scholar] [CrossRef]
- Nemes, S.; Gordon, M.; Rogmark, C.; Rolfson, O. Projections of total hip replacement in Sweden from 2013 to 2030. Acta Orthop. 2014, 85, 238–243. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Inacio, M.C.S.; Paxton, E.W.; Graves, S.E.; Namba, R.S.; Nemes, S. Projected increase in total knee arthroplasty in the United States-an alternative projection model. Osteoarthr. Cartil. 2017, 25, 1797–1803. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. 2018, 100, 1455–1460. [Google Scholar] [CrossRef]
- Losina, E.; Thornhill, T.S.; Rome, B.N.; Wright, J.; Katz, J.N. The Dramatic Increase in Total Knee Replacement Utilization Rates in the United States Cannot Be Fully Explained by Growth in Population Size and the Obesity Epidemic. J. Bone Jt. Surg. Am. 2012, 94, 201–207. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Rome, B.N.; Reichmann, W.M.; Collins, J.E.; Burbine, S.A.; Thornhill, T.S.; Wright, J.; Katz, J.N.; Losina, E. Estimating the Burden of Total Knee Replacement in the United States. J. Bone Jt. Surg. 2013, 95, 385–392. [Google Scholar] [CrossRef]
- Worlicek, M.; Koch, M.; Daniel, P.; Freigang, V.; Angele, P.; Alt, V.; Kerschbaum, M.; Rupp, M. A retrospective analysis of trends in primary knee arthroplasty in Germany from 2008 to 2018. Sci. Rep. 2021, 11, 5225. [Google Scholar] [CrossRef]
- Hamilton, D.F.; Howie, C.R.; Burnett, R.; Simpson, A.H.R.W.; Patton, J.T. Dealing with the predicted increase in demand for revision total knee arthroplasty: Challenges, risks and opportunities. Bone Jt. J. 2015, 97, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Dubin, J.A.; Bains, S.S.; Hameed, D.; Gottlich, C.; Turpin, R.; Nace, J.; Mont, M.; Delanois, R.E. Projected volume of primary total joint arthroplasty in the USA from 2019 to 2060. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 2663–2670. [Google Scholar] [CrossRef]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JBJS Open Access 2023, 8, e22.00112. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.C.S.; Graves, S.E.; Pratt, N.L.; Roughead, E.E.; Nemes, S. Increase in Total Joint Arthroplasty Projected from 2014 to 2046 in Australia: A Conservative Local Model With International Implications. Clin. Orthop. 2017, 475, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Moldovan, L.; Bataga, T. A Comprehensive Research on the Prevalence and Evolution Trend of Orthopedic Surgeries in Romania. Healthcare 2023, 11, 1866. [Google Scholar] [CrossRef]
- Pilz, V.; Hanstein, T.; Skripitz, R. Projections of primary hip arthroplasty in Germany until 2040. Acta Orthop. 2018, 89, 308–313. [Google Scholar] [CrossRef]
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of Primary TKA and THA in Germany From 2016 Through 2040. Clin. Orthop. 2020, 478, 1622–1633. [Google Scholar] [CrossRef]
- AAOS Annual Meeting. Available online: https://aaos-annualmeeting-presskit.org/2023/research-news/fact-sheet-orthopaedic-surgeons-will-need-to-double-total-joint-arthroplasty-caseload-to-meet-demand-by-2050/ (accessed on 17 August 2025).
- Sloan, M.; Premkumar, A.; Sheth, N.P. Future Demand for Total Joint Arthroplasty Drives Renewed Interest in Arthroplasty Fellowship. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2020, 16 (Suppl. S2), 210–215. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons. American Joint Replacement Registry (AJRR). Rosemont, Illinois. Available online: https://www.aaos.org/registries/publications/ajrr-annual-report/ (accessed on 13 July 2025).
- National Joint Registry: 17th Annual Report 2020. National Joint Registry. Available online: https://reports.njrcentre.org.uk/2019 (accessed on 13 July 2025).
- Clement, N.D.; Scott, C.E.H.; Murray, J.R.D.; Howie, C.R.; Deehan, D.J. The number of patients “worse than death” while waiting for a hip or knee arthroplasty has nearly doubled during the COVID-19 pandemic: A UK nationwide survey. Bone Jt. J. 2021, 103, 672–680. [Google Scholar] [CrossRef]
- Reddy, K.; Gharde, P.; Tayade, H.; Patil, M.; Reddy, L.S.; Surya, D. Advancements in Robotic Surgery: A Comprehensive Overview of Current Utilizations and Upcoming Frontiers. Cureus 2023, 15, e50415. [Google Scholar] [CrossRef]
- Lefetz, O.; Baste, J.M.; Hamel, J.F.; Mordojovich, G.; Lefevre-Scelles, A.; Coq, J.M. Robotic surgery and work-related stress: A systematic review. Appl. Ergon. 2024, 117, 104188. [Google Scholar] [CrossRef]
- Haffar, A.; Krueger, C.A.; Goh, G.S.; Lonner, J.H. Total Knee Arthroplasty With Robotic Surgical Assistance Results in Less Physician Stress and Strain Than Conventional Methods. J. Arthroplasty 2022, 37, S193–S200. [Google Scholar] [CrossRef]

| Australia | UK | Canada | Germany | Sweden | Hungary | Finland | Denmark | Romania | New Zealand | Switzerland | Scotland | The Netherlands | Czech Republic | Norway | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THA | |||||||||||||||
| 2015–19 | 111% | 111% | 114% | incomplete data | 114% | 92% | 119% | 113% | 124% | 104% | 110% | 99% | 112% | 116% | 124% |
| 2015–23 | 128% | 123% | 115% | incomplete data | 125% | 106% | 124% | 127% | 149% | 112% | 129% | 95% | 120% | 139% | 116% |
| 2019–23 | 115% | 111% | 101% | 116% | 110% | 115% | 104% | 112% | 121% | 108% | 118% | 96% | 107% | 120% | 93% |
| TKA | |||||||||||||||
| 2015–19 | 106% | 107% | 111% | incomplete data | 134% | 108% | 129% | 126% | 189% | 107% | 112% | 97% | 111% | 124% | 114% |
| 2015–23 | 140% | 118% | 108% | incomplete data | 157% | 120% | 140% | 152% | 284% | 135% | 142% | 88% | 127% | 140% | 134% |
| 2019–23 | 132% | 110% | 97% | 124% | 117% | 111% | 109% | 120% | 150% | 126% | 128% | 91% | 114% | 113% | 117% |
| Country | TKA—2011 OECD/Registry | TKA—2019 OECD/Registry | TKA—2021 OECD/Registry | THA—2011 OECD/Registry | THA—2019 OECD/Registry | THA—2021 OECD/Registry |
|---|---|---|---|---|---|---|
| Australia | 178/180 | 203/224 | 252/229 | 171/122 | 171/160 | 198/164 |
| Canada | 161/159 | 198/199 | 152/152 | 131/129 | 168/168 | 153/152 |
| Czech Republic | 110/n.a. | 121/117 | 108/77 | 160/116 | 173/152 | 198/126 |
| Denmark | 169/146 | 203/182 | 173/164 | 227/154 | 259/192 | 236/174 |
| Finland | 193/189 | 242/246 | 260/258 | 225/143 | 280/196 | 284/192 |
| Germany | 210/n.a. | 227/128 | 201/119 | 290/n.a. | 315/170 | 301/166 |
| Hungary | 55/64 | 94/123 | 28/82 | 113/124 | 147/162 | 79/100 |
| New Zealand | 93/143 | 109/168 | 103/193 | 142/165 | 160/189 | 147/208 |
| Norway | 89/n.a. | 117/134 | 122/138 | 243/n.a. | 268/201 | 255/173 |
| Romania | 13/7 | 27/28 | 18/21 | 52/28 | 77/44 | 65/38 |
| Scotland | n.a./123 | n.a./142 | n.a./63 | n.a./131 | n.a./146 | n.a./96 |
| Sweden | 129/127 | 141/165 | 100/120 | 238/169 | 249/191 | 215/167 |
| Switzerland | 214/n.a. | 260/181 | 273/191 | 300/n.a. | 313/235 | 323/252 |
| The Netherlands | 137/125 | 155/175 | n.a./153 | 226/145 | 254/188 | n.a./178 |
| UK | 140/125 | 142/154 | 108/115 | 177/113 | 182/142 | 180/125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stubnya, B.G.; Schulz, M.; Váncsa, S.; Szilágyi, G.S.; Szatmári, A.; Bejek, Z. Global Trends in Joint Arthroplasty: A Systematic Review and Future Projections. J. Clin. Med. 2025, 14, 8214. https://doi.org/10.3390/jcm14228214
Stubnya BG, Schulz M, Váncsa S, Szilágyi GS, Szatmári A, Bejek Z. Global Trends in Joint Arthroplasty: A Systematic Review and Future Projections. Journal of Clinical Medicine. 2025; 14(22):8214. https://doi.org/10.3390/jcm14228214
Chicago/Turabian StyleStubnya, Bence Gusztáv, Mercedes Schulz, Szilárd Váncsa, Gábor Sándor Szilágyi, Attila Szatmári, and Zoltán Bejek. 2025. "Global Trends in Joint Arthroplasty: A Systematic Review and Future Projections" Journal of Clinical Medicine 14, no. 22: 8214. https://doi.org/10.3390/jcm14228214
APA StyleStubnya, B. G., Schulz, M., Váncsa, S., Szilágyi, G. S., Szatmári, A., & Bejek, Z. (2025). Global Trends in Joint Arthroplasty: A Systematic Review and Future Projections. Journal of Clinical Medicine, 14(22), 8214. https://doi.org/10.3390/jcm14228214






