Prevalence of Familial Hypercholesterolemia and Its Association with Cardiovascular Risk in a Cross-Sectional Adult Population
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design
2.2. Study Population
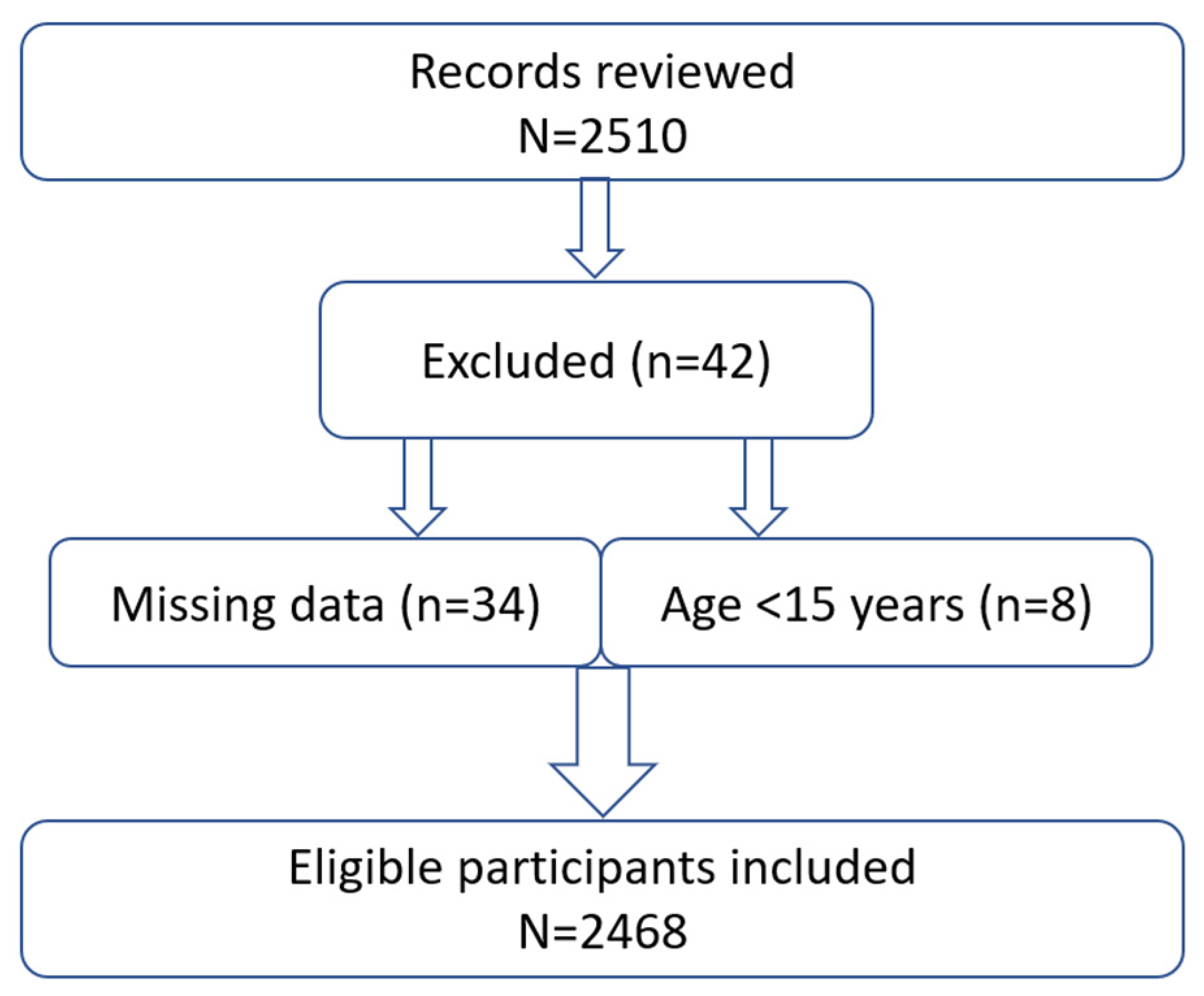
2.3. Sample Size and Recruitment
2.4. Variables and Definitions
2.5. Ethics
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings and Their Explanations
4.2. Future Implications and Directions
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FH | Familial hypercholesterolemia |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TC | Total cholesterol |
| TG | Triglyceride |
| CVD | Cardiovascular diseases |
| ASCVD | Atherosclerotic cardiovascular disease |
| DLCN | Dutch Lipid Clinic Network |
| HbA1c | Glycated hemoglobin |
| BMI | Body mass index |
| MI | Myocardial infarction |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
References
- Tokgozoglu, L.; Kayikcioglu, M. Familial Hypercholesterolemia: Global Burden and Approaches. Curr. Cardiol. Rep. 2021, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients with Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Isla, L.; Alonso, R.; Mata, N.; Saltijeral, A.; Muñiz, O.; Rubio-Marin, P.; Diaz-Diaz, J.L.; Fuentes, F.; de Andrés, R.; Zambón, D.; et al. Coronary Heart Disease, Peripheral Arterial Disease, and Stroke in Familial Hypercholesterolaemia: Insights from the SAFEHEART Registry (Spanish Familial Hypercholesterolaemia Cohort Study). Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Watts, G.F.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Familial hypercholesterolemia in the danish general population: Prevalence, coronary artery disease, and cholesterol-lowering medication. J. Clin. Endocrinol. Metab. 2012, 97, 3956–3964, Erratum in J. Clin. Endocrinol. Metab. 2014, 99, 4758–4759. [Google Scholar] [CrossRef] [PubMed]
- Bekbossynova, M.S.; Ivanova-Razumova, T.V.; Kali, A.; Sailybayeva, A.S.; Khamitov, S.R.; Oralbekova, Z.O. The Prevalence of Different Genotypic Forms of Familial Hypercholesterolemia in Relation to Race and Ethnicity. J. Clin. Med. Kaz. 2024, 21, 38–45. [Google Scholar] [CrossRef]
- Kou, H.; Wang, H.; Liu, P.; Wang, X.; Zhu, W.; Jiang, W.; Hu, X.; Deng, J. Prevalence, clinical features and prognosis of familial hypercholesterolemia in Chinese Han patients with acute coronary syndrome after a coronary event: A retrospective observational study. BMC Cardiovasc. Disord. 2024, 24, 144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duell, P.B.; Gidding, S.S.; Andersen, R.L.; Knickelbine, T.; Anderson, L.; Gianos, E.; Shrader, P.; Kindt, I.; O’Brien, E.C.; McCann, D.; et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: The CASCADE FH registry. Atherosclerosis 2019, 289, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Republic of Kazakhstan. Clinical Protocol for Diagnosis and Treatment of “Atherogenic Lipid Metabolism Disorders (Dyslipidemia)”. Available online: https://diseases.medelement.com (accessed on 15 July 2025).
- Harada-Shiba, M.; Arai, H.; Ohmura, H.; Okazaki, H.; Sugiyama, D.; Tada, H.; Dobashi, K.; Matsuki, K.; Minamino, T.; Yamashita, S.; et al. Guidelines for the Diagnosis and Treatment of Adult Familial Hypercholesterolemia 2022. J. Atheroscler. Thromb. 2023, 30, 558–586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Ferranti, S.D.; Rodday, A.M.; Mendelson, M.M.; Wong, J.B.; Leslie, L.K.; Sheldrick, R.C. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016, 133, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Akioyamen, L.E.; Genest, J.; Shan, S.D.; Reel, R.L.; Albaum, J.M.; Chu, A.; Tu, J.V. Estimating the prevalence of heterozygous familial hypercholesterolaemia: A systematic review and meta-analysis. BMJ Open 2017, 7, e016461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Sá, A.C.M.G.N.; Gomes, C.S.; Prates, E.J.S.; Brant, L.C.C.; Malta, D.C. Prevalence and factors associated with possible cases of familial hypercholesterolemia in Brazilian adults: A cross-sectional study. Sci. Rep. 2023, 13, 20459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toft-Nielsen, F.; Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. Clinical familial hypercholesterolemia—Factors influencing diagnosis and cardiovascular risk in the general population. Eur. J. Intern. Med. 2025, 136, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dron, J.S.; Bellows, B.K.; Khera, A.V.; Liu, J.; Balte, P.P.; Oelsner, E.C.; Amr, S.S.; Lebo, M.S.; Nagy, A.; et al. Familial Hypercholesterolemia Variant and Cardiovascular Risk in Individuals with Elevated Cholesterol. JAMA Cardiol. 2024, 9, 263–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banda, J.M.; Sarraju, A.; Abbasi, F.; Parizo, J.; Pariani, M.; Ison, H.; Briskin, E.; Wand, H.; Dubois, S.; Jung, K.; et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. npj Digit. Med. 2019, 2, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knowles, J.W. Statins in Familial Hypercholesterolemia: Translating Evidence to Action. J. Am. Coll. Cardiol. 2016, 68, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Luirink, I.K.; Wiegman, A.; Kusters, D.M.; Hof, M.H.; Groothoff, J.W.; de Groot, E.; Kastelein, J.J.P.; Hutten, B.A. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N. Engl. J. Med. 2019, 381, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Pyrpyris, N.; Iliakis, P.; Beneki, E.; Adamopoulou, E.; Papanikolaou, A.; Konstantinidis, D.; Fragkoulis, C.; Kollias, A.; Aznaouridis, K.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Patients Following Acute Coronary Syndromes: From Lipid Lowering and Plaque Stabilization to Improved Outcomes. J. Clin. Med. 2024, 13, 5040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso, R.; Perez de Isla, L.; Muñiz-Grijalvo, O.; Mata, P. Barriers to Early Diagnosis and Treatment of Familial Hypercholesterolemia: Current Perspectives on Improving Patient Care. Vasc. Health Risk Manag. 2020, 16, 11–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinnear, F.J.; Wainwright, E.; Perry, R.; Lithander, F.E.; Bayly, G.; Huntley, A.; Cox, J.; Shield, J.P.; Searle, A. Enablers and barriers to treatment adherence in heterozygous familial hypercholesterolaemia: A qualitative evidence synthesis. BMJ Open 2019, 9, e030290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, C.L.; Zordok, M.; Kullo, I.J. Familial hypercholesterolemia in Southeast and East Asia. Am. J. Prev. Cardiol. 2021, 6, 100157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amerizadeh, A.; Javanmard, S.H.; Sarrafzadegan, N.; Vaseghi, G. Familial Hypercholesterolemia (FH) Registry Worldwide: A Systematic Review. Curr. Probl. Cardiol. 2022, 47, 100999. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.L.; Tcheandjieu, C.; Hilliard, A.T.; Lee, K.M.; Lynch, J.; Chang, K.M.; Miller, D.; Knowles, J.W.; O’Donnell, C.; Tsao, P.S.; et al. Coronary Artery Disease Risk of Familial Hypercholesterolemia Genetic Variants Independent of Clinically Observed Longitudinal Cholesterol Exposure. Circ. Genom. Precis. Med. 2022, 15, e003501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variable Name | Definitions/Measurements |
|---|---|
| Familial Hypercholesterolemia (FH) | Classified by Dutch Lipid Clinic Network score: “probable FH” if score 6–8, “possible FH” if 3–5, “unlikely” if <3. |
| LDL-C, HDL-C, Total Cholesterol, Triglycerides | Fasting lipid profile (mmol/L) measured via standard enzymatic assays in certified labs. |
| HbA1c | Glycated hemoglobin (%), measured by high-performance liquid chromatography (NGSP/DCCT-aligned). |
| Body Mass Index (BMI) | Calculated as weight(kg)/[height(m)]2 from medical records. |
| Cardiovascular Disease (CVD) | History of coronary heart disease, myocardial infarction, stroke, or peripheral arterial disease (from medical records). |
| Family History | First-degree relative with hypertension or premature CVD (men <55 or women <60 years), recorded as yes/no for each. |
| Smoking Status | Categorized as current smoker, former smoker, or never smoker. |
| Alcohol Intake | Consumption of alcohol (yes/no). |
| Statin Use | Currently on statin medication (yes/no) as recorded in prescription list. |
| Aspirin Use | Currently on aspirin therapy (yes/no) as recorded in prescription list. |
| Characteristics | N (2468) | Percent (%) |
|---|---|---|
| Gender | ||
| female | 1596 | 64.67 |
| male | 872 | 35.33 |
| Age, years | 2468 | 45.2 (14.3) |
| Familial hypercholesterolemia status | ||
| no | 2292 | 92.87 |
| possible | 166 | 6.73 |
| probable | 10 | 0.41 |
| Education level | ||
| graduate | 1482 | 60.05 |
| postgraduate | 72 | 2.92 |
| school completed (11th grade) | 789 | 31.97 |
| school completed (9th grade) | 125 | 5.06 |
| Ethnicity | ||
| Kazakh | 1563 | 63.33 |
| Russian | 566 | 22.93 |
| other | 339 | 13.74 |
| Occupation | ||
| private worker | 1215 | 49.23 |
| public worker | 422 | 17.1 |
| retiree | 378 | 15.32 |
| student | 105 | 4.25 |
| unemployed | 348 | 14.1 |
| Smoking status | ||
| no | 2081 | 84.32 |
| yes | 387 | 15.68 |
| Alcohol intake | ||
| no | 1052 | 42.63 |
| yes | 1416 | 57.37 |
| CVD | ||
| no | 2010 | 81.44 |
| yes | 458 | 18.56 |
| Statin use | ||
| no | 2302 | 93.27 |
| yes | 166 | 6.73 |
| Hypertension among relatives | ||
| do not know | 36 | 1.46 |
| no | 1526 | 61.83 |
| yes | 906 | 36.71 |
| MI among relatives | ||
| do not know | 31 | 1.26 |
| no | 2101 | 85.13 |
| yes | 336 | 13.61 |
| HDL-C, mmol/L | 2468 | 1.37 (0.32) |
| LDL-C, mmol/L | 2468 | 3.34 (0.94) |
| Triglyceride, mmol/L | 2468 | 1.36 (1.06) |
| Total cholesterol, mmol/L | 2468 | 5.24 (1.32) |
| HbA1c, % | 2468 | 5.57 (0.93) |
| SBP, mmHg | 2468 | 124.01 (18.54) |
| DBP, mmHg | 2468 | 81.69 (11.01) |
| Body mass index | 2468 | 25.92 (4.75) |
| Characteristic | No FH (n = 2292) | Possible FH (n = 166) | Probable FH (n = 10) | p-Value |
|---|---|---|---|---|
| Age, years | 44.5 ± 14.3 | 55.2 ± 10.0 | 51.0 ± 9.7 | <0.001 |
| Female, % | 64.5% | 67.5% | 50.0% | 0.2834 |
| LDL-C, mmol/L | 3.20 ± 0.80 | 5.04 ± 0.63 | 6.74 ± 1.07 | <0.001 |
| HDL-C, mmol/L | 1.36 ± 0.39 | 1.53 ± 0.40 | 1.80 ± 0.47 | <0.001 |
| Triglycerides, mmol/L | 1.29 ± 0.77 | 2.03 ± 1.01 | 1.72 ± 0.58 | <0.001 |
| Total cholesterol, mmol/L | 5.06 ± 1.01 | 7.40 ± 1.07 | 9.70 ± 1.35 | <0.001 |
| HbA1c, % | 5.56 ± 0.87 | 5.67 ± 0.74 | 5.70 ± 0.64 | 0.3644 |
| BMI, kg/m2 | 25.9 ± 4.7 | 27.1 ± 5.1 | 25.4 ± 3.9 | <0.001 |
| SBP, mmHg | 122.9 ± 18.2 | 128.2 ± 18.3 | 134.7 ± 21.4 | <0.001 |
| DBP, mmHg | 81.0 ± 10.8 | 84.5 ± 11.9 | 80.2 ± 9.1 | <0.001 |
| Current smoker, % | 15.5% | 13.3% | 10.0% | 0.4021 |
| Statin use, % | 6.2% | 13.3% | 10.0% | 0.0011 |
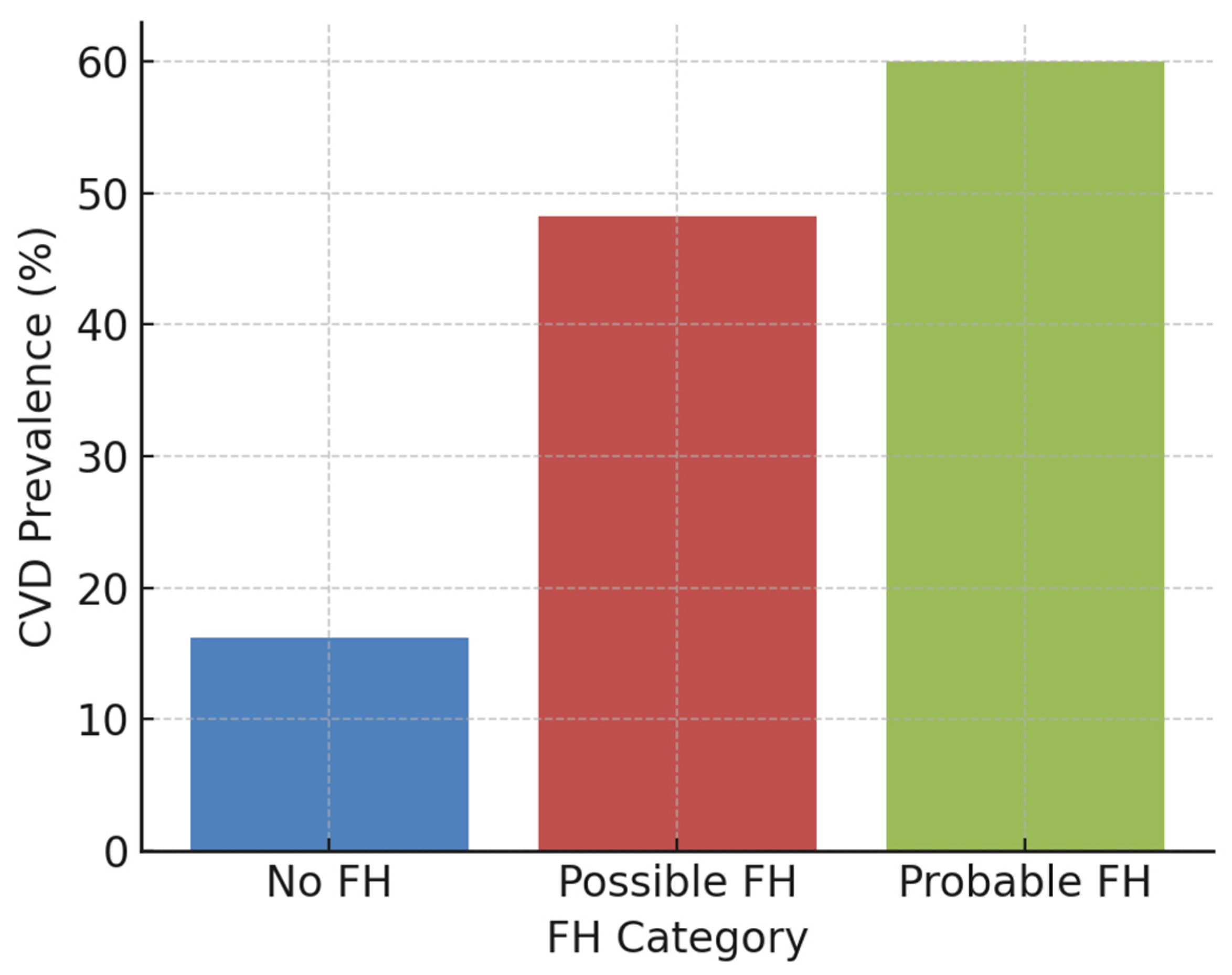
| CVD, % | 16.2% | 48.2% | 60% | <0.001 |
| Predictor | Adjusted OR (95% CI) | p-Value |
|---|---|---|
| Possible FH | 8.15 (5.30–12.53) | <0.0001 |
| Probable FH | 40.60 (9.15–180.2) | <0.0001 |
| Age, per 1 year | 1.035 (1.025–1.044) | <0.0001 |
| Male sex | 0.71 (0.52–0.90) | 0.0050 |
| Current smoker | 1.04 (0.74–1.44) | 0.8010 |
| Body mass index, per 1 kg/m2 | 1.04 (1.02–1.07) | 0.0010 |
| Systolic BP, per 1 mmHg | 1.005 (0.998–1.012) | 0.1540 |
| LDL-C, per 1 mmol/L | 0.61 (0.52–0.71) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletov, K.; Baibolsynova, I.; Umirbekov, N.; Auyelbekova, A.; Lee, S.; Kulmanbetov, R.; Kulimbet, M. Prevalence of Familial Hypercholesterolemia and Its Association with Cardiovascular Risk in a Cross-Sectional Adult Population. J. Clin. Med. 2025, 14, 8213. https://doi.org/10.3390/jcm14228213
Davletov K, Baibolsynova I, Umirbekov N, Auyelbekova A, Lee S, Kulmanbetov R, Kulimbet M. Prevalence of Familial Hypercholesterolemia and Its Association with Cardiovascular Risk in a Cross-Sectional Adult Population. Journal of Clinical Medicine. 2025; 14(22):8213. https://doi.org/10.3390/jcm14228213
Chicago/Turabian StyleDavletov, Kairat, Indira Baibolsynova, Nurdaulet Umirbekov, Ainagul Auyelbekova, Sergey Lee, Ruslan Kulmanbetov, and Mukhtar Kulimbet. 2025. "Prevalence of Familial Hypercholesterolemia and Its Association with Cardiovascular Risk in a Cross-Sectional Adult Population" Journal of Clinical Medicine 14, no. 22: 8213. https://doi.org/10.3390/jcm14228213
APA StyleDavletov, K., Baibolsynova, I., Umirbekov, N., Auyelbekova, A., Lee, S., Kulmanbetov, R., & Kulimbet, M. (2025). Prevalence of Familial Hypercholesterolemia and Its Association with Cardiovascular Risk in a Cross-Sectional Adult Population. Journal of Clinical Medicine, 14(22), 8213. https://doi.org/10.3390/jcm14228213







