Predicting Outcomes and Optimizing Therapy in Thrombotic Thrombocytopenic Purpura: Insights on Caplacizumab Use from a Romanian Hematology Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Subjects and Study Design
2.3. ADAMTS13 Activity Assays; Transition to Fully Automated FRET-Based Testing
2.4. Therapeutic Plasma Exchange
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Subgroup Analysis Based on Treatment Type
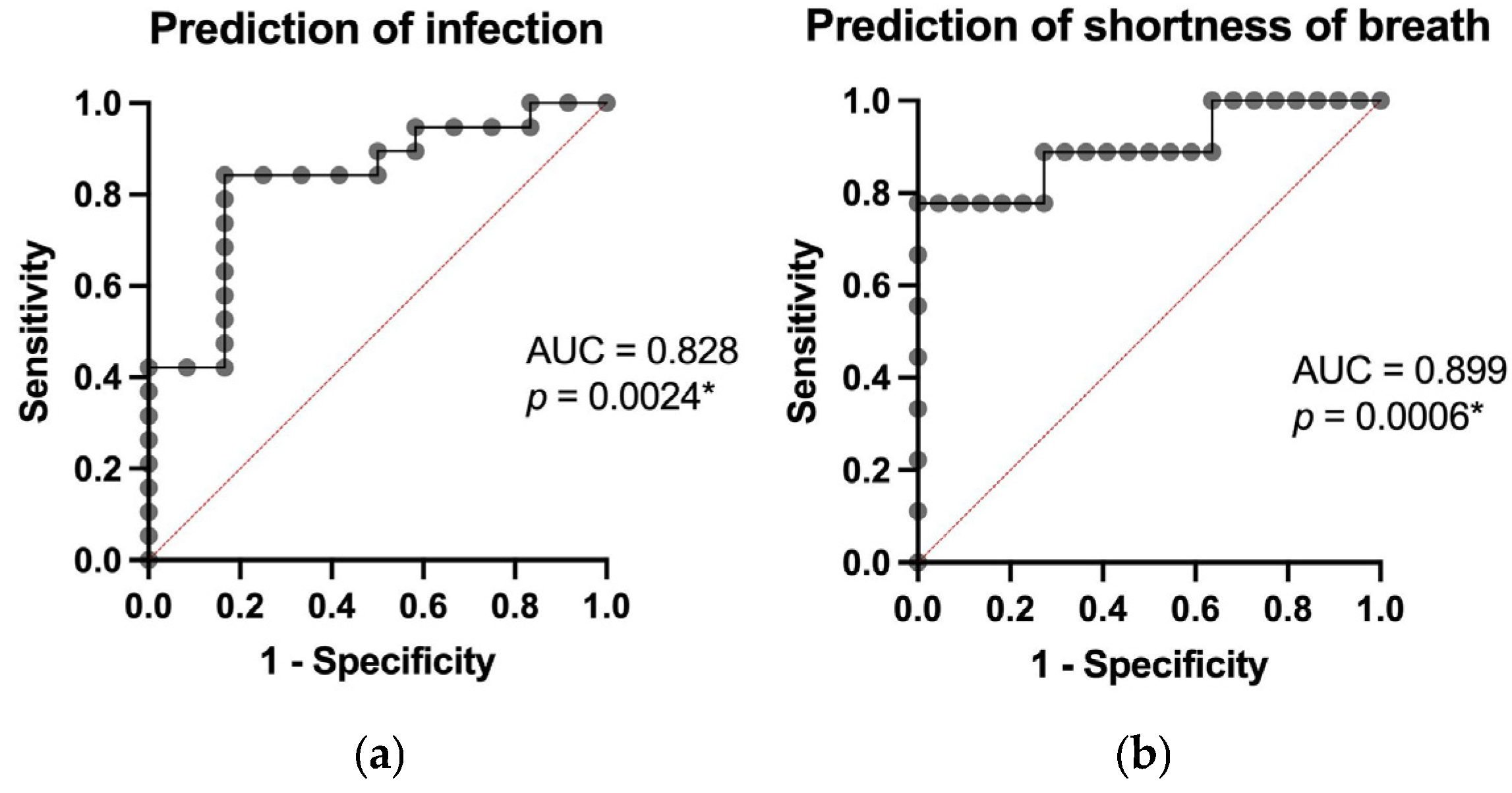
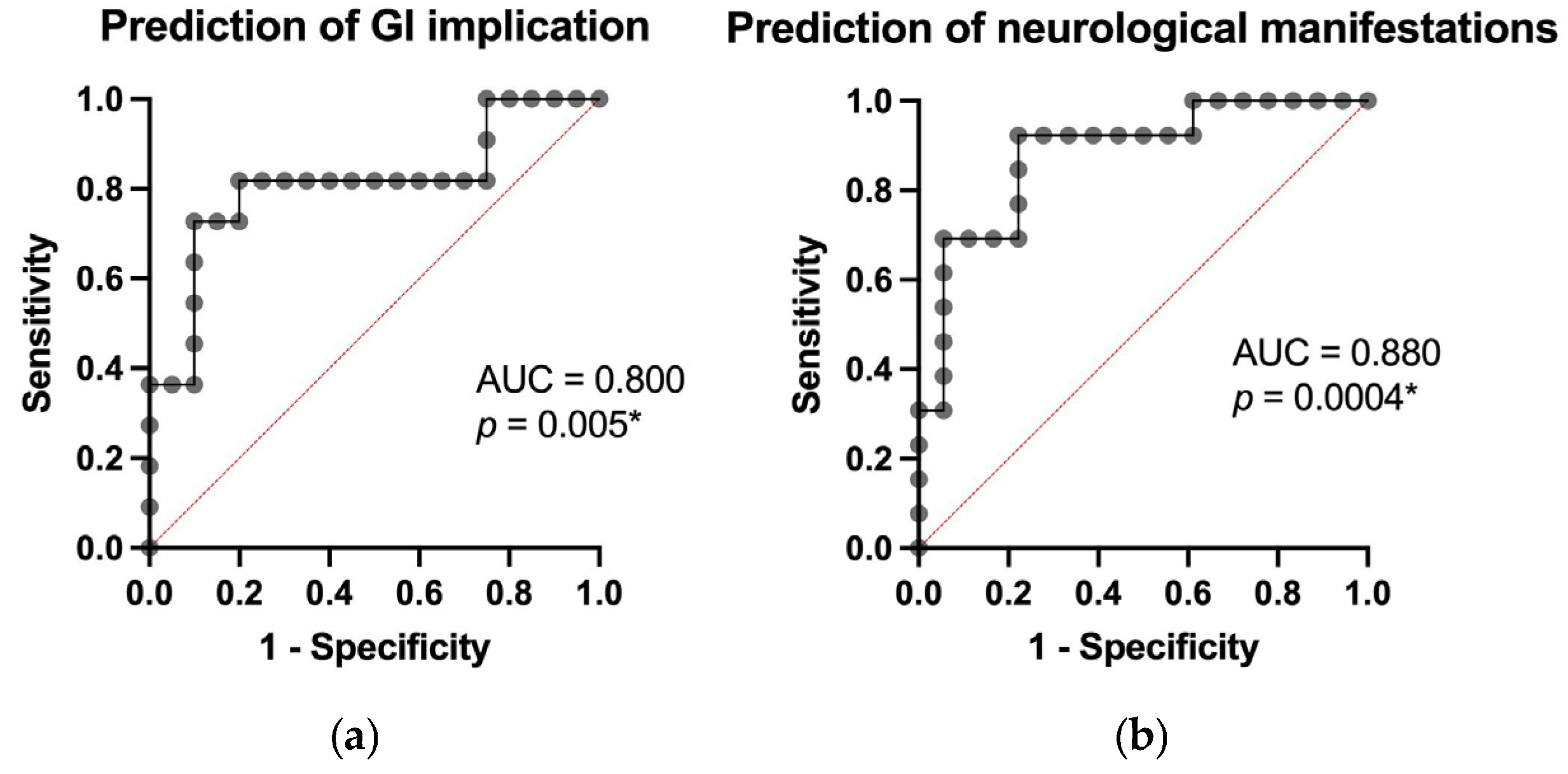
3.3. Predictive Modeling of Clinical Outcomes
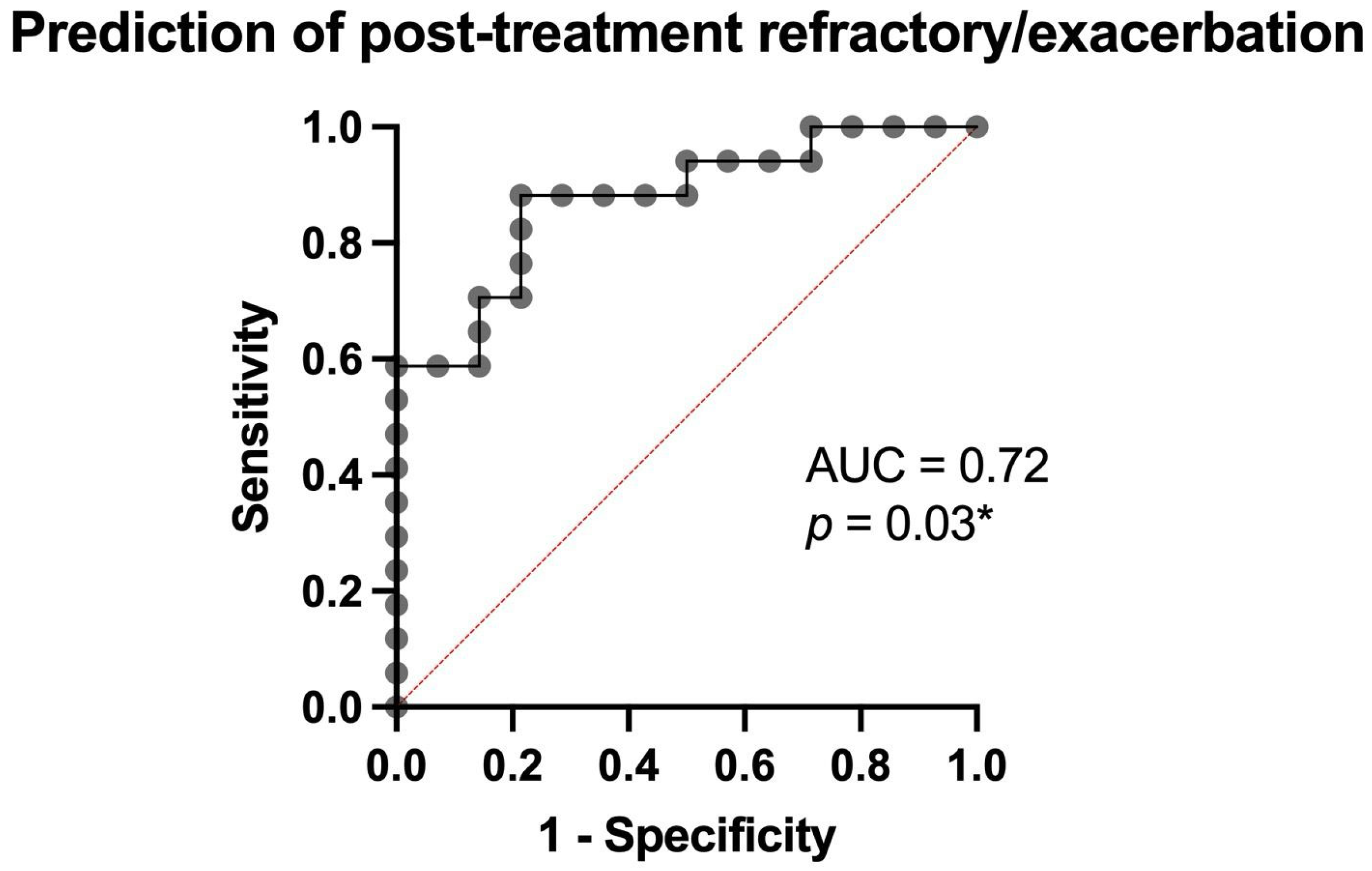
3.4. Prediction of Treatment Response and Post-Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FRET | Fluorescence resonance energy transfer |
| iTTP | Immune thrombotic thrombocytopenic purpura |
| TPE | therapeutic plasma exchange |
| CPP | Cryoprecipitate-poor plasma |
| TTP | Thrombotic thrombocytopenic purpura |
| TMAs | Thrombotic microangiopathies |
| vWF | von Willebrand factor |
| FFP | Fresh frozen plasma |
References
- Sukumar, S.; Lämmle, B.; Cataland, S.R. Clinical Medicine Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J. Clin. Med. 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; Hovinga, J.K.; Lämmle, B.; Matsumoto, M.; Pavenski, K.; Sadler, E.; et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 2017, 15, 312–322. Available online: https://www.jthjournal.org/action/showFullText?pii=S1538783622032500 (accessed on 14 March 2025). [CrossRef] [PubMed]
- Joly, B.S.; Coppo, P.; Veyradier, A. Thrombotic thrombocytopenic purpura. Blood 2017, 129, 2836–2846. [Google Scholar] [CrossRef]
- Amorosi, E.L.; Ultmann, J.E. Thrombotic Thrombocytopenic Purpura: Report of 16 Cases. Ann. Intern. Med. 1964, 61, 806. [Google Scholar] [CrossRef]
- Villarreal, A.E.; López, L. Thrombotic Thrombocytopenic Purpura. In The Brain of the Critically Ill Pregnant Woman; Academic Press: Cambridge, MA, USA, 2023; pp. 251–262. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430721/ (accessed on 14 March 2025).
- Mariotte, E.; Azoulay, E.; Galicier, L.; Rondeau, E.; Zouiti, F.; Boisseau, P.; Poullin, P.; De Maistre, E.; Provôt, F.; Delmas, Y.; et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): A cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016, 3, e237–e245. [Google Scholar] [CrossRef]
- Moschcowitz, E. An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries: An undescribed disease. Arch. Intern. Med. 1925, 36, 89–93. Available online: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/534853 (accessed on 13 March 2025). [CrossRef]
- Bendapudi, P.K.; Stefely, J.A.; Makar, R.S. Recombinant ADAMTS13 for Immune Thrombotic Thrombocytopenic Purpura. Reply. N. Engl. J. Med. 2024, 391, 383–384. Available online: http://www.ncbi.nlm.nih.gov/pubmed/39047257 (accessed on 4 June 2025).
- George, J.N. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 2010, 116, 4060–4069. [Google Scholar] [CrossRef]
- Scully, M.; Hunt, B.J.; Benjamin, S.; Liesner, R.; Rose, P.; Peyvandi, F.; Cheung, B.; Machin, S.J.; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br. J. Haematol. 2012, 158, 323–335. [Google Scholar] [CrossRef]
- Scully, M.; Yarranton, H.; Liesner, R.; Cavenagh, J.; Hunt, B.; Benjamin, S.; Bevan, D.; Mackie, I.; Machin, S. Regional UK TTP Registry: Correlation with laboratory ADAMTS 13 analysis and clinical features. Br. J. Haematol. 2008, 142, 819–826. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2141.2008.07276.x (accessed on 1 April 2025). [CrossRef]
- Lian, E.C.; Mui, P.T.; Siddiqui, F.A.; Chiu, A.Y.; Chiu, L.L. Inhibition of Platelet-aggregating Activity in Thrombotic Thrombocytopenic Purpura Plasma by Normal Adult Immunoglobulin G. J. Clin. Investig. 1984, 73, 548–555. [Google Scholar] [CrossRef][Green Version]
- McCarthy, L.J.; Dlott, J.S.; Orazi, A.; Waxman, D.; Miraglia, C.C.; Danielson, C.F.M. Thrombotic Thrombocytopenic Purpura: Yesterday, Today, Tomorrow. Ther. Apher. Dial. 2004, 8, 80–86. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1526-0968.2003.00113.x (accessed on 12 March 2025). [CrossRef]
- Moschcowitz, E. Hyaline thrombosis of the terminal arterioles and capillaries: A hitherto undescribed disease. Proc. NY Pathol. Soc. 1924, 24, 21–24. [Google Scholar]
- Kyttaris, V.C. Targeting B cells in Severe Thrombotic Thrombocytopenic Purpura-A road to cure? Crit. Care Med. 2012, 40, 317. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3243898/ (accessed on 2 April 2025). [CrossRef] [PubMed][Green Version]
- Brunskill, S.J.; Tusold, A.; Benjamin, S.; Stanworth, S.J.; Murphy, M.F. A systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Transfus. Med. 2007, 17, 17–35. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-3148.2006.00720.x (accessed on 2 April 2025). [CrossRef] [PubMed]
- Roose, E.; Joly, B.S. Current and Future Perspectives on ADAMTS13 and Thrombotic Thrombocytopenic Purpura. Hamostaseologie 2020, 40, 322–336. Available online: http://www.thieme-connect.com/products/ejournals/html/10.1055/a-1171-0473 (accessed on 2 April 2025). [CrossRef] [PubMed]
- Mafra, M.; Mora, M.M.R.; Castanha, E.; Godoi, A.; S, A.V. Comparing cryoprecipitate-poor plasma to fresh frozen plasma as replacement therapy in thrombotic thrombocytopenic purpura: An updated meta-analysis. Transfus. Apher. Sci. 2025, 64, 104040. [Google Scholar] [CrossRef]
- Stefanello, B.; De Paula, E.V.; Orsi, F.A.; Marques, J.F.C.; Roveri, E.G.; Colella, M.P.; Ozelo, M.C.; Annichino-Bizzacchi, J.M.; Addas-Carvalho, M. Safety and efficacy of cryoprecipitate-poor plasma as a replacement fluid for therapeutic plasma exchange in thrombotic thrombocytopenic purpura: A single center retrospective evaluation. J. Clin. Apher. 2014, 29, 311–315. [Google Scholar] [CrossRef]
- Altuntas, F.; Aydogdu, I.; Kabukcu, S.; Kocyigit, I.; Cıkım, K.; Sarı, I.; Erkut, M.A.; Eser, B.; Ozturk, A.; Kaya, E.; et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: A retrospective multicenter study. Transfus. Apher. Sci. 2007, 36, 57–67. [Google Scholar] [CrossRef]
- Nomura, S. Advances in diagnosis and treatments for immune thrombocytopenia. Clin. Med. Insights: Blood Disord. 2016, 9, 15–22. [Google Scholar] [CrossRef]
- Matsumoto, M.; Miyakawa, Y.; Kokame, K.; Ueda, Y.; Wada, H.; Higasa, S.; Yagi, H.; Ogawa, Y.; Sakai, K.; Miyata, T.; et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) in Japan 2023. Int. J. Hematol. 2023, 118, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Moake, J.L.; Rudy, C.K.; Troll, J.H.; Weinstein, M.J.; Colannino, N.M.; Azocar, J.; Seder, R.H.; Hong, S.L.; Deykin, D. Unusually Large Plasma Factor VIII: Von Willebrand Factor Multimers in Chronic Relapsing Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 1982, 307, 1432–1435. Available online: https://www.nejm.org/doi/abs/10.1056/NEJM198212023072306 (accessed on 14 March 2025). [CrossRef] [PubMed]
- de la Rubia, J.; Moscardó, F.; Gómez, M.J.; Guardia, R.; Rodríguez, P.; Sebrango, A.; Zamora, C.; Debén, G.; Goterris, R.; López, R.; et al. Efficacy and safety of rituximab in adult patients with idiopathic relapsing or refractory thrombotic thrombocytopenic purpura: Results of a Spanish multicenter study. Transfus. Apher. Sci. 2010, 43, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Cataland, S.R.; Jin, M.; Ferketich, A.K.; Kennedy, M.S.; Kraut, E.H.; George, J.N.; Wu, H.M. An evaluation of ciclosporin and corticosteroids individually as adjuncts to plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Br. J. Haematol. 2006, 136, 146–149. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2141.2006.06384.x (accessed on 2 April 2025). [CrossRef]
- Tsai, H.M. Thrombotic Thrombocytopenic Purpura: Beyond Empiricism and Plasma Exchange. Am. J. Med. 2019, 132, 1032–1037. [Google Scholar] [CrossRef]
- Butt, A.; Allen, C.; Purcell, A.; Ito, S.; Goshua, G. Global Health Resource Utilization and Cost-Effectiveness of Therapeutics and Diagnostics in Immune Thrombotic Thrombocytopenic Purpura (TTP). J. Clin. Med. 2023, 12, 4887. [Google Scholar] [CrossRef]
- Scully, M.; McDonald, V.; Cavenagh, J.; Hunt, B.J.; Longair, I.; Cohen, H.; Machin, S.J. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 2011, 118, 1746–1753. Available online: https://pubmed.ncbi.nlm.nih.gov/21636861/ (accessed on 4 June 2025). [CrossRef]
- Chaturvedi, S.; Antun, A.G.; Farland, A.M.; Woods, R.; Metjian, A.; Park, Y.A.; de Ridder, G.; Gibson, B.; Kasthuri, R.S.; Liles, D.K.; et al. Race, rituximab, and relapse in TTP. Blood 2022, 140, 1335–1344. [Google Scholar] [CrossRef]
- Owattanapanich, W.; Wongprasert, C.; Rotchanapanya, W.; Owattanapanich, N.; Ruchutrakool, T. Comparison of the Long-Term Remission of Rituximab and Conventional Treatment for Acquired Thrombotic Thrombocytopenic Purpura: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. 2019, 25, 1076029618825309. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6714958/ (accessed on 4 June 2025). [CrossRef]
- Banugaria, S.G.; Prater, S.N.; McGann, J.K.; Feldman, J.D.; Tannenbaum, J.A.; Bailey, C.; Gera, R.; Conway, R.L.; Viskochil, D.; Kobori, J.A.; et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: Lessons learned from Pompe disease. Genet. Med. 2013, 15, 123–131. Available online: https://pubmed.ncbi.nlm.nih.gov/23060045/ (accessed on 4 June 2025). [CrossRef]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; De La Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1806311 (accessed on 4 June 2025). [CrossRef]
- Peyvandi, F.; Scully, M.; Kremer Hovinga, J.A.; Cataland, S.; Knöbl, P.; Wu, H.; Artoni, A.; Westwood, J.-P.; Mansouri Taleghani, M.; Jilma, B.; et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2016, 374, 511–522. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1505533 (accessed on 4 June 2025). [CrossRef] [PubMed]
- Izquierdo, C.P.; Mingot-Castellano, M.E.; Fuentes, A.E.K.; Peinado, J.G.-A.; Cid, J.; Jimenez, M.M.; Valcarcel, D.; Gómez-Seguí, I.; de la Rubia, J.; Martin, P.; et al. Real-world effectiveness of caplacizumab vs. the standard of care in immune thrombotic thrombocytopenic purpura. Blood Adv. 2022, 6, 6219–6227. Available online: https://pubmed.ncbi.nlm.nih.gov/35930694/ (accessed on 4 June 2025). [CrossRef]
- Ulrichts, H.; Silence, K.; Schoolmeester, A.; de Jaegere, P.; Rossenu, S.; Roodt, J.; Priem, S.; Lauwereys, M.; Casteels, P.; Van Bockstaele, F.; et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood 2011, 118, 757–765. Available online: https://pubmed.ncbi.nlm.nih.gov/21576702/ (accessed on 4 June 2025). [CrossRef] [PubMed]
- Pavenski, K.; Scully, M.; Coppo, P.; Cataland, S.; Knöbl, P.; Peyvandi, F.; Hovinga, J.A.K.; de la Rubia, J.; Khan, U.; Marques, A.P.; et al. Caplacizumab improves clinical outcomes and is well tolerated across clinically relevant subgroups of patients with immune-mediated thrombotic thrombocytopenic purpura. Res. Pr. Thromb. Haemost. 2024, 8, 102512. Available online: https://pubmed.ncbi.nlm.nih.gov/39221451/ (accessed on 4 June 2025). [CrossRef]
- Zuno, J.A.N.; Khaddour, K. Thrombotic Thrombocytopenic Purpura Evaluation and Management. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470585/ (accessed on 4 June 2025).
- Kubo, M.; Matsumoto, M. Frontiers in pathophysiology and management of thrombotic thrombocytopenic purpura. Int. J. Hematol. 2023, 117, 331–340. Available online: https://link.springer.com/article/10.1007/s12185-023-03552-8 (accessed on 4 June 2025). [CrossRef]
- Coppo, P.; Bubenheim, M.; Benhamou, Y.; Völker, L.; Brinkkötter, P.; Kühne, L.; Knöbl, P.; Mingot-Castellano, M.E.; Pascual-Izquierdo, C.; de la Rubia, J.; et al. Caplacizumab use in immune-mediated thrombotic thrombocytopenic purpura: An international multicentre retrospective Cohort study (The Capla 1000+ project). eClinicalMedicine 2025, 82, 103168. [Google Scholar] [CrossRef]

| Age (yr) | HEMOGLOBIN (g/dL) | LEUKOCYTES (×103/µL) | PLATELETS (×103/µL) | CREATININE (μmol/L) | BILIRUBIN (μmol/L) | LDH (U/L) | PLASMIC SCORE (Points) | |
|---|---|---|---|---|---|---|---|---|
| OVERALL (n = 31) | 44 ± 11.16 | 7.79 ± 1.33 | 11.3 ± 4.48 | 16.5 ± 11.87 | 1.2 ± 0.56 | 2.8 ± 1.8 | 1925 ± 1142 | 6 ± 0.74 |
| CAPLACIZUMAB GROUP (n = 16) | 44 ± 11.25 | 7.9 ± 1.388 | 10.5 ± 3.85 | 14.8 ± 11.6 | 1.1 ± 0.5 | 2.3 ± 1.1 | 2000 ± 1280 | 5.9 ± 0.68 |
| PEX GROUP (n = 15) | 44.7 ± 11.46 | 7.6 ± 1.28 | 12.2 ± 5 | 18 ± 12.1 | 1.2 ± 0.57 | 3.4 ± 2.3 | 1837 ± 1011 | 6 ± 0.7 |
| Mann–Whitney (p) | 0.89 | 0.6 | 0.47 | 0.34 | 0.9 | 0.22 | 0.98 | 0.13 |
| PEX SESIONS (No.) | HOSPITAL STAY (Days) | ICU STAY (Days) | PLATELET RECOVERY (Days) | |
|---|---|---|---|---|
| OVERALL (n = 31) | 6.1 ± 3.46 | 15 ± 10.94 | 1.3 ± 3.2 | 6 ± 3.73 |
| CAPLACIZUMAB (n = 16) | 5.6 ± 3.1 | 10.4 ± 4.5 | 1.3 ± 3.4 | 4.6 ± 2.8 |
| PEX GROUP (n = 15) | 6.6 ± 3.7 | 20.8 ± 13.22 | 1.4 ± 3.1 | 7.7 ± 4 |
| Mann–Whitney (p) | 0.48 | 0.004 * | 0.66 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preda, D.O.; Himcinschi, M.E.; Vlad, A.; Gauianu, F.A.; Murariu, D.-N.; Croitoru, O.-R.; Coriu, D.; Badelita, S.N. Predicting Outcomes and Optimizing Therapy in Thrombotic Thrombocytopenic Purpura: Insights on Caplacizumab Use from a Romanian Hematology Center. J. Clin. Med. 2025, 14, 8211. https://doi.org/10.3390/jcm14228211
Preda DO, Himcinschi ME, Vlad A, Gauianu FA, Murariu D-N, Croitoru O-R, Coriu D, Badelita SN. Predicting Outcomes and Optimizing Therapy in Thrombotic Thrombocytopenic Purpura: Insights on Caplacizumab Use from a Romanian Hematology Center. Journal of Clinical Medicine. 2025; 14(22):8211. https://doi.org/10.3390/jcm14228211
Chicago/Turabian StylePreda, Diana Oana, Mihai Emanuel Himcinschi, Adelina Vlad, Florentina Adriana Gauianu, Daniel-Nicolae Murariu, Oana-Ruxandra Croitoru, Daniel Coriu, and Sorina Nicoleta Badelita. 2025. "Predicting Outcomes and Optimizing Therapy in Thrombotic Thrombocytopenic Purpura: Insights on Caplacizumab Use from a Romanian Hematology Center" Journal of Clinical Medicine 14, no. 22: 8211. https://doi.org/10.3390/jcm14228211
APA StylePreda, D. O., Himcinschi, M. E., Vlad, A., Gauianu, F. A., Murariu, D.-N., Croitoru, O.-R., Coriu, D., & Badelita, S. N. (2025). Predicting Outcomes and Optimizing Therapy in Thrombotic Thrombocytopenic Purpura: Insights on Caplacizumab Use from a Romanian Hematology Center. Journal of Clinical Medicine, 14(22), 8211. https://doi.org/10.3390/jcm14228211





